Soil and Sediments in Natural Underground Ecosystems as a Source of Culturable Micromycetes: A Case Study of the Brestovská Cave (Western Tatras, Slovakia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Isolation of Fungi from Samples
2.4. Fungal Identification
2.5. Data Analyses
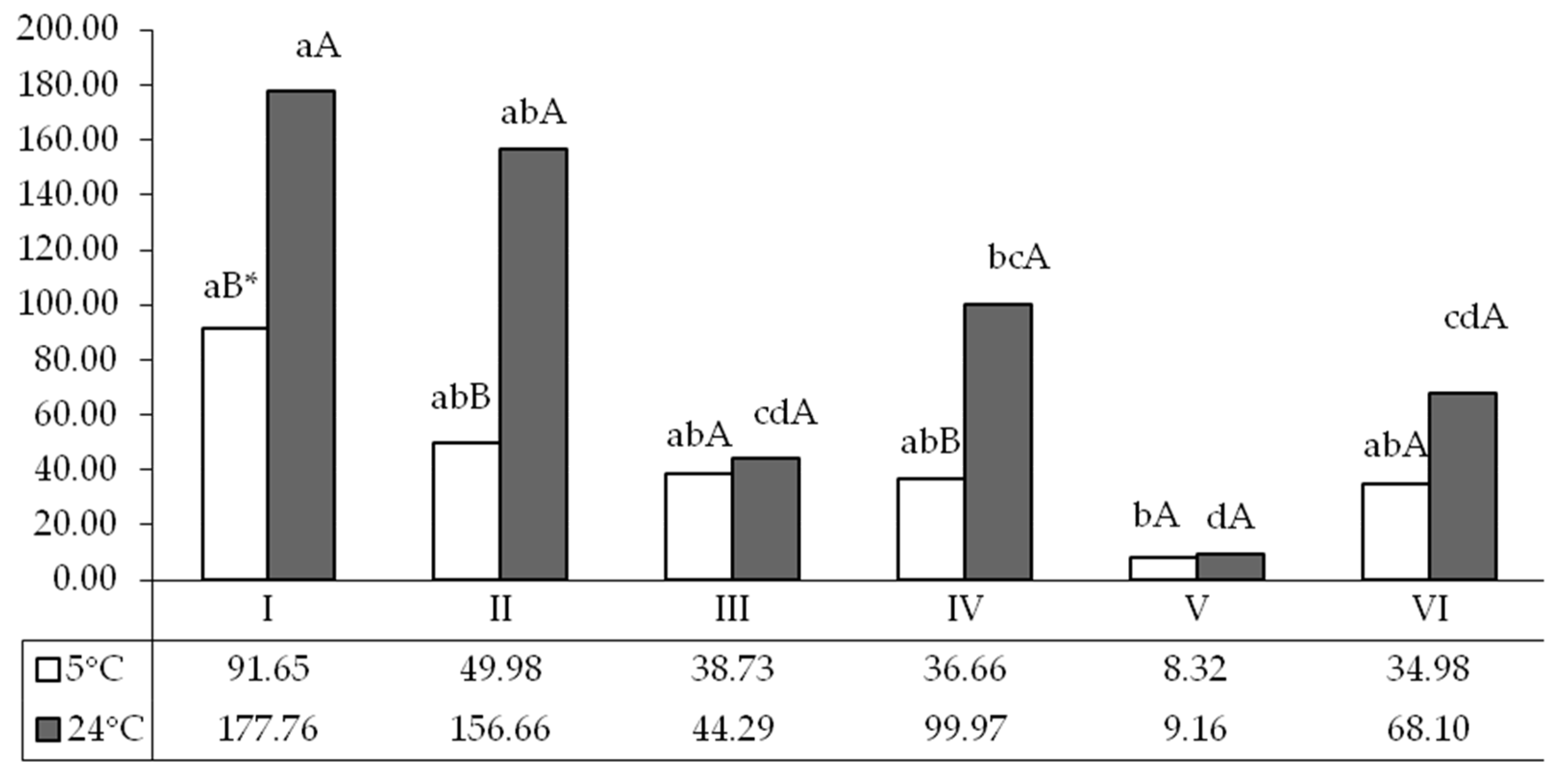
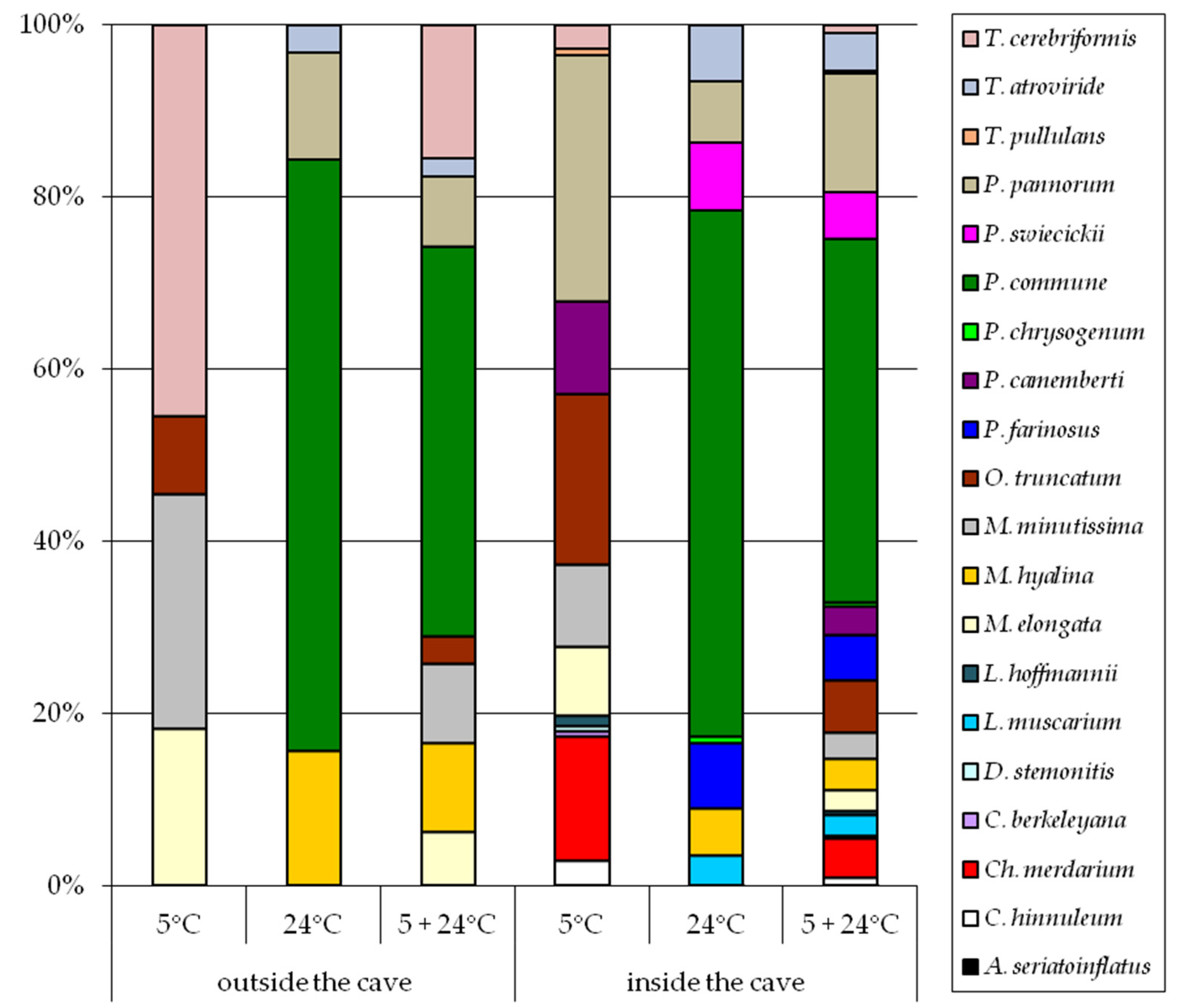
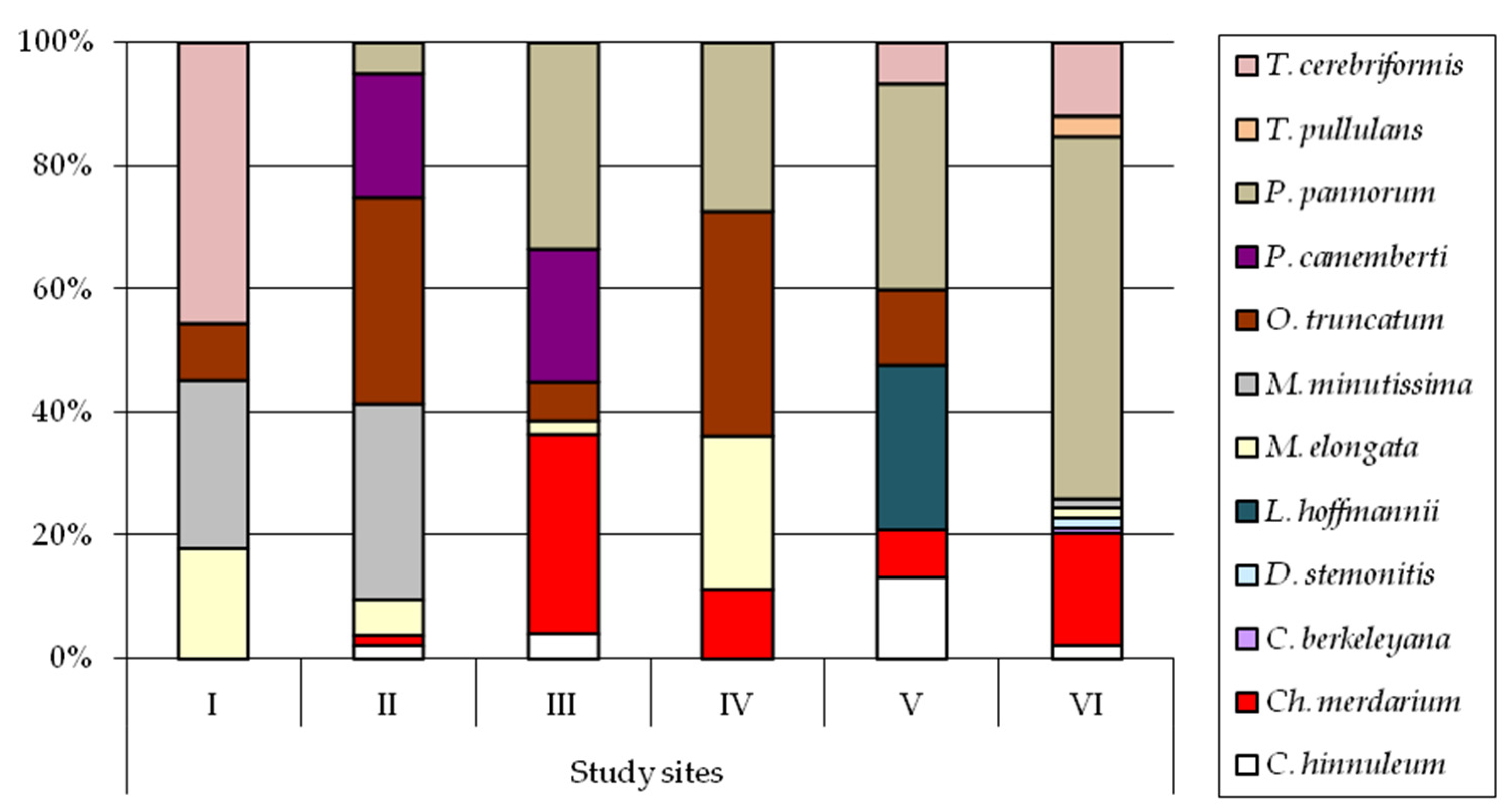
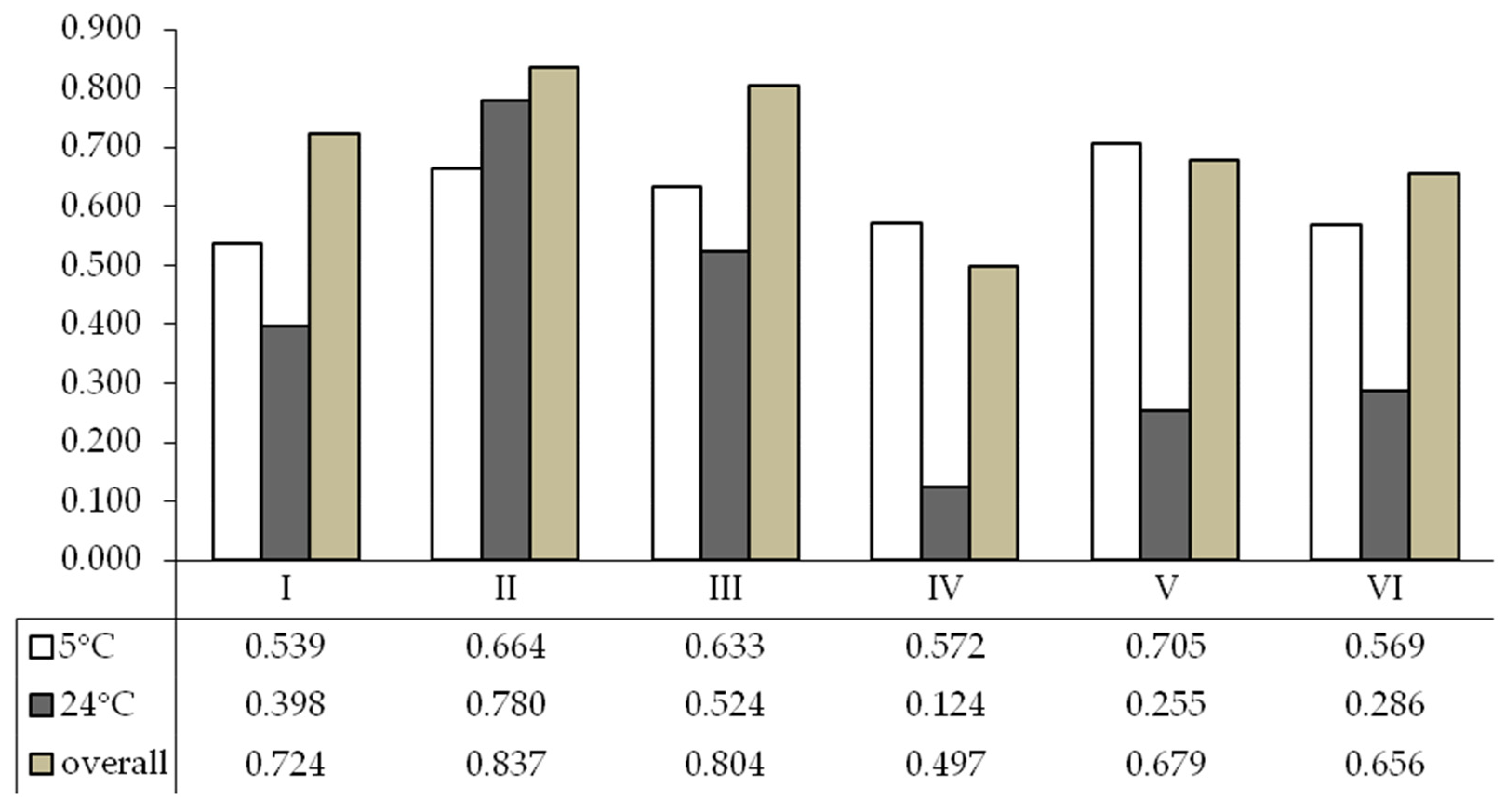
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Fungal Species | Fungi Cultured at 5 °C | Fungi Cultured at 24 °C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | I | II | III | IV | V | VI | |
| Ambomucor seriatoinflatus | —1 | — | — | — | — | — | — | — | — | — | — | 0.03 |
| Cephalotrichum hinnuleum | — | 1.25 | 1.67 | — | 1.11 | 0.83 | — | — | — | — | — | — |
| Chrysosporium merdarium | — | 0.83 | 12.50 | 4.17 | 0.66 | 6.39 | — | — | — | — | — | — |
| Cosmospora berkeleyana | — | — | — | — | — | 0.28 | — | — | — | — | — | — |
| Doratomyces stemonitis | — | — | — | — | — | 0.55 | — | — | — | — | — | — |
| Lecanicillium muscarium | — | — | — | — | — | — | — | 13.33 | — | — | — | — |
| Lecythophora hoffmannii | — | — | — | — | 2.22 | — | — | — | — | — | — | — |
| Mortierella elongata | 16.66 | 2.91 | 0.83 | 9.16 | — | 0.64 | — | — | — | — | — | — |
| Mortierella hyalina | — | — | — | — | — | 27.77 | 8.33 | — | 8.33 | — | 3.83 | |
| Mortierella minutissima | 25.00 | 15.83 | — | — | — | 0.47 | — | — | — | — | — | — |
| Oidiodendron truncatum | 8.33 | 16.66 | 2.50 | 13.33 | 1.00 | — | — | — | — | — | — | — |
| Paecilomyces farinosus | — | — | — | — | — | — | — | 25.00 | 2.50 | — | — | 0.87 |
| Penicillium camemberti | — | 10.00 | 8.33 | — | — | — | — | — | — | — | — | — |
| Penicillium chrysogenum | — | — | — | — | — | — | — | — | 2.91 | — | — | 0.14 |
| Penicillium commune | — | — | — | — | — | — | 122.22 | 50.00 | 27.50 | 91.67 | 6.66 | 55.55 |
| Penicillium swiecickii | — | — | — | — | — | — | — | 28.33 | 1.66 | — | — | — |
| Pseudogymnoascus pannorum | — | 2.50 | 12.9 | 10.00 | 2.78 | 20.55 | 22.22 | 16.67 | 7.64 | — | 2.50 | 0.18 |
| Tausonia pullulans | — | — | — | — | — | 1.11 | — | — | — | — | — | — |
| Trichoderma atroviride | — | — | — | — | — | — | 5.55 | 15.00 | 2.08 | — | — | 7.50 |
| Trichosporiella cerebriformis | 41.66 | — | — | — | 0.55 | 4.16 | — | — | — | — | — | — |
References
- Ogórek, R.; Speruda, M.; Borzęcka, J.; Piecuch, A.; Cal, M. First Speleomycological Study on the Occurrence of Psychrophilic and Psychrotolerant Aeromycota in the Brestovská Cave (Western Tatras Mts., Slovakia) and First Reports for Some Species at Underground Sites. Biology 2021, 10, 497. [Google Scholar] [CrossRef]
- Kuzmina, L.Y.; Galimzianova, N.F.; Abdullin, S.R.; Ryabova, A.S. Microbiota of the Kinderlinskaya Cave (South Urals, Russia). Microbiology 2012, 81, 251–258. [Google Scholar] [CrossRef]
- Gabriel, C.R.; Northup, D.E. Microbial ecology: Caves as an extreme habitat. In Cave Microbiomes: A Novel Resource for Drug Discovery; Cheeptham, N., Ed.; Springer Press: New York, NY, USA, 2013; pp. 85–108. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhao, P.; Cai, L. Origin of Cave Fungi. Front. Microbiol. 2018, 9, 1407. [Google Scholar] [CrossRef]
- Cigna, A.A. Modern trend(s) in cave monitoring. Acta Carsologica 2002, 31, 35–54. [Google Scholar] [CrossRef]
- Ogórek, R.; Pusz, W.; Zagożdżon, P.P. Abundance and diversity of psychrotolerant cultivable mycobiota in winter of a former aluminous shale mine. Geomicrobiol. J. 2017, 34, 823–833. [Google Scholar] [CrossRef]
- Ogórek, R.; Suchodolski, J.; Piecuch, A.; Przywara, K.; Višňovská, Z. Keratinophilic and Keratinolytic Fungi in Cave Ecosystems: A Culture-Based Study of Brestovská Cave and Demänovská Ľadová and Slobody Caves (Slovakia). Appl. Sci. 2022, 12, 1455. [Google Scholar] [CrossRef]
- Pusz, W.; Ogórek, R.; Knapik, R.; Kozak, B.; Bujak, H. The Occurrence of Fungi in the Recently Discovered Jarkowicka Cave in the Karkonosze Mts. (Poland). Geomicrobiol. J. 2014, 32, 59–67. [Google Scholar] [CrossRef]
- Ghosh, S.; Kuisiene, N.; Cheeptham, N. The cave microbiome as a source for drug discovery: Reality or pipe dream? Biochem. Pharmacol. 2017, 134, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Nováková, A.; Hubka, V.; Valinová, Š.; Kolařík, M.; Hillebrand-Voiculescu, A.M. Cultivable microscopic fungi from an underground chemosynthesis-based ecosystem: A preliminary study. Folia Microbiol. 2018, 63, 43–55. [Google Scholar] [CrossRef]
- Burow, K.; Grawunder, A.; Harpke, M.; Pietschmann, S.; Ehrhardt, R.; Wagner, L.; Voigt, K.; Merten, D.; Büchel, G.; Kothe, E. Microbiomes in an acidic rock–water cave system. FEMS Microbiol. Lett. 2019, 366, fnz167. [Google Scholar] [CrossRef]
- Poulson, T.L.; White, W.B. The cave environment. Science 1969, 165, 71–981. [Google Scholar] [CrossRef] [PubMed]
- Barton, H.A.; Northup, D.E. Geomicrobiology in cave environments: Past, current and future perspectives. J. Cave Karst Stud. 2007, 69, 163–178. [Google Scholar]
- Vanderwolf, K.J.; Malloch, D.; McAlpine, D.F.; Forbes, G. A world review of fungi, yeasts, and slime molds in caves. Int. J. Speleol. 2013, 142, 77–96. [Google Scholar] [CrossRef]
- Benoit, J.B.; Yoder, J.A.; Zettler, L.W.; Hobbs, H.H. Mycoflora of a trogloxenic Cave Cricket, Hadenoecus cumberlandicus (Orthoptera: Rhaphidophoridae), from two small caves in northeastern Kentucky. Ann. Entomol. Soc. Am. 2004, 97, 989–993. [Google Scholar] [CrossRef]
- Santamaria, S.; Faille, A. Rhachomyces (Ascomycota, Laboulbeniales) parasites on cave inhabiting Carabid beetles from the Pyrenees. Nova Hedwig. 2007, 85, 159–186. [Google Scholar] [CrossRef]
- Yoder, J.A.; Benoit, J.B.; Christensen, B.S.; Croxall, T.J.; Hobbs, H.H. Entomopathogenic fungi carried by the cave orb weaver spider, Meta ovalis (Araneae, Tetragnathidae), with implications for mycoflora transfer to cave crickets. J. Cave Karst Stud. 2009, 71, 116–120. [Google Scholar]
- Cubbon, B.D. Cave flora. In The Science of Speleology; Ford, T.D., Cullingford, C.H.D., Eds.; Academic Press: London, UK, 1976; pp. 423–452. [Google Scholar]
- Bindschedler, S.; Milliere, L.; Cailleau, G.; Job, D.; Verrecchia, E.P. An ultrastructural approach to analogies between fungal structures and needle fiber calcite. Geomicrobiol. J. 2012, 29, 301–313. [Google Scholar] [CrossRef]
- Engel, A.S. Microbial Diversity of Cave Ecosystems. In Geomicrobiology: Molecular and Environmental Perspective; Barton, L., Mandl, M., Loy, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 219–238. [Google Scholar] [CrossRef]
- Barton, H.A.; Jurado, V. What’s up down there? Microbial Diversity in Caves Microorganisms in caves survive under nutrient-poor conditions and are metabolically versatile and unexpectedly diverse. Microbe 2007, 2, 132–138. [Google Scholar]
- Jurado, V.; Sanchez-Moral, S.; Saiz-Jimenez, C. Entomogenous fungi and the conservation of the cultural heritage: A review. Int. Biodeterior. Biodegrad. 2008, 62, 325–330. [Google Scholar] [CrossRef]
- Ogórek, R.; Dyląg, M.; Kozak, B.; Višňovská, Z.; Tancinova, D.; Lejman, A. Fungi isolated and quantified from bat guano and air in Harmanecka’ and Driny Caves (Slovakia). J. Cave Karst Stud. 2016, 78, 41–49. [Google Scholar] [CrossRef]
- Borzęcka, J.; Piecuch, A.; Kokurewicz, T.; Lavoie, K.H.; Ogórek, R. Greater Mouse-Eared Bats (Myotis myotis) Hibernating in the Nietoperek Bat Reserve (Poland) as a Vector of Airborne Culturable Fungi. Biology 2021, 10, 593. [Google Scholar] [CrossRef] [PubMed]
- Ogórek, R.; Pusz, W.; Lejman, A.; Uklańska-Pusz, C. Microclimate effects on number and distribution of fungi in the Włodarz undeground complex in the Owl Mountains (Góry Sowie), Poland. J. Cave Karst Stud. 2014, 76, 146–153. [Google Scholar] [CrossRef]
- Ogórek, R.; Lejman, A.; Matkowski, K. Influence of the external environment on airborne fungi isolated from a cave. Pol. J. Environ. Stud. 2014, 23, 435–440. [Google Scholar]
- Shapiro, J.; Pringle, A. Anthropogenic Influences on the Diversity of Fungi Isolated from Caves in Kentucky and Tennessee. Am. Midl. Nat. 2010, 163, 76–86. [Google Scholar] [CrossRef]
- Sun, J.M.; Irzykowski, W.; Jędryczka, M.; Han, F.H. Analysis of the genetic structure of Sclerotinia sclerotiorum (Lib.) de Bary populations from different regions and host plants by Random Amplified Polymorphic DNA markers. J. Integr. Plant. Biol. 2005, 47, 385–395. [Google Scholar] [CrossRef]
- Treseder, K.K.; Lennon, J.T. Fungal traits that drive ecosystem dynamics on land. Microbiol. Mol. Biol. Rev. 2015, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Žifčáková, L.; Vetrovský, T.; Howe, A.; Baldrian, P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ. Microbiol. 2016, 18, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef]
- Lavelle, P.; Spain, A.V. Soil Organisms. In Soil Ecology; Springer: Dordrecht, The Netherlands, 2005; pp. 201–356. [Google Scholar] [CrossRef]
- Naga, K.; Suzuk, K.; Okada, G. Studies on the distribution of alkalophilic and alkali-tolerant soil fungi II: Fungal flora in two limestone caves in Japan. Mycoscience 1998, 39, 293–298. [Google Scholar] [CrossRef]
- Baldrian, P. Interactions of heavy metals with white-rot fungi. Enzym. Microb. Technol. 2003, 32, 78–91. [Google Scholar] [CrossRef]
- Creecy, J.P.; Caire, W.; Gilcrest, K.A. Examination of several Oklahoma bat hibernacula cave soils for Pseudogymnoascus destructans, the causative agent of White-Nose Syndrome. Southwest. Nat. 2015, 60, 213–217. [Google Scholar] [CrossRef]
- Reynolds, H.T.; Ingersoll, T.; Barton, H.A. Modeling the environmental growth of Pseudogymnoascus destructans and its impact on the White-Nose Syndrome Epidemic. J. Wildl. Dis. 2015, 51, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Droppa, A. Karst on Sivývrch. Ceskoslov. Kras 1972, 23, 77–98. (In Slovak) [Google Scholar]
- Brestovská Cave, Slovak Caves Administration. Available online: http://www.ssj.sk (accessed on 21 January 2024).
- van Tieghem, P. Troisième mémoire sur les Mucorinées. Ann. Sci. Nat. 1878, 4, 312–399. (In French) [Google Scholar]
- Karsten, P.A. Finlands mögelsvampar (Hyphomyctes fennici). Bidrag till Kännedom av Finlands Natur och Folk. Fin. Litt.-Sällskapets Tryckeri 1892, 51, 343–534. (In Swedish) [Google Scholar]
- Thom, C. Fungi in Cheese Ripening; Camembert and Roquefort; US Department of Agriculture, Bureau of Animal Industry: Washington, DC, USA, 1906; Volume 82, 39p. [Google Scholar]
- Thom, C. Cultural Studies of Species of Penicillium; US Department of Agriculture, Bureau of Animal Industry: Washington, DC, USA, 1910; Volume 118, 107p. [Google Scholar]
- Linnemann, G. Die Mucorineen-Gattung Mortierella Coemans. Pflanzenforschung 1941, 23, 1–64. (In German) [Google Scholar]
- Brown, A.H.S.; Smith, G. The genus Paecilomyces Bainier and its perfect stage Byssochlamys Westling. Trans. Br. Mycol. Soc. 1957, 40, 17–89. [Google Scholar] [CrossRef]
- Barron, G.L. New species and new records of Oidiodendron. Canad. J. Bot. 1962, 40, 589–607. [Google Scholar] [CrossRef]
- Carmichael, J.W. Chrysosporium and some other aleuriosporic Hyphomycetes. Canad. J. Bot. 1962, 40, 1137–1173. [Google Scholar] [CrossRef]
- Morton, F.J.; Smith, G. The genera Scopulariopsis Bainier, Microascus Zukal and Doratomyces Corda. Mycol. Pap. 1963, 86, 1–96. [Google Scholar]
- von Arx, J.A. Über die Typusart, zwei neue und einige weitere Arten der Gattung Sporotrichum. Persoonia 1971, 6, 179–184. (In German) [Google Scholar]
- Matsushima, T. Icones Microfungorum: A Matsushima Lectorum; Matsushima: Kobe, Japan, 1975; p. 63. [Google Scholar]
- Gams, W.; McGinnis, M.R. Phialemonium, a new anamorph genus intermediate between Phialophora and Acremonium. Mycologia 1983, 75, 977–987. [Google Scholar] [CrossRef]
- Zare, R.; Gams, W. A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium. Nova Hedwig. 2001, 73, 1–50. [Google Scholar] [CrossRef]
- Gräfenhan, T.; Schroers, H.J.; Nirenberg, H.I.; Seifert, K.A. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Stud. Mycol. 2011, 68, 79–113. [Google Scholar] [CrossRef] [PubMed]
- Minnis, A.M.; Lindner, D.L. Phylogenetic evaluation of Geomyces and allies reveals no close relatives of Pseudogymnoascus destructans, comb. nov., in bat hibernacula of eastern North America. Fungal Biol. 2013, 117, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.Y.; Liu, X.Y. Ambomucor gen. & spp. nov. from China. Mycotaxon 2013, 126, 7–108. [Google Scholar] [CrossRef]
- Visagie, C.M.; Hirooka, Y.; Tanney, J.B.; Whitfield, E.; Mwange, K.; Meijer, M.; Amend, A.S.; Seifert, K.A.; Samson, R.A. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Stud. Mycol. 2014, 78, 63–139. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Zheng, R.Y. New taxa of Ambomucor (Mucorales, Mucoromycotina) from China. Mycotaxon 2015, 130, 165–171. [Google Scholar] [CrossRef]
- Liu, X.Z.; Wang, Q.M.; Göker, M. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud. Mycol. 2015, 81, 85–147. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Guarro, J.; Cano-Lira, J.F.; Sutton, D.A.; Wiederhold, N.P.; de Hoog, G.S.; Abbott, S.P.; Decock, C.; Sigler, L.; Gené, J. Phylogeny and taxonomic revision of Microascaceae with emphasis on synnematous fungi. Stud. Mycol. 2016, 83, 193–233. [Google Scholar] [CrossRef]
- Kepler, R.M.; Luangsa-Ard, J.J.; Hywel-Jones, N.L.; Quandt, C.A.; Sung, G.H.; Rehner, S.A.; Aime, M.C.; Henkel, T.W.; Sanjuan, T.; Zare, R.; et al. A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 2017, 8, 335–353. [Google Scholar] [CrossRef] [PubMed]
- Dyląg, M.; Sawicki, A.; Ogórek, R. Diversity of Species and Susceptibility Phenotypes toward Commercially Available Fungicides of Cultivable Fungi Colonizing Bones of Ursus spelaeus on Display in Niedźwiedzia Cave (Kletno, Poland). Diversity 2019, 11, 224. [Google Scholar] [CrossRef]
- Vandepol, N.; Liber, J.; Desiró, A.; Na, H.; Kennedy, M.; Barry, K.; Grigoriev, I.V.; Miller, A.N.; O’Donnell, K.; Stajich, J.E.; et al. Resolving the Mortierellaceae phylogeny through synthesis of multi-gene phylogenetics and phylogenomics. Fungal Divers. 2020, 104, 267–289. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Ogórek, R.; Dyląg, M.; Kozak, B. Dark stains on rock surfaces in Driny Cave (Little Carpathian Mountains, Slovakia). Extremophiles 2016, 20, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wei, X.L.; Zhang, Y.Q.; Liu, H.Y.; Yu, L.Y. Diversity and distribution of lichen-associated fungi in the Ny-Ålesund Region (Svalbard, High Arctic) as revealed by pyrosequencing. Sci. Rep. 2015, 14, 14850. [Google Scholar] [CrossRef]
- Spellerberg, I.F.; Fedor, P. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Kokurewicz, T.; Ogórek, R.; Pusz, W.; Matkowski, K. Bats increase the number of cultivable airborne fungi in the “Nietoperek” bat reserve in Western Poland. Microb. Ecol. 2016, 72, 36–48. [Google Scholar] [CrossRef]
- Ogórek, R.; Višňovská, Z.; Tančinová, D. Mycobiota of underground habitats: Case study of Harmanecká Cave in Slovakia. Microb. Ecol. 2016, 71, 87–99. [Google Scholar] [CrossRef]
- Ogórek, R. Fungal communities on rock surfaces in Demänovská Ice Cave and Demänovská Cave of Liberty (Slovakia). Geomicrobiol. J. 2018, 35, 266–276. [Google Scholar] [CrossRef]
- Ogórek, R.; Kozak, B.; Višňovská, Z.; Tančinová, D. Phenotypic and genotypic diversity of airborne fungal spores in Demänovská Ice Cave (Low Tatras, Slovakia). Aerobiologia 2018, 34, 13–28. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Van Pelt, S.; Moore-Kucera, J.; Baddock, M.C.; Zobeck, T.M. Microbiology of wind-eroded sediments: Current knowledge and future research directions. Aeolian Res. 2015, 18, 99–113. [Google Scholar] [CrossRef]
- Low, C.Y.; Rotstein, C. Emerging fungal infections in immunocompromised patients. F1000 Med. Rep. 2011, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Lee, J.W.; Kim, S.H.; Park, J.H.; You, Y.H.; Lim, Y.W. Penicillium from Rhizosphere Soil in Terrestrial and Coastal Environments in South Korea. Mycobiology 2020, 48, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Pusz, W.; Baturo-Cieśniewska, A.; Zagożdżon, P.; Ogórek, R. Mycobiota of the disused ore mine of Marcinków in Śnieżnik Masiff (western Poland). J. Mt. Sci. 2017, 14, 2448–2457. [Google Scholar] [CrossRef]
- Duncan, S.M.; Farrell, R.L.; Thwaites, J.M.; Held, B.W.; Arenz, B.E.; Jurgens, J.A.; Blanchette, R.A. Endoglucanase-producing fungi isolated from Cape Evans historic expedition hut on Ross Island, Antarctica. Environ. Microbial. 2006, 8, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Gawas-Sakhalkar, P.; Singh, S.M.; Simantini, N.; Ravindra, R. High-temperature optima phosphatases from the cold-tolerant Arctic fungus Penicillium citrinum. Polar Res. 2012, 31, 11105. [Google Scholar] [CrossRef]
- Hassan, N.; Rafiq, M.; Hayat, M.; Shah, A.A.; Hasan, F. Psychrophilic and psychrotrophic fungi: A comprehensive review. Rev. Environ. Sci. Biotechnol. 2016, 15, 147–172. [Google Scholar] [CrossRef]
- Cheong, E.Y.L.; Sandhu, A.; Jayabalan, J.; Le, T.T.; Nhiep, N.T.; Ho, H.T.; Zwielehner, J.; Bansal, N.; Turner, M.S. Isolation of lactic acid bacteria with antifungal activity against the common cheese spoilage mould Penicillium commune and their potential as biopreservatives in cheese. Food Control 2014, 46, 91–97. [Google Scholar] [CrossRef]
- Wagener, R.E.; Davis, N.D.; Diener, U.L. Penitrem A and Roquefortine Production by Penicillium commune. Appl. Environ. Microbiol. 1980, 39, 882–887. [Google Scholar] [CrossRef]
- Rundberget, T.; Skaar, I.; Flåøyen, A. The presence of Penicillium and Penicillium mycotoxins in food wastes. Int. J. Food Microbiol. 2004, 90, 181–188. [Google Scholar] [CrossRef]
- Pickard, C.; Fortin, J.S.; Holmes, D.; Buchweitz, J.P.; Lehner, A.F. A novel chemical marker of tremorgenic mycotoxicosis detected by gas-chromatography/mass-spectrometry. World Mycotoxin J. 2021, 15, 223–240. [Google Scholar] [CrossRef]
- Christen-Zaech, S.; Patel, S.; Mancini, A.J. Recurrent cutaneous Geomyces pannorum infection in three brothers with ichthyosis. J. Am. Acad. Dermatol. 2008, 58, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.; DeFiglio, H.; Chaturvedi, S. Phenotype profiling of white-nose syndrome pathogen Pseudogymnoascus destructans and closely-related Pseudogymnoascus pannorum reveals metabolic differences underlying fungal lifestyles. F1000Research 2018, 7, 665. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A. Aerial Transport of Keratinaceous Substrate and Distribution of the Fungus Geomyces pannorum in Antarctic Soils. Microb. Ecol. 1998, 36, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Chabasse, D.; de Gentile, L.; Bouchara, J.P. Pathogenicity of some Chrysosporium species isolated in France. Mycopathologia 1989, 106, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, W.G.; Krishtul, A.; Bottone, E.J.; Phelps, R.; Cohen, S. Cutaneous adiaspiromycosis: A distinct dermatologic entity associated with Chrysosporium species. J. Am. Acad. Dermatol. 2004, 51, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Palma, M.A.; Avila Espín, L.; Fernández Pérez, A.; Moreno León, J.A. Micosis nasosinusal invasiva por Chrysosporium tropicum [Invasive sinusal mycosis due to Chrysosporium tropicum]. Acta Otorrinolaringol. Esp. 2007, 58, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; You, Y.H.; Lee, S.Y.; Jung, H.Y. A New Species of Thelonectria and a New Record of Cephalotrichum hinnuleum from Gunwi and Ulleungdo in Korea. Mycobiology 2020, 48, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Lechat, C.; Fournier, J. Cosmospora xylariae (Nectriaceae), a new species from France, Germany and U.K., with notes on C. berkeleyana, now Sphaerostilbella berkeleyana, and C. scruposae. Ascomycete.Org 2021, 13, 189–196. [Google Scholar] [CrossRef]
- Sakaeyama, S.; Sano, A.; Murata, Y.; Kamei, K.; Nishimura, K.; Hatai, K. Lecythophora hoffmannii isolated from a case of canine osteomyelitis in Japan. Med. Mycol. 2007, 45, 267–272. [Google Scholar] [CrossRef]
- Kabtani, J.; Militello, M.; Ranque, S. Coniochaeta massiliensis sp. nov. Isolated from a Clinical Sampl28. J. Fungi 2022, 8, 999. [Google Scholar] [CrossRef]
- Peterson, R.; Grinyer, J.; Nevalainen, H. Secretome of the Coprophilous Fungus Doratomyces stemonitis C8, Isolated from Koala Feces. Appl. Environ. Microbiol. 2011, 77, 3793–3801. [Google Scholar] [CrossRef]
- Chouikhi, S.; Assadi, B.H.; Lebdi, K.G.; Belkadhi, M.S. Efficacy of the entomopathogenic fungus, Beauveria bassiana and Lecanicillium muscarium against two main pests, Bemisia tabaci (Genn.) and Tetranychus urticae (Koch), under geothermal greenhouses of Southern Tunisia. Egypt. J. Biol. Pest Control 2022, 32, 125. [Google Scholar] [CrossRef]
- Fenice, M. The Psychrotolerant Antarctic Fungus Lecanicillium muscarium CCFEE 5003: A Powerful Producer of Cold-Tolerant Chitinolytic Enzymes. Molecules 2016, 21, 447. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, D.; Luan, Y.; Gu, Q.; Zhu, T. Cytotoxic metabolites from the antarctic psychrophilic fungus Oidiodendron truncatum. J. Nat. Prod. 2012, 75, 920–927. [Google Scholar] [CrossRef]
- Weng, Q.; Zhang, X.; Chen, W.; Hu, Q. Secondary Metabolites and the Risks of Isaria fumosorosea and Isaria farinosa. Molecules 2019, 24, 664. [Google Scholar] [CrossRef] [PubMed]
- Kurek, E.; Kornillowicz-Kowalska, T.; Slomka, A.; Melke, J. Characteristics of soil filamentous fungi communities isolated from various micro-relief forms in the high Arctic tundra (Bellsund region, Spitsbergen). Pol. Polar Res. 2007, 28, 57–73. [Google Scholar]
- Young, J.M.; Liddicoat, C.; van Dijk, K.J.; Tabernero, P.; Caillet, C.; White, N.J.; Linacre, A.; Austin, J.J.; Newton, P.N. Environmental DNA as an innovative technique to identify the origins of falsified antimalarial tablets—A pilot study of the pharmabiome. Sci. Rep. 2022, 12, 21997. [Google Scholar] [CrossRef]
- Miteva-Staleva, J.; Krumova, E.; Stoyancheva, G.; Kostadinova, N.; Grozdanov, P.; Spassova, B.; Angelova, M. Isolation, Identification and Proteolytic Activity of Filamentous Fungi from Alaska. Acta Microbiol. Bulg. 2022, 38, 26–30. [Google Scholar]
- Weinstein, R.N.; Montiel, P.O.; Johnstone, K. Influence of Growth Temperature on Lipid and Soluble Carbohydrate Synthesis by Fungi Isolated from Fellfield Soil in the Maritime Antarctic. Mycologia 2000, 92, 222–229. [Google Scholar] [CrossRef]
- Gams, W.; Chien, C.-Y.; Domsch, K.H. Zygospore Formation by the Heterothallic Mortierella elongata and a Related Homothallic Species, M. epigama sp. nov. Trans. Br. Mycol. Soc. 1972, 58, 5–13. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Park, S.W.; Pangging, M.; Lee, H.B. Molecular and Morphological Confirmation of Three Undescribed Species of Mortierella from Korea. Mycobiology 2019, 47, 31–39. [Google Scholar] [CrossRef]
- Trytek, M.; Fiedurek, J. A novel psychrotrophic fungus, Mortierella minutissima, for D-limonene biotransformation. Biotechnol. Lett. 2005, 27, 149–153. [Google Scholar] [CrossRef]
- Tsuji, M. Genetic diversity of yeasts from East Ongul Island, East Antarctica and their extracellular enzymes secretion. Polar Biol. 2018, 41, 249–258. [Google Scholar] [CrossRef]
- Trochine, A.; Bellora, N.; Nizovoy, P.; Duran, R.; Greif, G.; de García, V.; Batthyany, C.; Robello, C.; Libkind, D. Genomic and proteomic analysis of Tausonia pullulans reveals a key role for a GH15 glucoamylase in starch hydrolysis. Appl. Microbiol. Biotechnol. 2022, 106, 4655–4667. [Google Scholar] [CrossRef]
- Meletiadis, J.; Meis, J.F.G.M.; Mouton, J.W.; Verweij, P.E. Analysis of growth characteristics of filamentous fungi in different nutrient media. J. Clin. Microbiol. 2001, 39, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Fraatz, M.A.; Naeve, S.; Hausherr, V.; Zorn, H.; Blank, L.M. A minimal growth medium for the basidiomycete Pleurotus sapidus for metabolic flux analysis. Fungal Biol. Biotechnol. 2014, 1, 9. [Google Scholar] [CrossRef]


| Fungi Isolated from Soil and Sediment Samples | Identity with Sequence from GenBank | |||||||
|---|---|---|---|---|---|---|---|---|
| Isolate Number | Identified Species | Phylum | Family | GenBank Accession No. | The Sequence Length (bp) | Query Cover % | Identity % | Accession |
| UWR_314 | Ambomucor seriatoinflatus | Mucoromycota | Mucoraceae | OQ073897 | 494 | 100 | 100.00 | MG827311.1 |
| UWR_315 | Cephalotrichum hinnuleum | Ascomycota | Microascaceae | OQ073898 | 503 | 100 | 100.00 | LC519564.1 |
| UWR_316 | Chrysosporium merdarium | Ascomycota | Onygenaceae | OQ073899 | 454 | 100 | 100.00 | MH859164.1 |
| UWR_317 | Cosmospora berkeleyana | Ascomycota | Nectriaceae | OQ073900 | 428 | 100 | 100.00 | MH859038.1 |
| UWR_318 | Doratomyces stemonitis | Ascomycota | Microascaceae | OQ073901 | 506 | 100 | 99.60 | LN850985.1 |
| UWR_319 | Lecanicillium muscarium | Ascomycota | Cordycipitaceae | OQ073902 | 526 | 100 | 100.00 | MF467854.1 |
| UWR_320 | Lecythophora hoffmannii | Ascomycota | Coniochaetaceae | OQ073903 | 428 | 100 | 100.00 | FJ903377.1 |
| UWR_321 | Mortierella elongata (Linnemannia elongata) 1 | Mortierellomycota | Mortierellaceae | OQ073904 | 470 | 100 | 99.79 | MT366011.1 |
| UWR_322 | Mortierella hyalina (Linnemannia hyalina) | Mortierellomycota | Mortierellaceae | OQ073905 | 584 | 100 | 100.00 | MT003063.1 |
| UWR_323 | Mortierella minutissima (Podila minutissima) | Mortierellomycota | Mortierellaceae | OQ073906 | 552 | 100 | 100.00 | MK513846.1 |
| UWR_324 | Oidiodendron truncatum | Ascomycota | Myxotrichaceae | OQ073907 | 398 | 100 | 100.00 | KF835845.1 |
| UWR_325 | Paecilomyces farinosus(Cordyceps farinosa) | Ascomycota | Cordycipitaceae | OQ073908 | 376 | 100 | 100.00 | AF368793.1 |
| UWR_326 | Penicillium camemberti | Ascomycota | Aspergillaceae | OQ073909 | 507 | 100 | 100.00 | MT530220.1 |
| UWR_327 | Penicillium chrysogenum | Ascomycota | Aspergillaceae | OQ073910 | 496 | 100 | 100.00 | MT328526.1 |
| UWR_328 | Penicillium commune | Ascomycota | Aspergillaceae | OQ073911 | 520 | 100 | 100.00 | KU936231.1 |
| UWR_329 | Penicillium swiecickii | Ascomycota | Aspergillaceae | OQ073912 | 493 | 100 | 100.00 | MH865783.1 |
| UWR_330 | Pseudogymnoascus pannorum | Ascomycota | Pseudeurotiaceae | OQ073913 | 472 | 100 | 100.00 | MT573491.1 |
| UWR_231 | Tausonia pullulans | Basidiomycota | Mrakiaceae | OQ073910 | 392 | 100 | 98.98 | MK782486.1 |
| UWR_232 | Trichoderma atroviride | Ascomycota | Hypocreaceae | OQ073915 | 545 | 100 | 100.00 | MN533771.1 |
| UWR_233 | Trichosporiella cerebriformis | Ascomycota | Dermateaceae | OQ073916 | 552 | 99 | 99.28 | MH865134.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogórek, R.; Borzęcka, J.; Spychała, K.; Piecuch, A.; Suchodolski, J. Soil and Sediments in Natural Underground Ecosystems as a Source of Culturable Micromycetes: A Case Study of the Brestovská Cave (Western Tatras, Slovakia). Appl. Sci. 2024, 14, 3517. https://doi.org/10.3390/app14083517
Ogórek R, Borzęcka J, Spychała K, Piecuch A, Suchodolski J. Soil and Sediments in Natural Underground Ecosystems as a Source of Culturable Micromycetes: A Case Study of the Brestovská Cave (Western Tatras, Slovakia). Applied Sciences. 2024; 14(8):3517. https://doi.org/10.3390/app14083517
Chicago/Turabian StyleOgórek, Rafał, Justyna Borzęcka, Klaudyna Spychała, Agata Piecuch, and Jakub Suchodolski. 2024. "Soil and Sediments in Natural Underground Ecosystems as a Source of Culturable Micromycetes: A Case Study of the Brestovská Cave (Western Tatras, Slovakia)" Applied Sciences 14, no. 8: 3517. https://doi.org/10.3390/app14083517







