Synthesis and Electrical Properties of a New Bipolar Material Using Spacer Moiety
Abstract
:1. Introduction
2. Materials and Methods
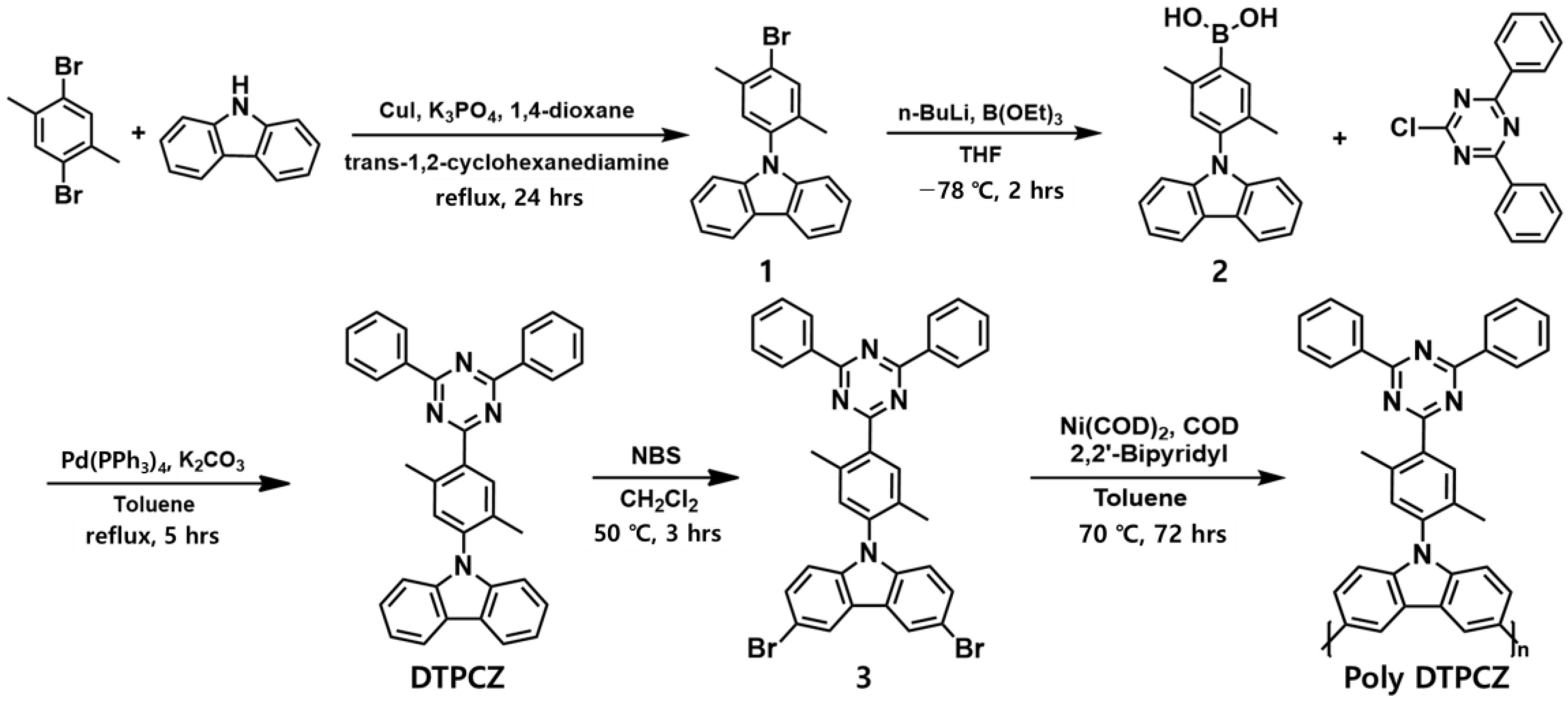
2.1. Synthesis of 9-(4-Bromo-2,5-dimethyl-phenyl)-9H-carbazole (1)
2.2. Synthesis of 9-(4-Boronic acid-2,5-dimethyl-phenyl)-9H-carbazole (2)
2.3. Synthesis of 9-[4-(4,6-Diphenyl-[1,3,5]triazin-2-yl)-2,5-dimethyl-phenyl]-9H-carbazole (DTPCZ)
2.4. Synthesis of 3,6-Dibromo-9-(4-(4,6-diphenyl-1,3,5-triazin-2-yl)-2,5-dimethylphenyl)-9H-carbazole (3)
2.5. Polymerization of Compound (Poly DTPCZ)
3. Results and Discussion
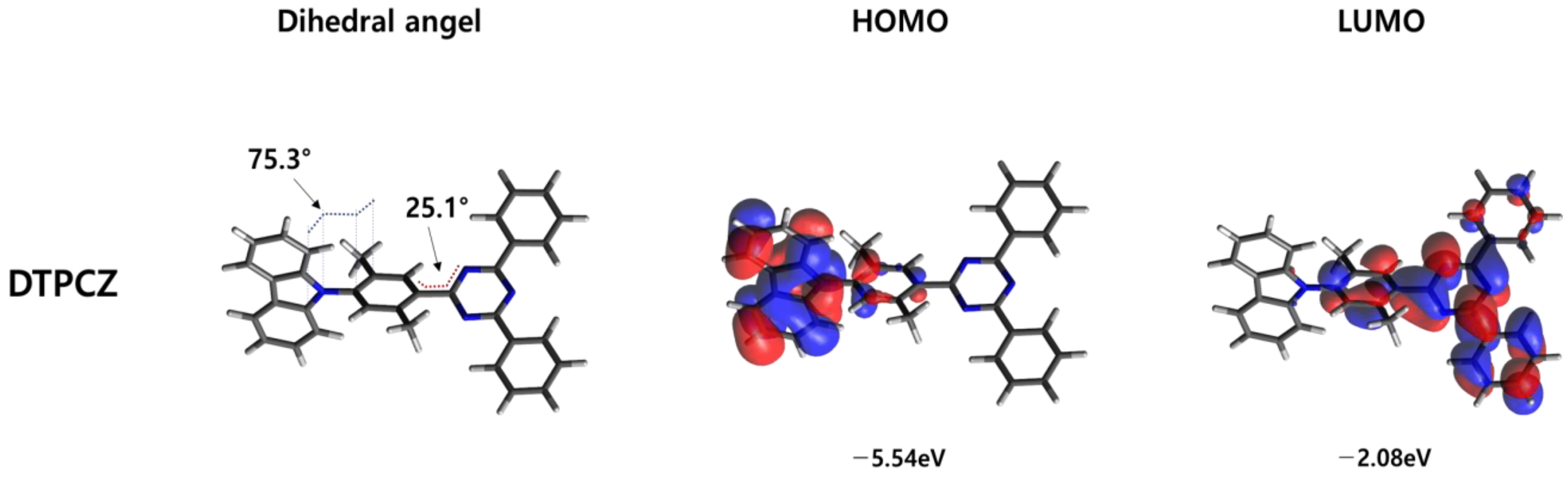
3.1. Optical Properties and Theoretical Calculations
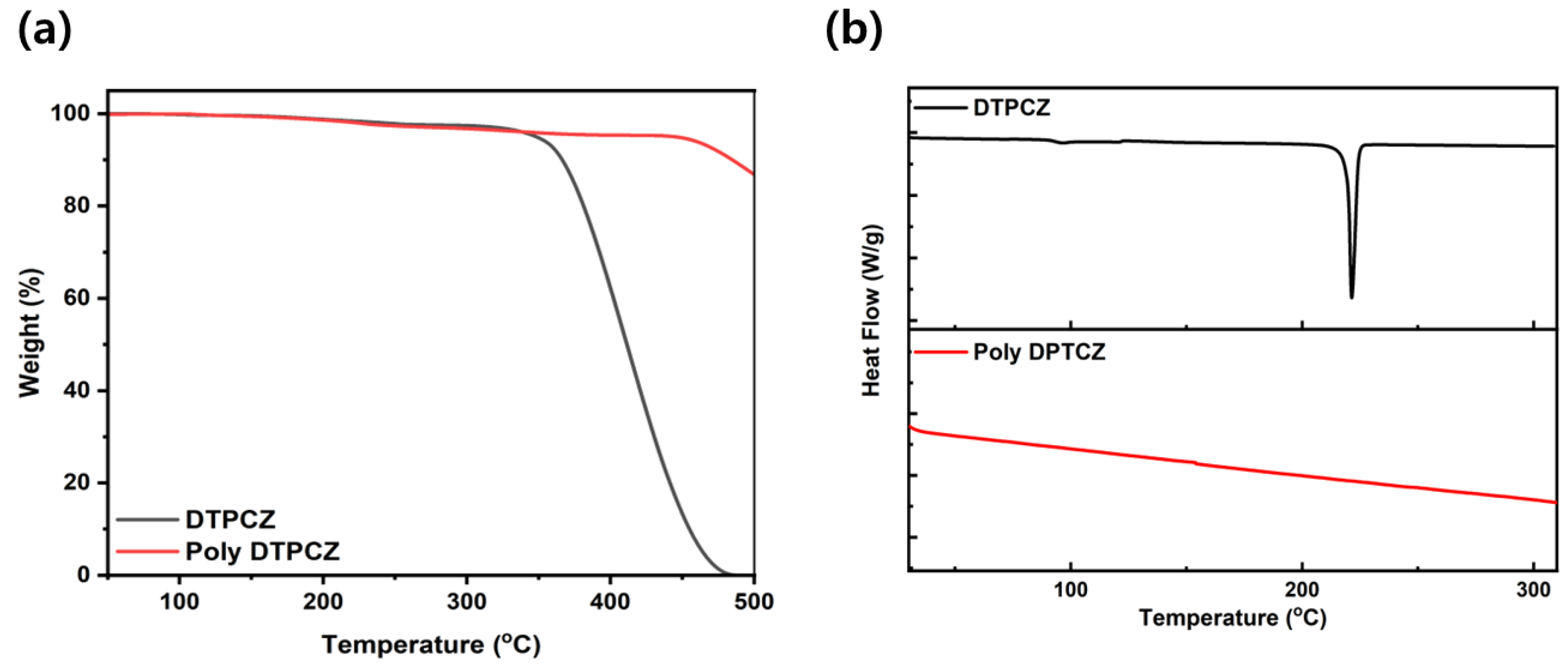
3.2. Thermal Properties
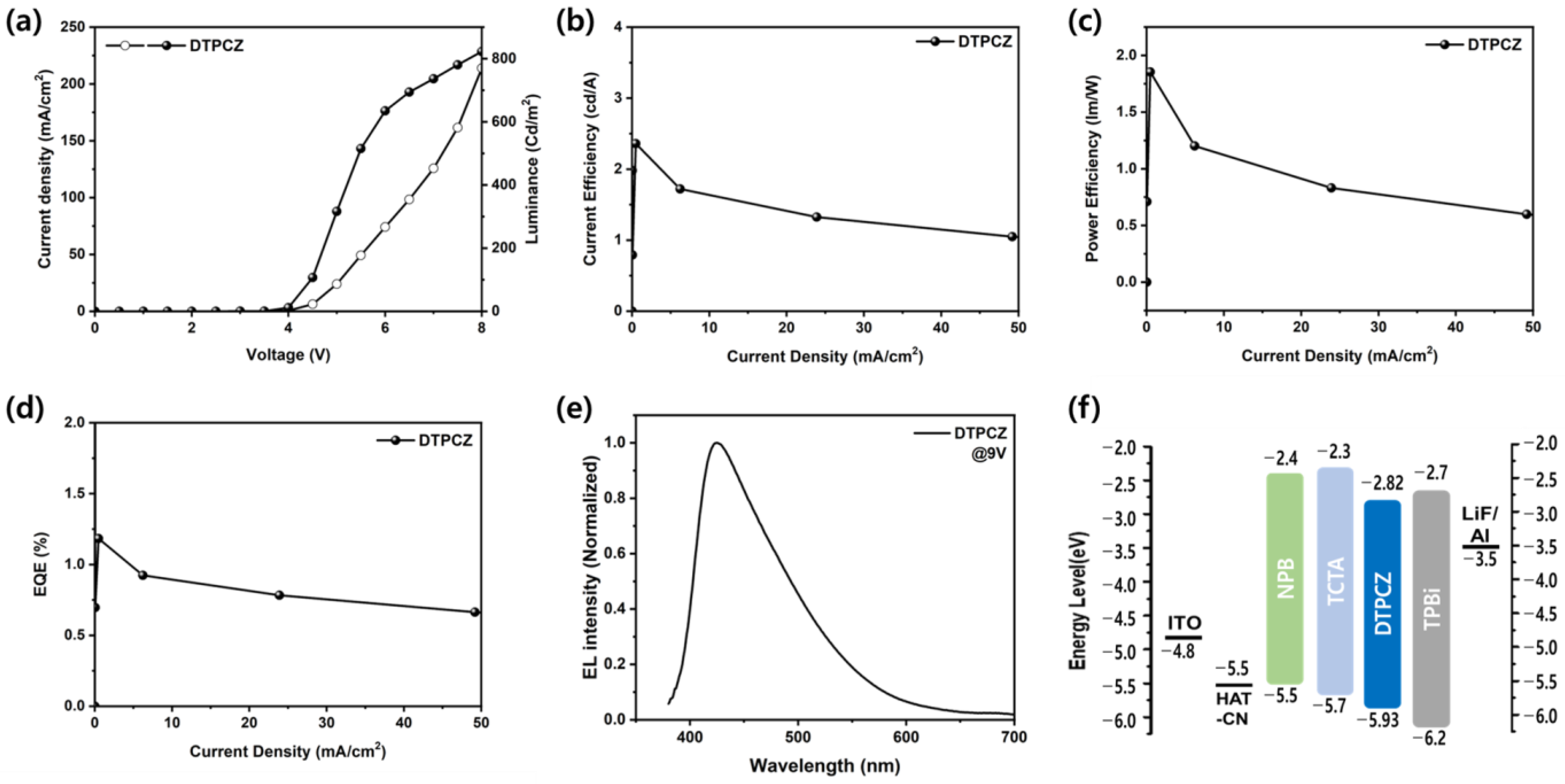
3.3. Electroluminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, S.; Jung, H.; Lee, H.; Park, S.; Kim, J.; Park, J. Highly efficient dual-core derivatives with EQEs as high as 8.38% at high brightness for OLED blue emitters. J. Mater. Chem. C 2019, 7, 14709–14716. [Google Scholar] [CrossRef]
- Ashok Kumar, S.; Shankar, J.S.; Periyasamy, B.K.; Nayak, S.K. Device engineering aspects of Organic Light-Emitting Diodes (OLEDs). Polym.-Plast. Technol. Mater. 2019, 58, 1597–1624. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, H.; Jeong, J.E.; Park, S.H.; Koh, C.W.; Kim, C.W.; Woo, H.Y.; Cho, M.J.; Park, S.; Choi, D.H. Ultra-Deep-Blue Aggregation-Induced Delayed Fluorescence Emitters: Achieving Nearly 16% EQE in Solution-Processed Nondoped and Doped OLEDs with CIE y < 0.1. Adv. Funct. Mater. 2021, 31, 2102588. [Google Scholar] [CrossRef]
- Reddy, S.S.; Sree, V.G.; Cho, W.; Jin, S.H. Achieving Pure Deep-Blue Electroluminescence with CIE y</=0.06 via a Rational Design Approach for Highly Efficient Non-Doped Solution-Processed Organic Light-Emitting Diodes. Chem. Asian J. 2016, 11, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-Y.; Chi, L.-C.; Chen, W.-J.; Chen, Y.-M.; Chou, S.-H.; Wong, K.-T. A new benzimidazole/carbazole hybrid bipolar material for highly efficient deep-blue electrofluorescence, yellow–green electrophosphorescence, and two-color-based white OLEDs. J. Mater. Chem. 2010, 20, 10113–10119. [Google Scholar] [CrossRef]
- Peng, L.; Huo, Y.; He, S.; Liu, Y.; Ren, Z.; Ying, S.; Yan, S. A linear deep-blue bipolar fluorescent material with the CIEy < 0.065 serving as the emitter and host for high-performance monochromatic and hybrid white OLEDs. J. Mater. Chem. C 2022, 10, 11642–11653. [Google Scholar] [CrossRef]
- Tan, Y.; Zhao, Z.; Shang, L.; Liu, Y.; Wei, C.; Li, J.; Wei, H.; Liu, Z.; Bian, Z.; Huang, C. A novel bipolar D–π–A type phenanthroimidazole/carbazole hybrid material for high efficiency nondoped deep-blue organic light-emitting diodes with NTSC CIEy and low efficiency roll-off. J. Mater. Chem. C 2017, 5, 11901–11909. [Google Scholar] [CrossRef]
- Kim, M.K.; Kwon, J.; Kwon, T.-H.; Hong, J.-I. A bipolar host containing 1,2,3-triazole for realizing highly efficient phosphorescent organic light-emitting diodes. New J. Chem. 2010, 34, 1317–1322. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, L. Polycyclic Aromatic Hydrocarbon Derivatives toward Ideal Electron-Transporting Materials for Organic Light-Emitting Diodes. J. Phys. Chem. Lett. 2019, 10, 2528–2537. [Google Scholar] [CrossRef]
- Chi, C.-C.; Chiang, C.-L.; Liu, S.-W.; Yueh, H.; Chen, C.-T.; Chen, C.-T. Achieving high-efficiency non-doped blue organic light-emitting diodes: Charge-balance control of bipolar blue fluorescent materials with reduced hole-mobility. J. Mater. Chem. 2009, 19, 5561–5571. [Google Scholar] [CrossRef]
- Yu, P.; Xiao, Y. Non-Doped Deep-Blue OLEDs Based on Carbazole-pi-Imidazole Derivatives. Materials 2021, 14, 2349. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, Y.; Fan, S.; Wu, S.; Zhao, X.; Wang, S.; Li, X. A novel bipolar carbazole/phenanthroimidazole derivative for high efficiency nondoped deep-blue organic light-emitting diodes. Org. Electron. 2019, 64, 259–265. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, C.; Park, S.H.; Kang, D.W.; Kim, H.; Jeong, J.-E.; Woo, H.Y.; Hong, C.S.; Park, S.; Cho, M.J.; et al. Pyrimidine-based bipolar host materials for high efficiency solution processed green thermally activated delayed fluorescence OLEDs. J. Mater. Chem. C 2020, 8, 2196–2204. [Google Scholar] [CrossRef]
- Ye, J.; Chen, Z.; Fung, M.-K.; Zheng, C.; Ou, X.; Zhang, X.; Yuan, Y.; Lee, C.-S. Carbazole/Sulfone Hybrid D-π-A-Structured Bipolar Fluorophores for High-Efficiency Blue-Violet Electroluminescence. Chem. Mater. 2013, 25, 2630–2637. [Google Scholar] [CrossRef]
- Suzuki, K.; Kubo, S.; Shizu, K.; Fukushima, T.; Wakamiya, A.; Murata, Y.; Adachi, C.; Kaji, H. Triarylboron-Based Fluorescent Organic Light-Emitting Diodes with External Quantum Efficiencies Exceeding 20. Angew. Chem. Int. Ed. Engl. 2015, 54, 15231–15235. [Google Scholar] [CrossRef]
- Chen, C.-H.; Huang, W.-S.; Lai, M.-Y.; Tsao, W.-C.; Lin, J.T.; Wu, Y.-H.; Ke, T.-H.; Chen, L.-Y.; Wu, C.-C. Versatile, Benzimidazole/Amine-Based Ambipolar Compounds for Electroluminescent Applications: Single-Layer, Blue, Fluorescent OLEDs, Hosts for Single-Layer, Phosphorescent OLEDs. Adv. Funct. Mater. 2009, 19, 2661–2670. [Google Scholar] [CrossRef]
- Gibson, V.C.; Spitzmesser, S.K.; White, A.J.; Williams, D.J. Synthesis and reactivity of 1, 8-bis (imino) carbazolide complexes of iron, cobalt and manganese. Dalton Trans. 2003, 13, 2718–2727. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Orecchia, P.; Petkova, D.S.; Goetz, R.; Rominger, F.; Hashmi, A.S.K.; Schaub, T. Pd-Catalysed Suzuki–Miyaura cross-coupling of aryl chlorides at low catalyst loadings in water for the synthesis of industrially important fungicides. Green. Chem. 2021, 23, 8169–8180. [Google Scholar] [CrossRef]
- Zhang, Z.-B.; Fujiki, M.; Tang, H.-Z.; Motonaga, M.; Torimitsu, K. The first high molecular weight poly (N-alkyl-3, 6-carbazole)s. Macromolecules 2002, 35, 1988–1990. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Liu, D.; Huang, T.; Li, D. Effects of Electron Affinity and Steric Hindrance of the Trifluoromethyl Group on the pi-Bridge in Designing Blue Thermally Activated Delayed Fluorescence Emitters. Chem. Eur. J. 2020, 26, 6899–6909. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Yao, Z.F.; Xu, Z.; Kong, H.K.; Wang, X.Y.; Li, Q.Y.; Wang, J.Y.; Pei, J. Controlling Solution-State Aggregation and Solid-State Microstructures of Conjugated Polymers by Tuning Backbone Conformation. Macromol. Rapid Commun. 2022, 43, e2200069. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Qiu, W.; Yuan, P.; Wang, F.; Liu, Y.; Xu, S.; Su, S.J.; Cao, S. Thermally activated delayed fluorescence polymers for high-efficiency solution-processed non-doped OLEDs: Convenient synthesis by binding TADF units and host units to the pre-synthesized polycarbazole-based backbone via click reaction. Polymer 2022, 240, 124468. [Google Scholar] [CrossRef]
- Huang, T.; Jiang, W.; Duan, L. Recent progress in solution processable TADF materials for organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 5577–5596. [Google Scholar] [CrossRef]
- Usta, H.; Risko, C.; Wang, Z.; Huang, H.; Deliomeroglu, M.K.; Zhukhovitskiy, A.; Facchetti, A.; Marks, T.J. Design, Synthesis, and Characterization of Ladder-Type Molecules and Polymers. Air-Stable, Solution-Processable n-Channel and Ambipolar Semiconductors for Thin-Film Transistors via Experiment and Theory. J. Am. Chem. Soc. 2009, 131, 5586–5608. [Google Scholar] [CrossRef] [PubMed]
- Karelson, M.; Zerner, M.C. On the n-pi.* blue shift accompanying solvation. J. Am. Chem. Soc. 1990, 112, 9405–9406. [Google Scholar] [CrossRef]
- Jung, H.; Shin, H.; Kim, S.; Kim, J.; An, B.-K.; Lee, J.-H.; Ihee, H.; Park, J. High electroluminescence efficiency and long device lifetime of a fluorescent green-light emitter using aggregation-induced emission. J. Ind. Eng. Chem. 2020, 87, 213–221. [Google Scholar] [CrossRef]
- Kang, S.; Jung, H.; Lee, H.; Lee, S.; Jung, M.; Lee, J.; Kim, Y.C.; Park, J. Blue light emission of new anthracene derivatives produced using optimized side group link positions. Dye. Pigment. 2018, 156, 369–378. [Google Scholar] [CrossRef]
- Yang, T.; Liang, B.; Cheng, Z.; Li, C.; Lu, G.; Wang, Y. Construction of Efficient Deep-Red/Near-Infrared Emitter Based on a Large π-Conjugated Acceptor and Delayed Fluorescence OLEDs with External Quantum Efficiency of over 20%. J. Phys. Chem. C 2019, 123, 18585–18592. [Google Scholar] [CrossRef]
- Wu, C.; Liu, W.; Li, K.; Cheng, G.; Xiong, J.; Teng, T.; Che, C.M.; Yang, C. Face-to-Face Orientation of Quasiplanar Donor and Acceptor Enables Highly Efficient Intramolecular Exciplex Fluorescence. Angew. Chem. Int. Ed. 2021, 60, 3994–3998. [Google Scholar] [CrossRef]
- Tao, S.; Lee, C.S.; Lee, S.-T.; Zhang, X. Efficient blue and white organic light-emitting devices based on a single bipolar emitter. Appl. Phys. Lett. 2007, 91, 013507. [Google Scholar] [CrossRef]
- Lee, J.-H.; Tsai, H.-H.; Leung, M.-K.; Yang, C.-C.; Chao, C.-C. Phosphorescent organic light-emitting device with an ambipolar oxadiazole host. Appl. Phys. Lett. 2007, 90, 243501. [Google Scholar] [CrossRef]




| Compound | Solution a | Film b | HO MO c (eV) | LU MO c (eV) | Band Gap d (eV) | ||||
|---|---|---|---|---|---|---|---|---|---|
| λAbs (nm) | λPL (FWHM) (nm) | ΦF e (%) | λAbs (nm) | λPL (FWHM) (nm) | ΦF e (%) | ||||
| DTPCZ | 340 | 421 (54) | 43.2 | 341 | 425 (56) | 18.9 | −5.9 | −2.8 | 3.1 |
| Poly DTPCZ | 380 | 461 (68) | 32.4 | 387 | 475 (84) | 9.1 | −5.5 | −2.6 | 2.9 |
| PLmax (nm) | |||||
|---|---|---|---|---|---|
| Hexane (1.88) a | Toluene (2.38) a | Chloroform (4.81) a | THF (7.58) a | DMSO (46.7) a | |
| DTPCZ | 397 | 421 | 451 | 457 | 522 |
| Poly DTPCZ | NA b | 466 | 526 | 524 | 559 |
| @ 10 mA/cm2 | Von a (V) | CE (cd/A) | EQE (%) | PE (lm/W) | ELmax (nm) | FWHM (nm) | CIE (x, y) |
|---|---|---|---|---|---|---|---|
| CBP: 10% DTPCZ | 3.9 | 0.48 | 1.07 | 0.30 | 430 | 61 | (0.16, 0.06) |
| CBP: 20% DTPCZ | 3.9 | 1.17 | 2.32 | 0.68 | 430 | 61 | (0.16, 0.06) |
| CBP: 30% DTPCZ | 3.9 | 0.97 | 2.05 | 0.59 | 430 | 61 | (0.17, 0.09) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.; Park, S.; Kwon, H.; Lee, H.; Lee, K.; Park, J. Synthesis and Electrical Properties of a New Bipolar Material Using Spacer Moiety. Appl. Sci. 2024, 14, 3593. https://doi.org/10.3390/app14093593
Jo S, Park S, Kwon H, Lee H, Lee K, Park J. Synthesis and Electrical Properties of a New Bipolar Material Using Spacer Moiety. Applied Sciences. 2024; 14(9):3593. https://doi.org/10.3390/app14093593
Chicago/Turabian StyleJo, Seunghyeon, Sangwook Park, Hyukmin Kwon, Hayoon Lee, Kiho Lee, and Jongwook Park. 2024. "Synthesis and Electrical Properties of a New Bipolar Material Using Spacer Moiety" Applied Sciences 14, no. 9: 3593. https://doi.org/10.3390/app14093593





