Phase Behavior and Rheological Properties of AES/CAPB/H2O Ternary System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation for Phase Diagram
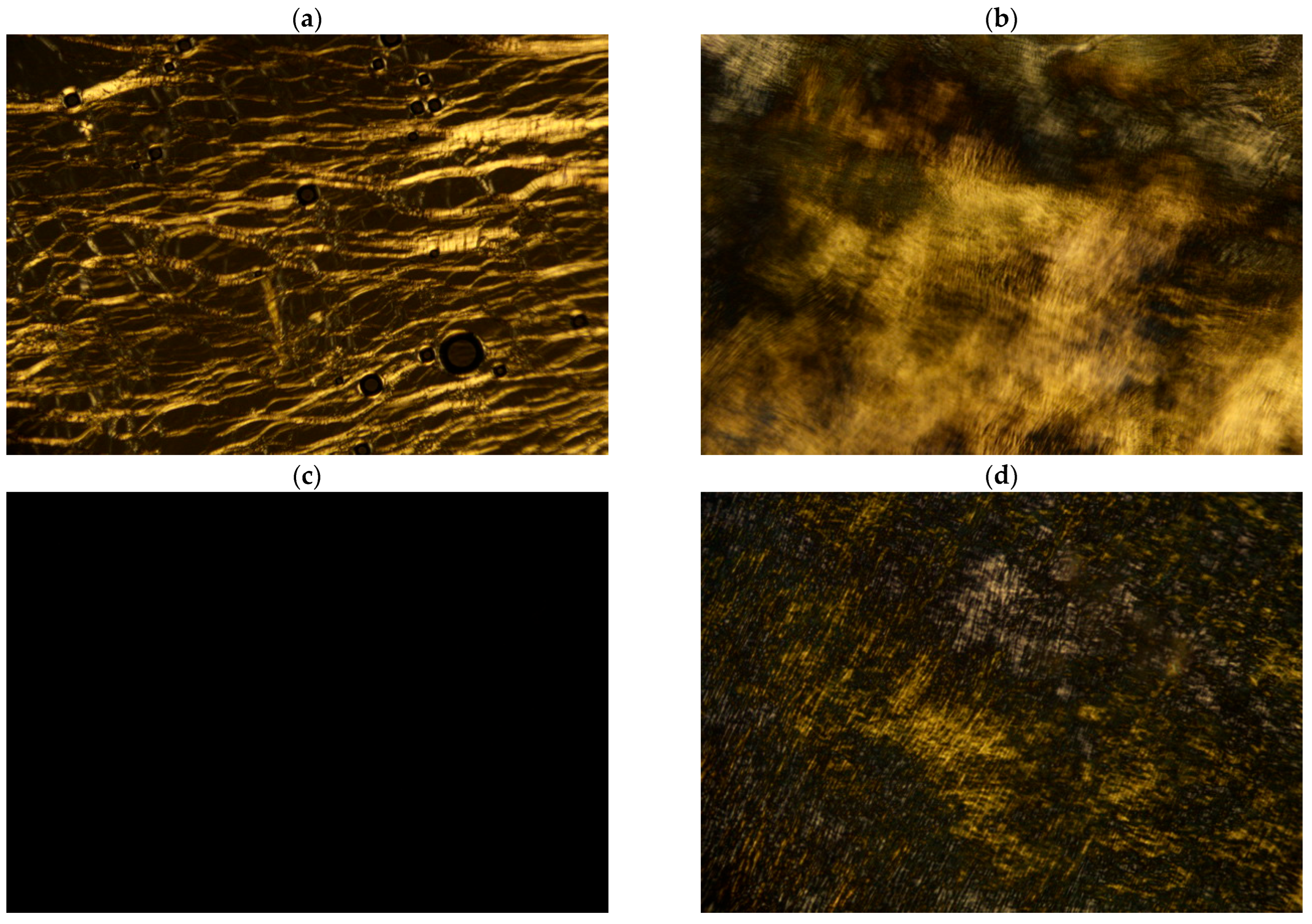
2.3. Polarizing Optical Microscopy
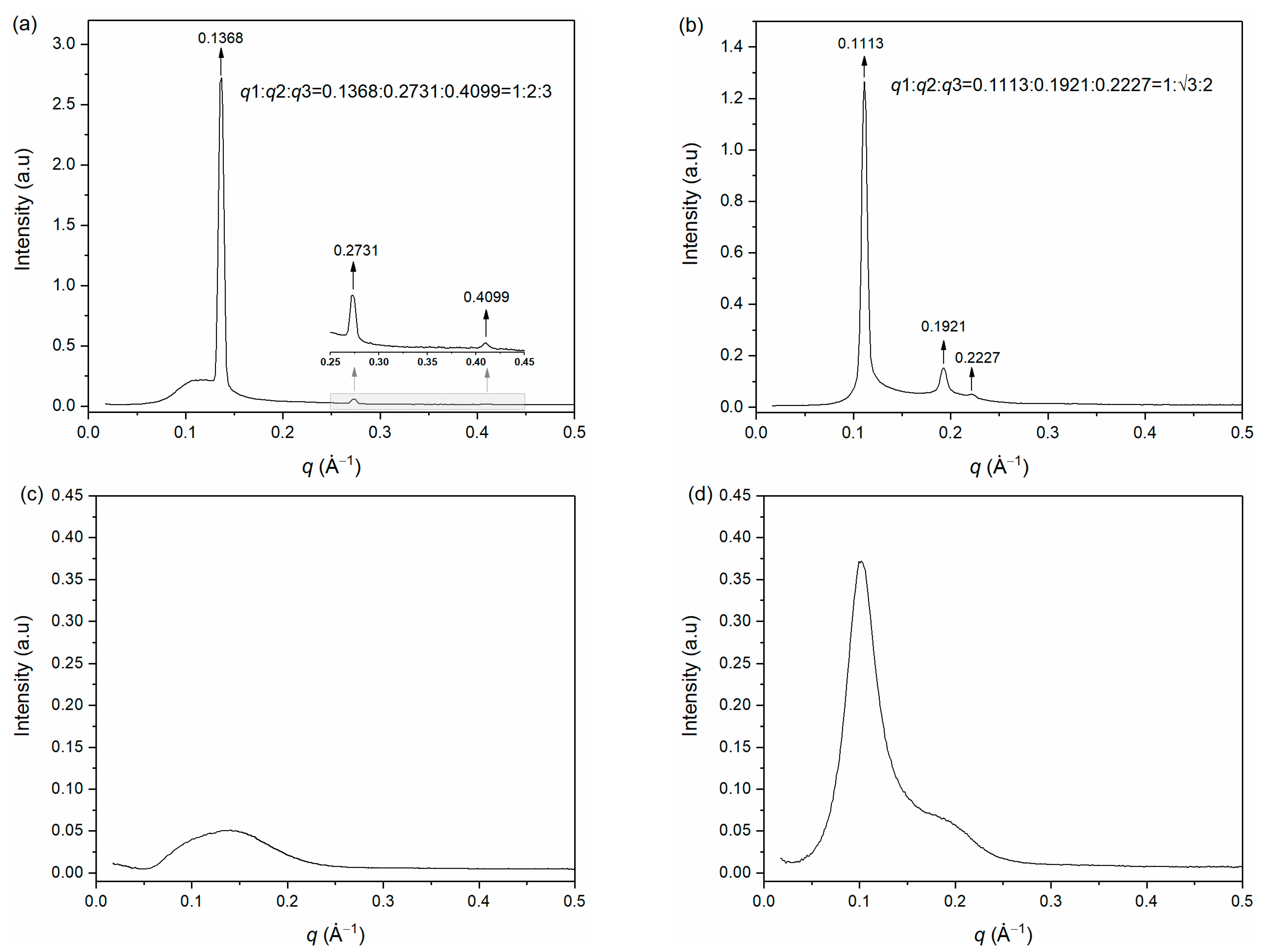
2.4. Small Angle X-ray Scattering
2.5. Viscosity and Rheological Measurements
3. Results and Discussion
3.1. Preliminary Identification of Phases
3.2. Small Angle X-ray Scattering Measurement
3.3. Phase Behavior of AES/CAPB/H2O Ternary System
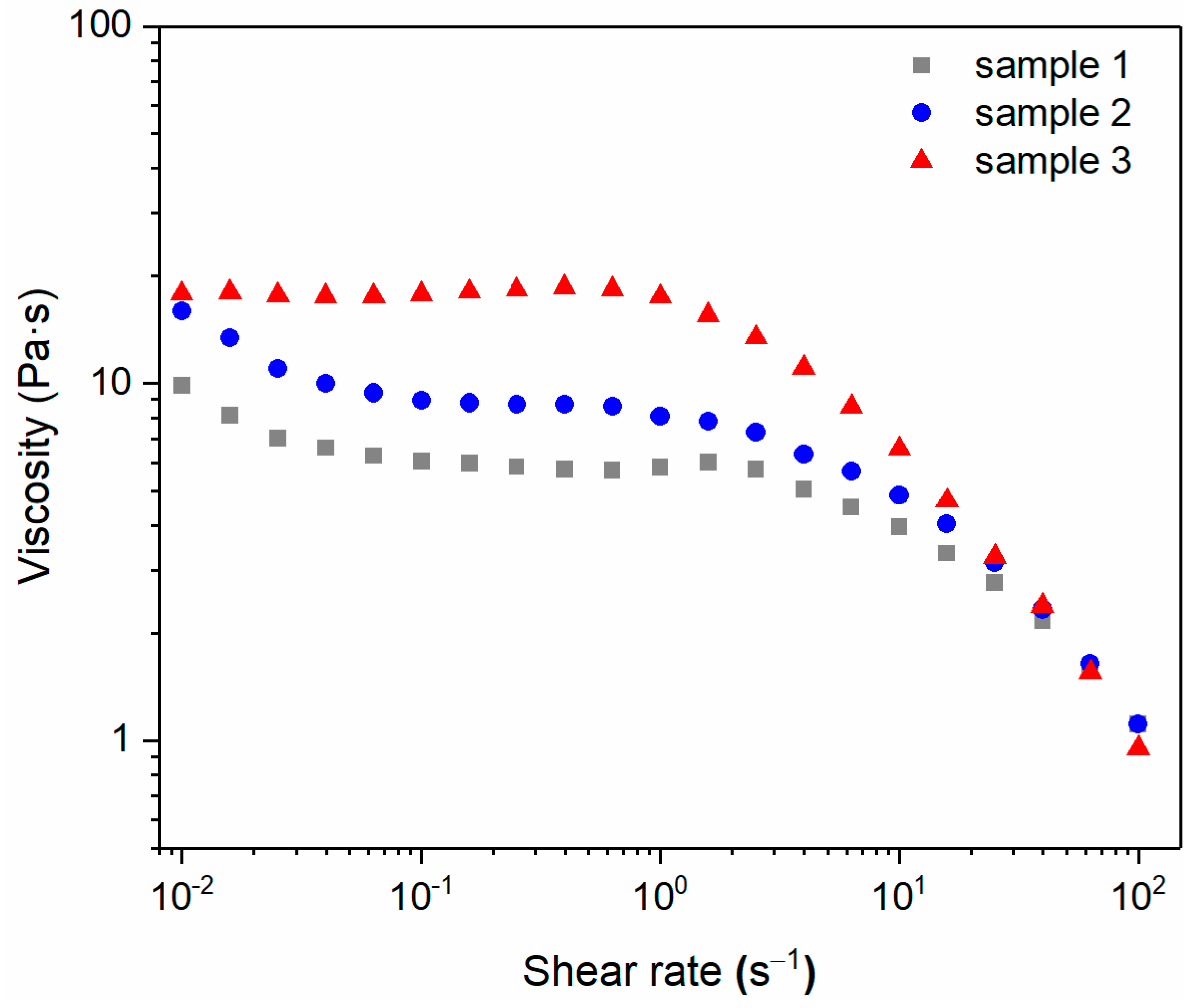
3.4. Viscosity Distribution of AES/CAPB/H2O Ternary System
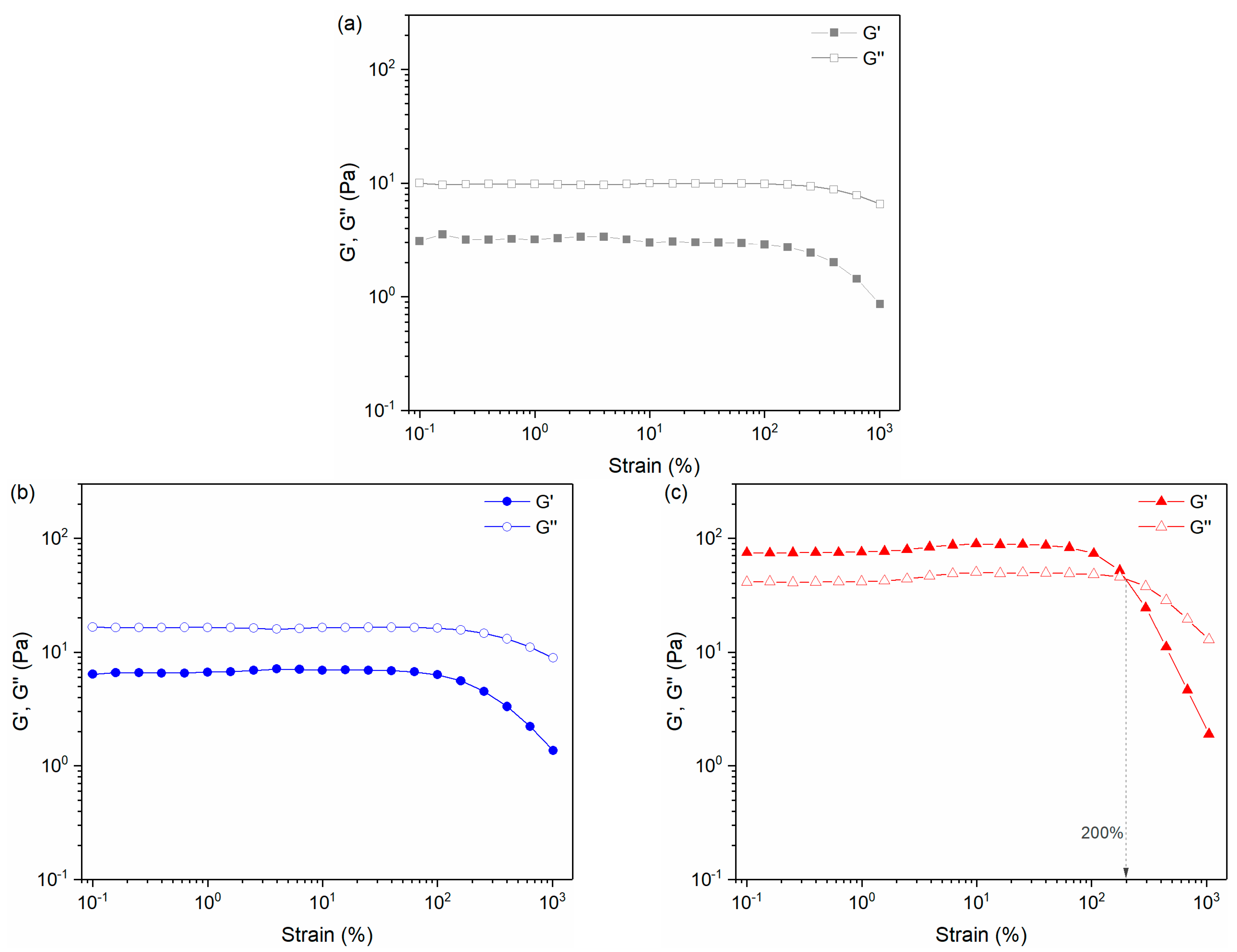
3.5. Rheological Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Ramos, C.; Ballesteros, O.; Zafra-Gómez, A.; Camino-Sánchez, F.J.; Blanc, R.; Navalón, A.; Pérez-Trujillo, J.P.; Vílchez, J.L. Evaluation of the levels of alcohol sulfates and ethoxysulfates in marine sediments near wastewater discharge points along the coast of Tenerife Island. Mar. Pollut. Bull. 2014, 79, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Sasi, S.; Rayaroth, M.P.; Aravindakumar, C.T.; Aravind, U.K. Alcohol ethoxysulfates (AES) in environmental matrices. Environ. Sci. Pollut. Res. 2021, 28, 34167–34186. [Google Scholar] [CrossRef] [PubMed]
- Sibila, M.A.; Garrido, M.C.; Perales, J.A.; Quiroga, J.M. Ecotoxicity and biodegradability of an alkyl ethoxysulphate surfactant in coastal waters. Sci. Total Environ. 2008, 394, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Topliceanu, S.; Almeida, M.; Oliveira, M.; Cogălniceanu, D.; Lopes, I. The Number of Ethylene Oxide Groups of Sulphate-Based Surfactants Influences the Cytotoxicity of Mixed Micelles to an Amphibian Cell Line. Appl. Sci. 2023, 13, 8745. [Google Scholar] [CrossRef]
- Acharya, D.P.; Varade, D.; Aramaki, K. Effect of temperature on the rheology of wormlike micelles in a mixed surfactant system. J. Colloid Interface Sci. 2007, 315, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Savignano, L.; Fabozzi, A.; Vitiello, R.; Fornasier, M.; Murgia, S.; Guido, S.; Guida, V.; Paduano, L.; D’Errico, G. Effect of tail branching on the phase behavior and the rheological properties of amine oxide/ethoxysulfate surfactant mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126091. [Google Scholar] [CrossRef]
- Wang, Z.; Diao, Z.; Liu, F.; Li, G.; Zhang, G. Microstructure and rheological properties of liquid crystallines formed in Brij 97/water/IPM system. J. Colloid Interface Sci. 2006, 297, 813–818. [Google Scholar] [CrossRef]
- Alexandridis, P.; Olsson, U.; Lindman, B. A Record Nine Different Phases (Four Cubic, Two Hexagonal, and One Lamellar Lyotropic Liquid Crystalline and Two Micellar Solutions) in a Ternary Isothermal System of an Amphiphilic Block Copolymer and Selective Solvents (Water and Oil). Langmuir 1998, 14, 2627–2638. [Google Scholar] [CrossRef]
- Alam, M.M.; Ushiyama, K.; Aramaki, K. Phase Behavior, Formation, and Rheology of Cubic Phase and Related Gel Emulsion in Tween80/Water/Oil Systems. J. Oleo Sci. 2009, 58, 361–367. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, L.; Tan, H.; Wei, H. Phase Behavior of Commercial AES/Ethanol/H2O Ternary System. J. Dispers. Sci. Technol. 2016, 37, 633–639. [Google Scholar] [CrossRef]
- Godoy, C.A.; Valiente, M.; Muñoz, W.; Bonilla, P.; Pons, R.; Montalvo, G. Microstructural and Viscoelastic Properties of Liquid Crystal Phases of Soybean Lecithin with Olive and Castor Oils at Low Water Contents. J. Chem. Eng. Data 2023, 68, 1534–1542. [Google Scholar] [CrossRef]
- Li, H.; Dang, L.; Yang, S.; Li, J.; Wei, H. The study of phase behavior and rheological properties of lyotropic liquid crystals in the LAS/AES/H2O system. Colloids Surf. A Physicochem. Eng. Asp. 2016, 495, 221–228. [Google Scholar] [CrossRef]
- Pan, Y.; Dang, L.; Wei, H. Phase Behavior and Rheological Properties of Poly(vinyl alcohol)/AES/Water System. J. Phys. Chem. B 2021, 125, 3230–3237. [Google Scholar] [CrossRef]
- Montalvo, G.; Valiente, M.; Rodenas, E. Rheological Properties of the L Phase and the Hexagonal, Lamellar, and Cubic Liquid Crystals of the CTAB/Benzyl Alcohol/Water System. Langmuir 1996, 12, 5202–5208. [Google Scholar] [CrossRef]
- Różańska, S. Rheology of wormlike micelles in mixed solutions of cocoamidopropyl betaine and sodium dodecylbenzenesulfonate. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 394–402. [Google Scholar] [CrossRef]
- Yang, J. Viscoelastic wormlike micelles and their applications. Curr. Opin. Colloid Interface Sci. 2002, 7, 276–281. [Google Scholar] [CrossRef]
- Fundarò, S.P.; Salti, G.; Malgapo, D.M.H.; Innocenti, S. The Rheology and Physicochemical Characteristics of Hyaluronic Acid Fillers: Their Clinical Implications. Int. J. Mol. Sci. 2022, 23, 10518. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, R.; Lei, Z.; Dang, L. Synergistic Effect of Cocamidopropyl Betaine and Sodium Lauroyl Sarcosinate. Trans. Tianjin Univ. 2021, 27, 366–376. [Google Scholar] [CrossRef]
- Stancheva, T.N.; Georgiev, M.T.; Radulova, G.M.; Danov, K.D.; Marinova, K.G. Rheology of saturated micellar networks: Wormlike micellar solutions vs. bicontinuous micellar phases. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129927. [Google Scholar] [CrossRef]
- Stanimirova, R.D.; Kralchevsky, P.A.; Danov, K.D.; Xu, H.; Ung, Y.W.; Petkov, J.T. Oil drop deposition on solid surfaces in mixed polymer-surfactant solutions in relation to hair- and skin-care applications. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 53–61. [Google Scholar] [CrossRef]
- Yavrukova, V.I.; Radulova, G.M.; Danov, K.D.; Kralchevsky, P.A.; Xu, H.; Ung, Y.W.; Petkov, J.T. Rheology of mixed solutions of sulfonated methyl esters and betaine in relation to the growth of giant micelles and shampoo applications. Adv. Colloid Interface Sci. 2020, 275, 102062. [Google Scholar] [CrossRef]
- Anachkov, S.E.; Georgieva, G.S.; Abezgauz, L.; Danino, D.; Kralchevsky, P.A. Viscosity Peak due to Shape Transition from Wormlike to Disklike Micelles: Effect of Dodecanoic Acid. Langmuir 2018, 34, 4897–4907. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final Report of the Cosmetic Ingredient Review Expert Panel on the Safety Assessment of Cocamidopropyl betaine (CAPB). Int. J. Toxicol. 2012, 31, 77S–111S. [Google Scholar] [CrossRef]
- Cowan-Ellsberry, C.; Belanger, S.; Dorn, P.; Dyer, S.; McAvoy, D.; Sanderson, H.; Versteeg, D.; Ferrer, D.; Stanton, K. Environmental Safety of the Use of Major Surfactant Classes in North America. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1893–1993. [Google Scholar] [CrossRef]
- Tzocheva, S.S.; Kralchevsky, P.A.; Danov, K.D.; Georgieva, G.S.; Post, A.J.; Ananthapadmanabhan, K.P. Solubility limits and phase diagrams for fatty acids in anionic (SLES) and zwitterionic (CAPB) micellar surfactant solutions. J. Colloid Interface Sci. 2012, 369, 274–286. [Google Scholar] [CrossRef]
- Georgieva, G.S.; Anachkov, S.E.; Lieberwirth, I.; Koynov, K.; Kralchevsky, P.A. Synergistic Growth of Giant Wormlike Micelles in Ternary Mixed Surfactant Solutions: Effect of Octanoic Acid. Langmuir 2016, 32, 12885–12893. [Google Scholar] [CrossRef]
- Mitrinova, Z.; Tcholakova, S.; Golemanov, K.; Denkov, N.; Vethamuthu, M.; Ananthapadmanabhan, K.P. Surface and foam properties of SLES+CAPB+fatty acid mixtures: Effect of pH for C12–C16 acids. Colloids Surf. A Physicochem. Eng. Asp. 2013, 438, 186–198. [Google Scholar] [CrossRef]
- Selivanova, N.; Gubaidullin, A.; Galyametdinov, Y. Structural transformations and phase transitions in hexagonal La-containing lyomesophases. Fluid Phase Equilibria 2023, 568, 113732. [Google Scholar] [CrossRef]
- Barai, M.; Manna, E.; Sultana, H.; Mandal, M.K.; Guchhait, K.C.; Manna, T.; Patra, A.; Chang, C.-H.; Moitra, P.; Ghosh, C.; et al. Micro-structural investigations on oppositely charged mixed surfactant gels with potential dermal applications. Sci. Rep. 2021, 11, 15527. [Google Scholar] [CrossRef]
- Li, X.; Hong, B.; Schwiedernoch, R.; Streiff, S.; Xu, Y. Self-assembly of Symmetric Double Chain Surfactants Derived from Internal Ketone in an Aqueous System. Ind. Eng. Chem. Res. 2022, 61, 7275–7283. [Google Scholar] [CrossRef]
- Boltenhagen, P.; Lavrentovich, O.; Kleman, M. Oily streaks and focal conic domains in Lα lyotropic liquid crystals. J. Phys. II France 1991, 1, 1233–1252. [Google Scholar]
- Rodríguez-Abreu, C.; García-Roman, M.; Kunieda, H. Rheology and Dynamics of Micellar Cubic Phases and Related Emulsions. Langmuir 2004, 20, 5235–5240. [Google Scholar] [CrossRef]
- Hyde, S.T. Identification of lyotropic liquid crystalline mesophases. In Handbook of Applied Surface and Colloid Chemistry; Wiley: New York, NY, USA, 2001; Volume 2, pp. 299–332. [Google Scholar]
- Dierking, I.; Al-Zangana, S. Lyotropic Liquid Crystal Phases from Anisotropic Nanomaterials. Nanomaterials 2017, 7, 305. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Sun, S.; Liu, Y.; Zhu, C.; Li, J.; Sun, J.; Wang, S.; Zhang, H. Characterization of Crystal Microstructure Based on Small Angle X-ray Scattering (SAXS) Technique. Molecules 2020, 25, 443. [Google Scholar] [CrossRef]
- Gupta, R.; Muralidhara, H.S.; Davis, H.T. Structure and Phase Behavior of Phospholipid-Based Micelles in Nonaqueous Media. Langmuir 2001, 17, 5176–5183. [Google Scholar] [CrossRef]
- Xiao, X.; Qi, J.; Zhou, J.; Sun, Y.; Huang, J.; Yan, Y. Enhanced salt thickening effect of the aqueous solution of peaked-distribution alcohol ether sulfates (AES). Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128146. [Google Scholar] [CrossRef]
- Jin, P.; Wu, J.; Shi, R.; Dai, L.; Li, Y. Parabolic Viscosity Behavior of NaCl-Thickened Surfactant Systems upon Temperature Change. ACS Omega 2023, 8, 37511–37520. [Google Scholar] [CrossRef]
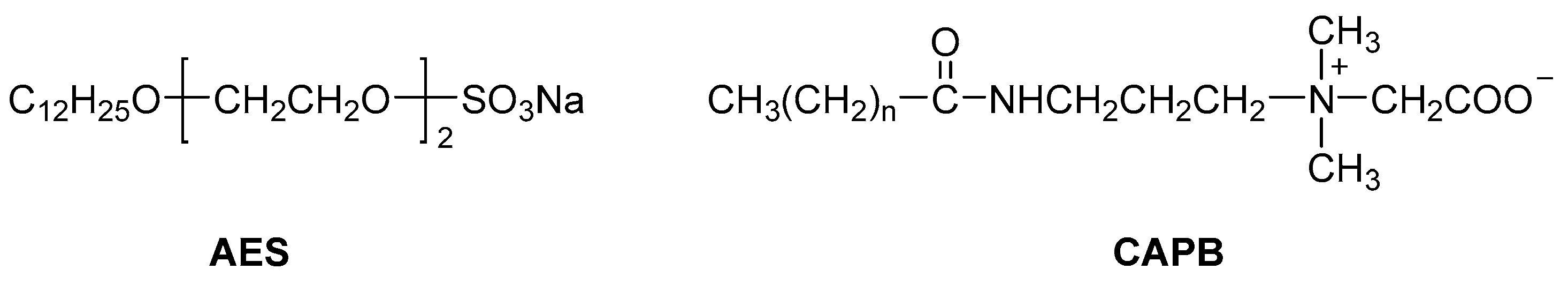
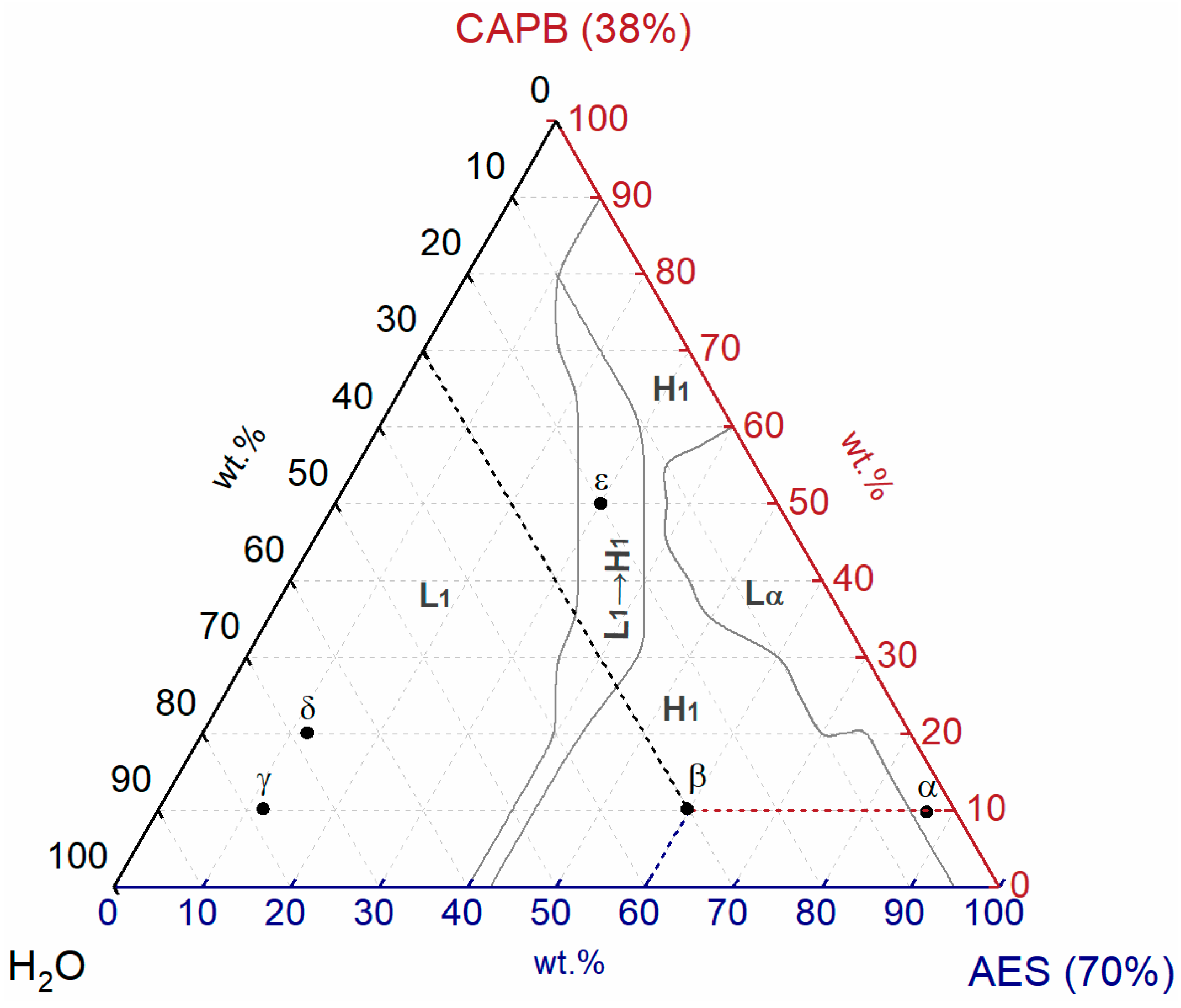


| Sample | AES (70%) (wt.%) | CAPB (38%) (wt.%) | H2O (wt.%) | Phase | Viscosity (Pa∙s) |
|---|---|---|---|---|---|
| α | 87.5 | 10 | 2.5 | Lα | 374.51 |
| β | 60 | 10 | 30 | H1 | 753.48 |
| γ | 12 | 10 | 78 | L1 | 0.02 |
| δ | 12 | 20 | 68 | L1 | 29.88 |
| ε | 30 | 50 | 20 | L1 → H1 | 47.28 |
| Sample | CAPB (38%) (wt.%) | AES (70%) (wt.%) | H2O (wt.%) | Viscosity (Pa∙s) | η0 * | R2 |
|---|---|---|---|---|---|---|
| 1 | 8 | 12 | 80 | 5.80 | 5.99 | 0.9922 |
| 2 | 9 | 12 | 79 | 8.06 | 9.13 | 0.9977 |
| 3 | 10 | 12 | 78 | 17.53 | 18.69 | 0.9961 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Zhang, G.; Wang, P. Phase Behavior and Rheological Properties of AES/CAPB/H2O Ternary System. Appl. Sci. 2024, 14, 3605. https://doi.org/10.3390/app14093605
Wu X, Zhang G, Wang P. Phase Behavior and Rheological Properties of AES/CAPB/H2O Ternary System. Applied Sciences. 2024; 14(9):3605. https://doi.org/10.3390/app14093605
Chicago/Turabian StyleWu, Xinran, Guangyan Zhang, and Peng Wang. 2024. "Phase Behavior and Rheological Properties of AES/CAPB/H2O Ternary System" Applied Sciences 14, no. 9: 3605. https://doi.org/10.3390/app14093605





