Development of Surface-Modified Polyacrylonitrile Fibers and Their Selective Sorption Behavior of Precious Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polyacrylonitrile (PAN) Fiber
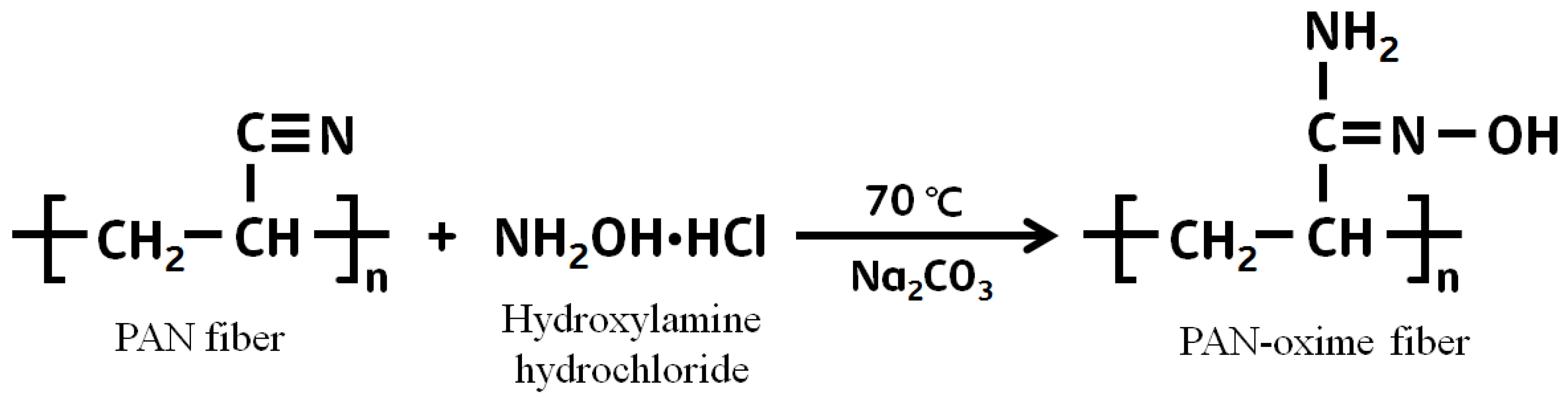
2.3. Chemical Modification of PAN Fiber
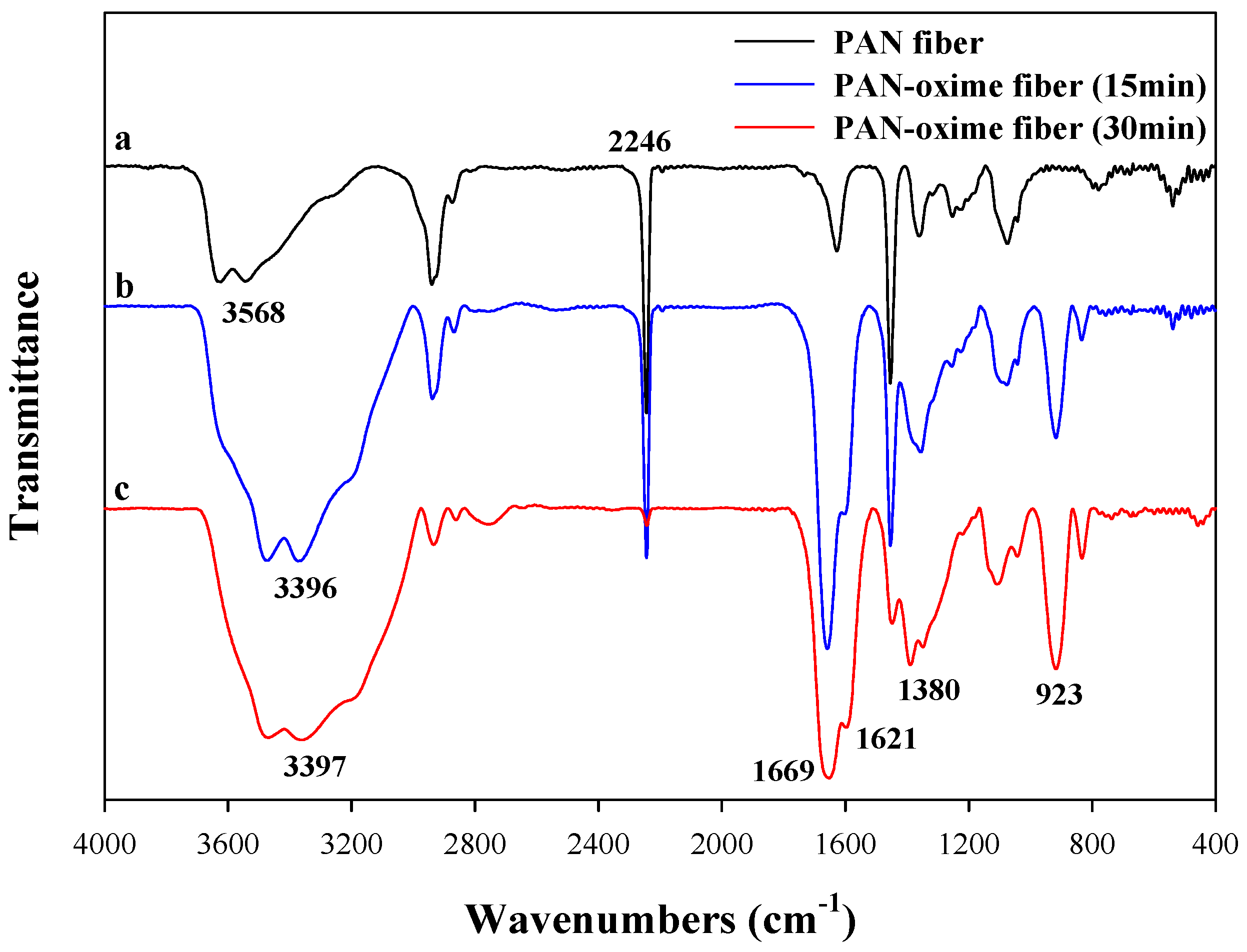
2.4. Characterization of PAN–Oxime Fiber
2.5. Batch Adsorption Experiments
3. Results and Discussion
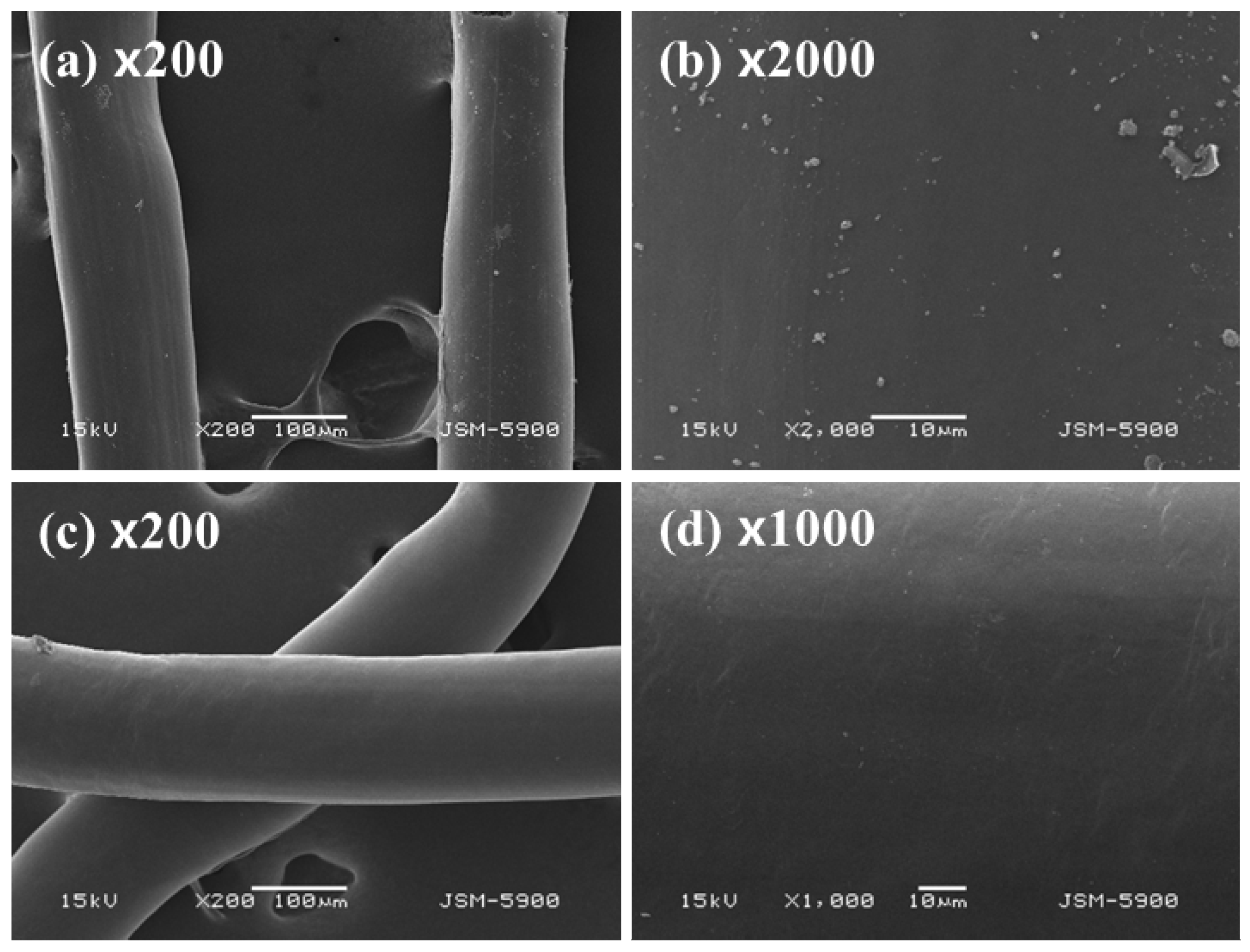
3.1. The Effect on Amidoximation on the Preparation of PAN–Oxime Fiber
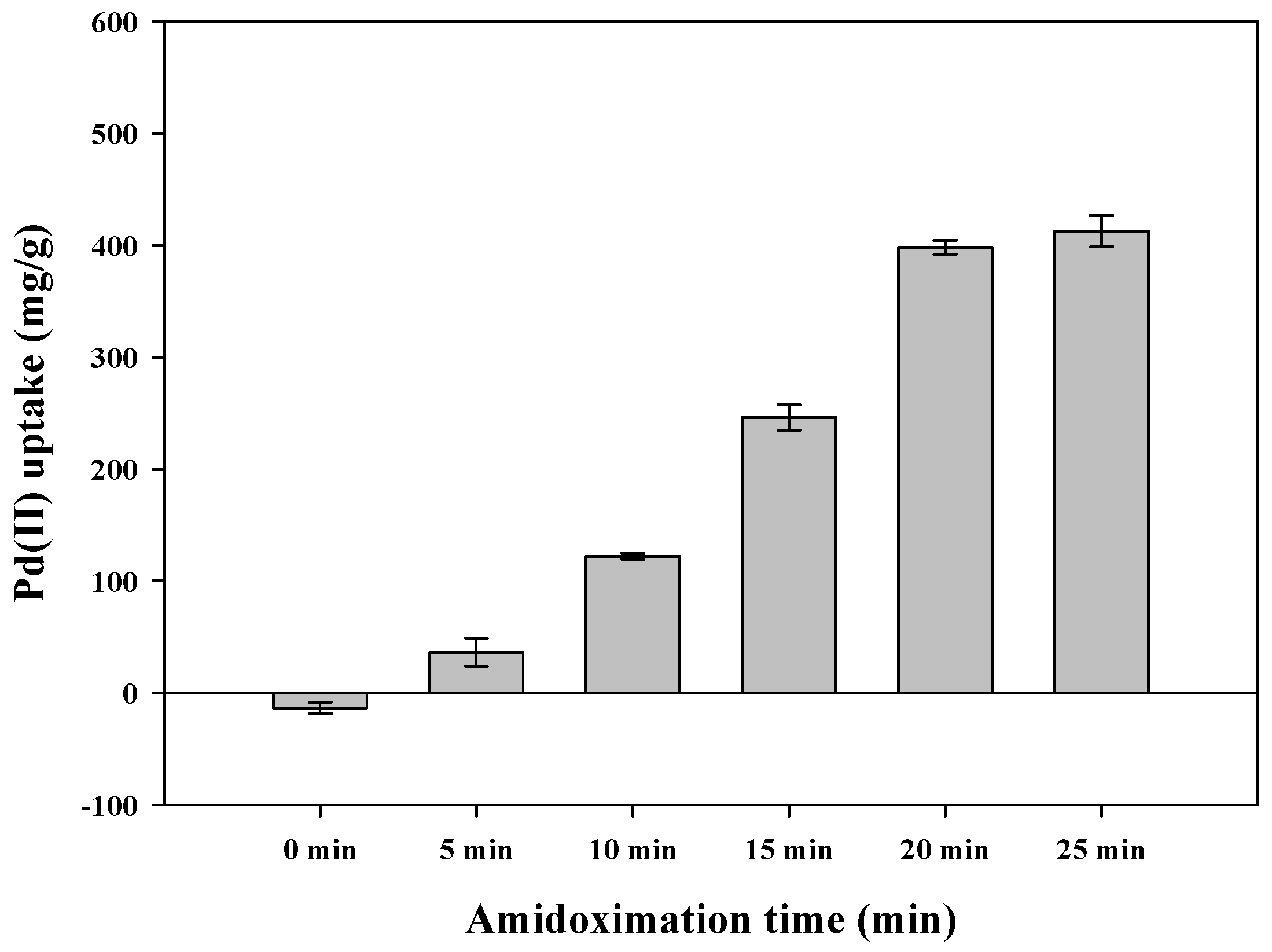
3.2. Effect of Amidoximation Time on Adsorption Capacity
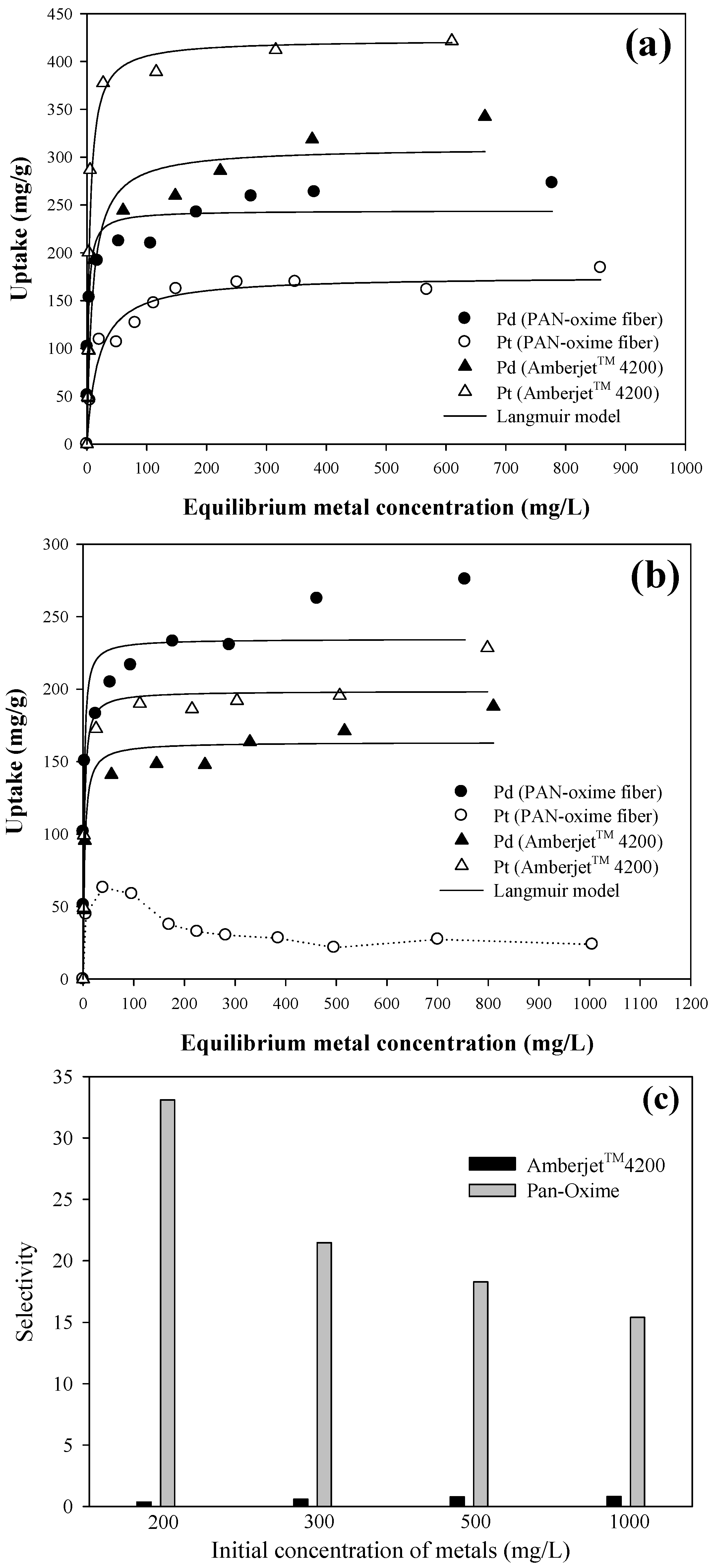
3.3. Isotherms Study
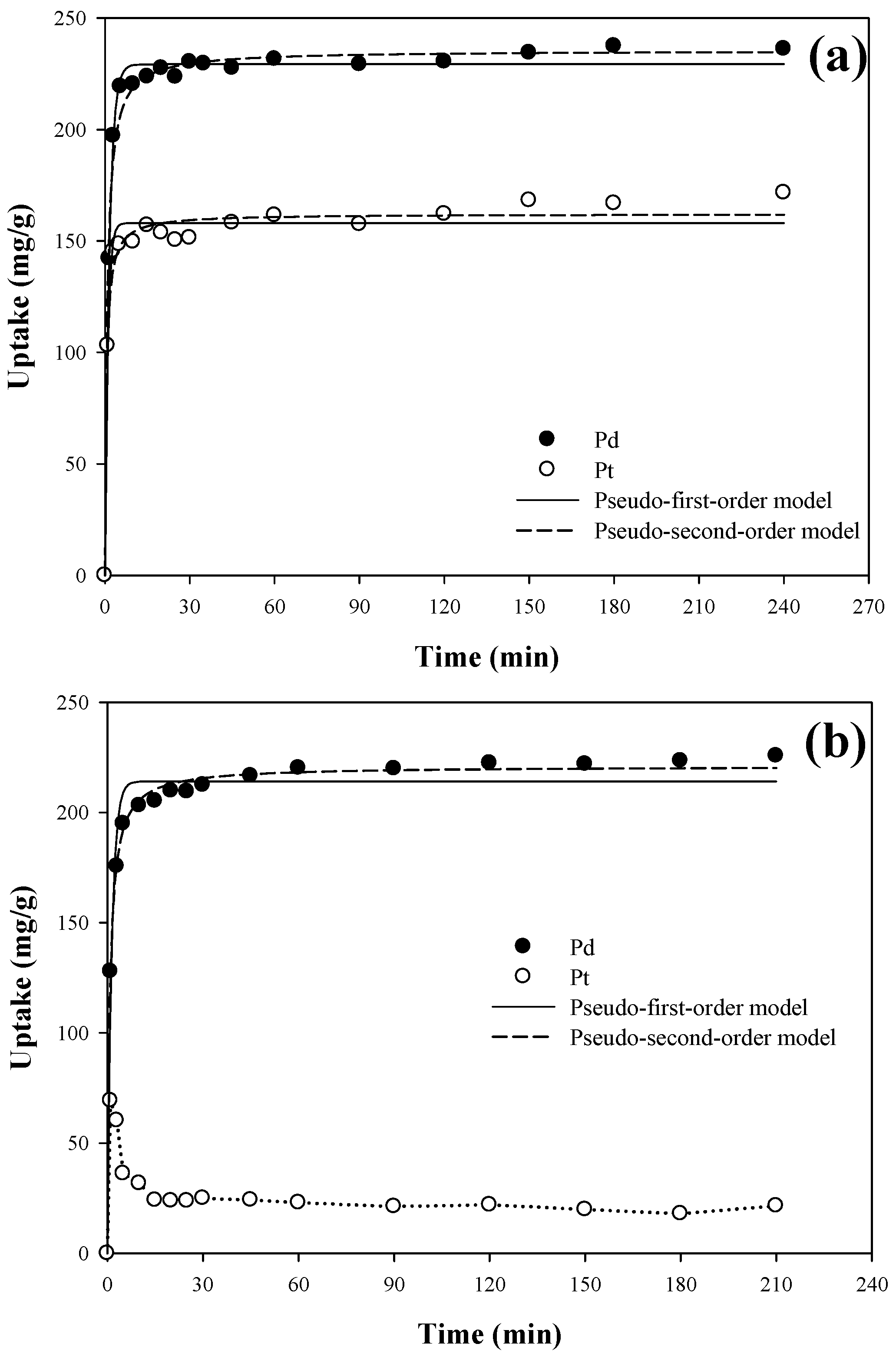
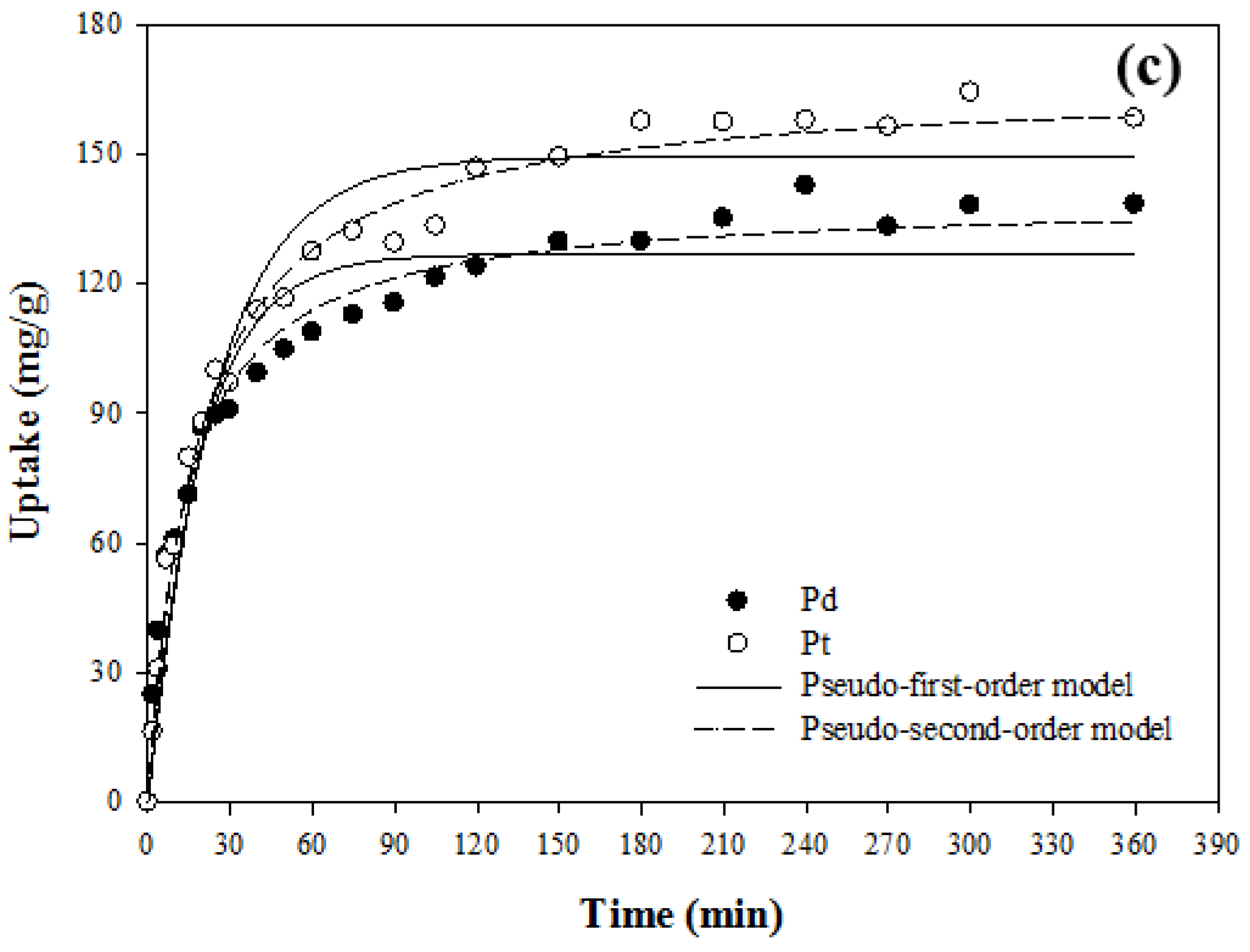
3.4. Kinetic Study
4. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Ravindra, K.; Bencs, L.; Van Grieken, R. Platinum group elements in the environment and their health risk. Sci. Total Environ. 2004, 318, 1–43. [Google Scholar] [CrossRef]
- Demopoulos, G.P. Solvent extraction in precious metals refining. JOM 1986, 38, 13–17. [Google Scholar] [CrossRef]
- Lee, J.Y.; Raju, B.; Kumar, B.N.; Kumar, J.R.; Park, H.K.; Reddy, B.R. Solvent extraction separation and recovery of palladium and platinum from chloride leach liquors of spent automobile catalyst. Sep. Purif. Technol. 2010, 73, 213–218. [Google Scholar] [CrossRef]
- Golunski, S.; Rajaram, R.; Hodge, N.; Hutchings, G.J.; Kiely, C.J. Low-temperature redox activity in co-precipitated catalysts: A comparison between gold and platinum-group metals. Catal. Today 2002, 72, 107–113. [Google Scholar] [CrossRef]
- Schreier, G.; Edtmaier, C. Separation of Ir, Pd and Rh from secondary Pt scrap by precipitation and calcination. Hydrometallurgy 2003, 68, 69–75. [Google Scholar] [CrossRef]
- Dabrowski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Gaita, R.; Al-Bazi, S.J. An ion-exchange method for selective separation of palladium, platinum and rhodium from solutions obtained by leaching automotive catalytic converters. Talanta 1995, 42, 249–255. [Google Scholar] [CrossRef]
- Birinci, E.; Gülfen, M.; Aydın, A.O. Separation and recovery of palladium(II) from base metal ions by melamine–formaldehyde–thiourea (MFT) chelating resin. Hydrometallurgy 2009, 95, 15–21. [Google Scholar] [CrossRef]
- Mack, C.; Wilhelmi, B.; Duncan, J.R.; Burgess, J.E. Biosorption of precious metals. Biotechnol. Adv. 2007, 25, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Koduru, J.R.; Lee, K.D. Separation studies of Pd(II) from acidic chloride solutions of Pt(IV), Ni(II) and Rh(III) by using 4-aroyl-3-phenyl-5-isoxazolones. J. Chem. 2012, 9, 756–765. [Google Scholar]
- Coşkun, R.; Soykan, C. Preparation of amidoximated polyester fiber and competitive adsorption of some heavy metal ions from aqueous solution onto this fiber. J. Appl. Polym. Sci. 2009, 112, 1798–1807. [Google Scholar] [CrossRef]
- Lin, W.; Hsieh, Y.-L. Kinetics of metal ion absorption on ion-exchange and chelating fibers. Ind. Eng. Chem. Res. 1996, 35, 3817–3821. [Google Scholar] [CrossRef]
- Yan, W.; Watson, V.J.; Logan, B.E. Substantial humic acid adsorption to activated carbon air cathodes produces a small reduction in catalytic activity. Environ. Sci. Technol. 2016, 50, 8904–8909. [Google Scholar]
- Deng, H.; Ning, J.; Wang, X. Amino-functionalized cotton fiber for enhanced adsorption of active brilliant red X-38 from aqueous solution. Microsc. Res. Tech. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.O.; Bakaev, V.A.; Banerjee, J.; Mueller, K.T.; Pantano, C.G. Characterization and reactivity of sodium aluminoborosilicate glass fiber surfaces. Appl. Surf. Sci. 2016, 370, 328–334. [Google Scholar] [CrossRef]
- Oyloa, Y.; Dai, S. High surface-area amidoxime-based polymer fibers co-grafted with various acid monomers yielding increased adsorption capacity for the extraction of uranium from weawater. Dalton Trans. 2016, 45, 8824–8834. [Google Scholar] [CrossRef] [PubMed]
- Nam, C.-W.; Kim, Y.-H.; Ko, S.-W. Modification of polyacrylonitrile (PAN) fiber by blending with N-(2-hydroxy)propyl-3-trimethyl-ammonium chitosan chloride. J. Appl. Polym. Sci. 1999, 74, 2258–2265. [Google Scholar] [CrossRef]
- George, J.; Sreekala, M.S.; Thomas, S. A review on interface modification and characterization of natural fiber reinforced plastic composites. Polym. Eng. Sci. 2001, 41, 1471–1485. [Google Scholar] [CrossRef]
- Saeed, K.; Haider, S.; Oh, T.-J.; Park, S.-Y. Preparation of amidoxime-modified polyacrylonitrile (PAN-oxime) nanofibers and their applications to metal ions adsorption. J. Membr. Sci. 2008, 322, 400–405. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Chiou, M.-S.; Li, H.-Y. Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. J. Hazard. Mater. 2002, 93, 233–248. [Google Scholar] [CrossRef]

| Adsorbents | Conditions | Metals | Parameters | ||
|---|---|---|---|---|---|
| qm (mg/g) | b (L/mg) | R2 | |||
| PAN–oxime fiber | Single | Pd(II) | 244.06 ± 10.68 | 0.435 ± 0.138 | 0.928 |
| Pt(IV) | 175.64 ± 6.62 | 0.052 ± 0.012 | 0.960 | ||
| Binary | Pd(II) | 234.54 ± 11.53 | 0.589 ± 0.214 | 0.905 | |
| Pt(IV) | - | - | - | ||
| Amberjet™ 4200 | Single | Pd(II) | 310.05 ± 13.25 | 0.107 ± 0.026 | 0.964 |
| Pt(IV) | 422.65 ± 21.33 | 0.239 ± 0.053 | 0.955 | ||
| Binary | Pd(II) | 163.35 ± 6.09 | 0.340 ± 0.093 | 0.955 | |
| Pt(IV) | 198.61 ± 6.64 | 0.473 ± 0.116 | 0.966 | ||
| Adsorbents | Conditions | Metals | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|---|
| q1 (mg/g) | k1 (min−1) | R2 | q2 (mg/g) | k2 × 10−3 (g/mg·min) | R2 | |||
| PAN–oxime fiber | Single | Pd(II) | 229.26 ± 1.22 | 0.644 ± 0.028 | 0.994 | 235.52 ± 1.86 | 5.5 ± 0.5 | 0.990 |
| Pt(IV) | 158.04 ± 1.93 | 1.008 ± 0.117 | 0.973 | 162.16 ± 1.72 | 11.5 ± 1.7 | 0.983 | ||
| Binary | Pd(II) | 214.09 ± 2.74 | 0.757 ± 0.084 | 0.972 | 221.00 ± 1.05 | 6.0 ± 0.3 | 0.997 | |
| Pt(IV) | - | - | - | - | - | - | ||
| Amberjet™ 4200 | Binary | Pd(II) | 126.84 ± 3.10 | 0.053 ± 0.006 | 0.933 | 139.48 ± 2.11 | 0.5 ± 0.04 | 0.984 |
| Pt(IV) | 149.40 ± 3.22 | 0.041 ± 0.004 | 0.959 | 166.82 ± 2.19 | 0.3 ± 0.02 | 0.991 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, A.; Song, M.-H.; Cho, C.-W.; Yun, Y.-S. Development of Surface-Modified Polyacrylonitrile Fibers and Their Selective Sorption Behavior of Precious Metals. Appl. Sci. 2016, 6, 378. https://doi.org/10.3390/app6120378
Lim A, Song M-H, Cho C-W, Yun Y-S. Development of Surface-Modified Polyacrylonitrile Fibers and Their Selective Sorption Behavior of Precious Metals. Applied Sciences. 2016; 6(12):378. https://doi.org/10.3390/app6120378
Chicago/Turabian StyleLim, Areum, Myung-Hee Song, Chul-Woong Cho, and Yeoung-Sang Yun. 2016. "Development of Surface-Modified Polyacrylonitrile Fibers and Their Selective Sorption Behavior of Precious Metals" Applied Sciences 6, no. 12: 378. https://doi.org/10.3390/app6120378







