1. Introduction
UV light can be generated as a consequence of electronic transitions of light sources through an Hg discharge. In low-pressure Hg discharge, the main emission line is located at a wavelength of 254 nm. This light is invisible and harmful to human bodies, so it has to be converted into visible light, which can be done with a combination of luminescent materials. These luminescent materials can strongly absorb light of that wavelength and efficiently convert it into visible light. Recently, white light emitting diodes (LEDs) have become popular because they have several advantages, including high efficiency, long lifetime, and low power consumption. Red phosphors are also helpful for generating white light when they are excited by blue or near-UV lights. To obtain phosphors with highly efficient emissions, it is important to choose the right compound materials and ensure they have outstanding physical and chemical stability. Numerous studies have explored different luminescent materials to enable the development of suitable phosphors. When lanthanide contraction ions are introduced into host materials, unfilled 4f
N electron orbitals result, and these have attracted considerable attention. The resulting phosphors emit very luminescent emissions with specific light wavelengths because of variations in the energy level of some free electrons [
1,
2,
3,
4]. These phosphors have been the most promising candidates for applications in fluorescent lamps and flat panel display devices, such as electroluminescence panels, plasma display panels, and field emission displays.
A large number of isotropic compounds with perovskite structures, such as double perovskite oxides, have the general formula
A2BB’O
6, in which
BO
6 and
B’O
6 octahedra are corner-shared, alternately. The great flexibility of
A and
B(
B’) sites in
A2BB’O
6 allows very rich substitutions, and this framework forms cube-octahedral cavities filled by A-site cations [
5,
6]. Double perovskites with the formula
A2BB’O
6, where
A uses an alkaline earth,
B and
B’ are metal transition magnetic and nonmagnetic ions, and O is oxygen, have been investigated as magnetic materials for many years. For example, Sr
2CrMoO
6 has been studied as a half-metallic system [
7]. Kobayashi et al. recently reported room-temperature low-field magnetic resistance in the ordered double perovskite Sr
2FeMoO
6 [
8]. Complete ordering of Fe and Mo on the
B and
B’ sites of this metallic
A2BB’O
6 double perovskite is predicted to give half-metallic ferromagnetism with localized majority-spin electrons on the Fe atoms [
9,
10]. Recently, the study of
A2BB’O
6-based materials has increased due to various technological applications, such as inorganic oxide luminescent materials.
The emitting materials are usually composed of activators and a host lattice. Some host lattice materials can produce light themselves, and some can produce light when doped with rare-earth activators (ions). Rare-earth ions are known to exist in various valence states, although the trivalent state is the most prevalent. Rare-earth ions can be applied in lighting devices and display panels due to their abundant energy levels across a wide spectrum range, from ultraviolet to near infrared. Sm- and Eu-based ions are the most commonly used dopants because they are stable in trivalent (Sm
3+ and Eu
3+), as well as divalent (Eu
2+), states. The luminescence of rare-earth ions doped in perovskite-type ceramics was actively investigated in the 1960s and 1970s because of interest in their ferroelectricity, phase transitions, and semiconducting properties [
11]. Recently, many studies have shown that the double perovskite structure with a composition of
A2BMO
6 (
A = Ba, Sr;
B = Ca, Zn;
M = Mo, W) is activated by trivalent europium ions (Eu
3+) [
12,
13,
14,
15]. Phosphors activated by Eu
3+ are considered ideal red sources because of their sharp emission lines in the red region [
12,
13,
14,
15,
16]. Eu
3+-doped double-perovskite materials have a broad excitation band ranging from UV to visible light, and they also show highly efficient red luminescence. For that, Eu
3+-doped double molybdenum (Mo)-based double perovskite oxides have attracted significant attention for their possible application as luminescent materials, such as Sr
2MgMo
xW
1−xO
6: Eu
3+ [
17], Sr
2Ca(Mo/W)O
6: Eu
3+ [
18], Sr
2CaMoO
6: Eu
3+ [
19], (Ba,Sr)
2CaMoO
6: Eu
3+, Yb
3+ [
20], Ca
2LaMO
6: Eu
3+ (
M = Sb, Nb, Ta) [
21], and A
2CaMoO
6 (
A = Sr, Ba) [
6], respectively.
To the best of our knowledge, the luminescent characteristics of concentrated Eu
3+ ions in Sr
2−xEu
xZnMoO
6 phosphors, which are molybdenum-based double-perovskite oxides, have not been reported. In this study, the red-emitting phosphors of Eu
3+-doped Mo-based double-perovskite Sr
2−xEu
xZnMoO
6 oxides were synthesized by the conventional high-temperature solid state reaction method. We found that Eu
3+ concentration and the synthesizing temperature of the Sr
2−xEu
xZnMoO
6 phosphors had a large effect on their luminescent characteristics. The concentration quenching effect of Eu
3+ ions in Sr
2−xEu
xZnMoO
6 phosphors was found and would be well discussed [
22]. In this study, the first important novelty is that we have not found any similar studies about Sr
2−xEu
xZnMoO
6 phosphors. The second important novelty is that we found a linear relationship between the synthesizing temperature and lifetime of Sr
2−xEu
xZnMoO
6 phosphors. We also investigated the relationship between the synthesized temperature and lifetime of Sr
2−xEu
xZnMoO
6 phosphors.
3. Results and Discussion
To achieve high PL properties, the preparation of Sr
2−xEu
xZnMoO
6 powders forming the double-perovskite phase is very important, because crystallization of Sr
2−xEu
xZnMoO
6 powders influences their photoluminescent properties.
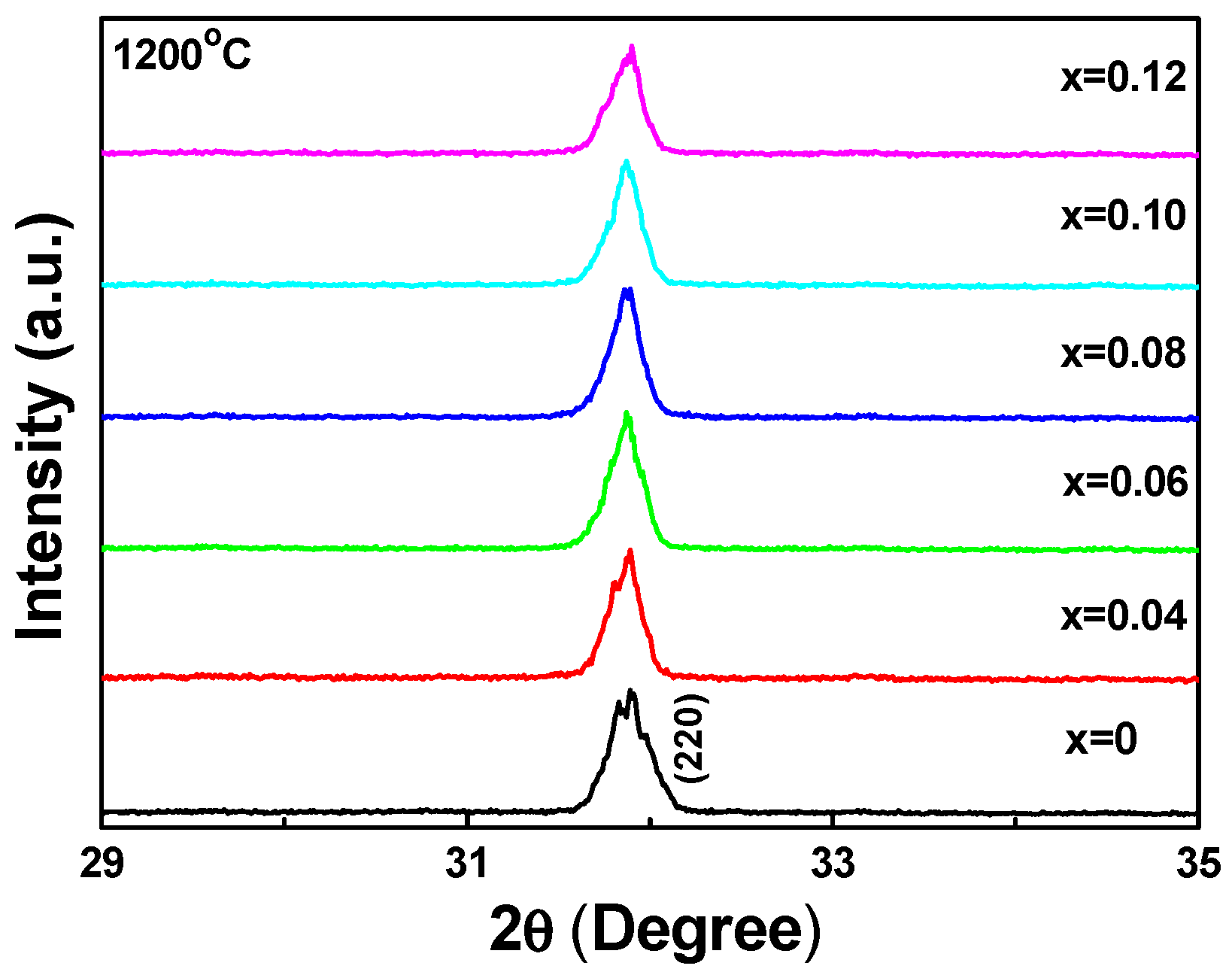
Figure 1 shows the XRD patterns of our Sr
2−xEu
xZnMoO
6 powders as a function of the synthesizing temperature. The strong peaks occurred at around 31.9° for the (220) diffraction peak of the six host lattices. Those results suggest that the XRD patterns showed stable double-perovskite features regardless of the synthesizing temperature and Eu
3+ concentration.
As
Figure 1a shows, when the synthesizing temperature of the Sr
2−xEu
xZnMoO
6 powders was 900 °C and as the
x value increased from 0 to 0.12, the 2θ value of the (220) diffraction peak shifted from 31.85 to 31.87 and the full width at half maximum (FWHM) values for the (220) diffraction peak were in the range of 2θ = 0.25°–0.27°. When the synthesizing temperature was 1200 °C, the 2θ value of the (220) diffraction peak shifted from 31.88 to 31.90, and the FWHM values for the (220) diffraction peak of the Sr
2−xEu
xZnMoO
6 powders were in the range of 2θ = 0.19–0.20°, as the
x value of the Sr
2−xEu
xZnMoO
6 powders increased from 0 to 0.12. The results in
Figure 1a–d show that the 2θ of the (220) diffraction peak shifted to a higher value and the FWHM values for the (220) diffraction peaks of the Sr
2−xEu
xZnMoO
6 powders decreased as the synthesizing temperature increased.
The ideal cubic double-perovskite structure (with the same space group Pm
m (221)) can be described by a faced-centered cubic (fcc) lattice with lattice constant 2
a [
23]. The
B(
B’) ion is coordinated by the
B’(
B) ion using an O ion as an intermediate in the middle, and the lengths of
B–O and
B’–O are considered to be equal. After relaxation, both lattice constants and atomic positions reduce the ideal cubic structure (space group
Fmm) to a tetragonal structure (space group
I4/
mmm). There are two O
1 atoms located on the
z-axis with
B and
B’ atoms sitting between, and the four O
2 atoms are located on the
xy-plane; the same as the
B and
B’ atoms. The angle of the
B–O–
B’ remains at 180° during structural optimization, whereas the lattice constant and bond length change. The lattice
a can be calculated by using (1) the reflection peaks (011), (111), (200), and (220) from the XRD patterns in
Figure 1; and (2) the closeness of the
c/
a ratio to the ideal value of
. As the synthesizing temperature was 900 °C, the Sr
2−xEu
xZnMoO
6 powders exhibited a cubic crystal structure with the cell parameters changing from
a =
b =
c/
= 0.3968 nm to
a =
b =
c/
= 0.3971 nm as the concentration of Eu
3+ ions increased from 0 to 0.12. The cell parameters for Sr
2−xEu
xZnMoO
6 powders synthesized at 1200 °C were also calculated from the XRD patterns shown in
Figure 1, and the cell parameters changed from
a =
b =
c/
= 0.3971 nm to
a =
b =
c/
= 0.3974 nm as the concentration of Eu
3+ ions increased from 0 to 0.12. These results were in good agreement with those from the Joint Committee on Powder Diffraction Standards (JCPDS) file number 742474.
As the results of the X-ray diffraction (XRD) patterns showed in
Figure 1 were compared, these results indicated that the diffraction intensities of unknown phases for peaks located at around 2θ = 28.44° and 33.09° increased as the concentration of Eu
3+ ions increased and decreased as the synthesizing temperature increased. Those peaks are in good agreement with the (222) and (400) peaks of JCPDS file number 120393 for cubic Eu
2O
3. The decrease in the diffraction intensities of peaks located at around 2θ = 28.44° and 33.09° prove that more Eu
3+ ions will substitute the sites of Sr
2+ ions as the synthesizing temperature is raised. Even when the synthesizing temperature was 1100 °C, secondary or unknown phases were observed in the Sr
2−xEu
xZnMoO
6 ceramic powders. These Eu
2O
3 and secondary or unknown phases were not observed when the synthesizing temperature was 1200 °C. These results suggest that when the same synthesizing temperature is used, the concentration of Eu
3+ ions has no apparent effect on the crystallization of Sr
2−xEu
xZnMoO
6 powders; hence, the synthesizing temperature is an important factor in determining the crystalline properties of Sr
2−xEu
xZnMoO
6 powders. Additionally, the concentration of Eu
3+ ions and the synthesizing temperature affect the photoluminescent properties of Sr
2−xEu
xZnMoO
6 phosphors.
XRD patterns for Sr
2−xEu
xZnMoO
6 phosphors synthesized at 1200 °C for 4 h in the narrow range of 29–35° are shown in
Figure 2. These results are significant. Initially, the splitting of the (220) diffraction peak was observed in the Sr
2ZnMoO
6 and Sr
1.98Eu
0.02ZnMoO
6 phosphors, but it was not observed in other Sr
2−xEu
xZnMoO
6 phosphors. The two split peaks of the Sr
2ZnMoO
6 phosphors were located at 2θ = 31.83° and 31.88°, and the two split peaks of the Sr
1.98Eu
0.02ZnMoO
6 phosphors were located at 2θ = 31.81° and 31.89°. These results suggest that the Sr
2ZnMoO
6 and Sr
1.98Eu
0.02ZnMoO
6 phosphors revealed a perovskite structure with the tetragonal phase. As the concentration of Eu
3+ was more than 0.02, the Sr
2−xEu
xZnMoO
6 phosphors would have transformed from the tetragonal phase to the (pseudo-)cubic phase, since the splitting of the (220) diffraction peak was not observed. Since the Sr
2−xEu
xZnMoO
6 phosphors were tetragonal phase or (pseudo-)cubic phase, even when the concentration of Eu
3+ ions was increased to 0.12, this result also proves that the Eu
3+ ions would have substituted into the sites of Ba
2+ ions. If the Eu
3+ ions had substituted into the sites of Zn
2+ (or Mo) ions, the Sr
2−xEu
xZnMoO
6 phosphors would have revealed other crystalline phases, or more secondary phases would have been revealed in the Sr
2−xEu
xZnMoO
6 phosphors rather than in the double-perovskite features.
Previously, Lin et al. successfully synthesized novel near-UV and blue-excited Eu
3+, Tb
3+-co-doped one-dimensional strontium germanate full-color nanophosphors by a simple sol-hydrothermal method. They found that incorporation of the Eu
3+ and Tb
3+ ions into strontium germanate resulted in a slight shrinkage of the lattice constants and the unit cell volume because Eu
3+ and Tb
3+ had smaller radii than Sr
2+, indicating the Eu
3+ and Tb
3+ ions had been incorporated into the host lattice of SrGe
4O
9 and did not change the crystal structure [
24]. Those results also suggested that the Eu
3+ ions substituted into the sites of Sr
2+ ions. The 2θ value of the (220) diffraction peak was shifted to a higher value, as the concentration of Eu
3+ was equal to, or greater than, 0.02. The ion radius of Sr
2+ is 0.130 nm and the ion radius of Eu
3+ is 0.1087 nm. Hence, as more Sr
2+ would have been substituted by Eu
3+, the ionic radius of the Sr
2−xEu
xZnMoO
6 phosphors would have decreased, increasing the 2θ value of the (220) diffraction peak. The ion radius of Eu
2+ is 0.131 nm, which is thought to be the same as the ion radius of Sr
2+. If Eu
2O
3 exists as Eu
2+ ions, the cell parameters of the Sr
2−xEu
xZnMoO
6 phosphors would not have been changed. The shift of the (220) diffraction peak to a higher 2θ value or the decrease in the cell parameters can prove that Eu
2O
3 existed in the Sr
2−xEu
xZnMoO
6 phosphors and in the Eu
3+ state.
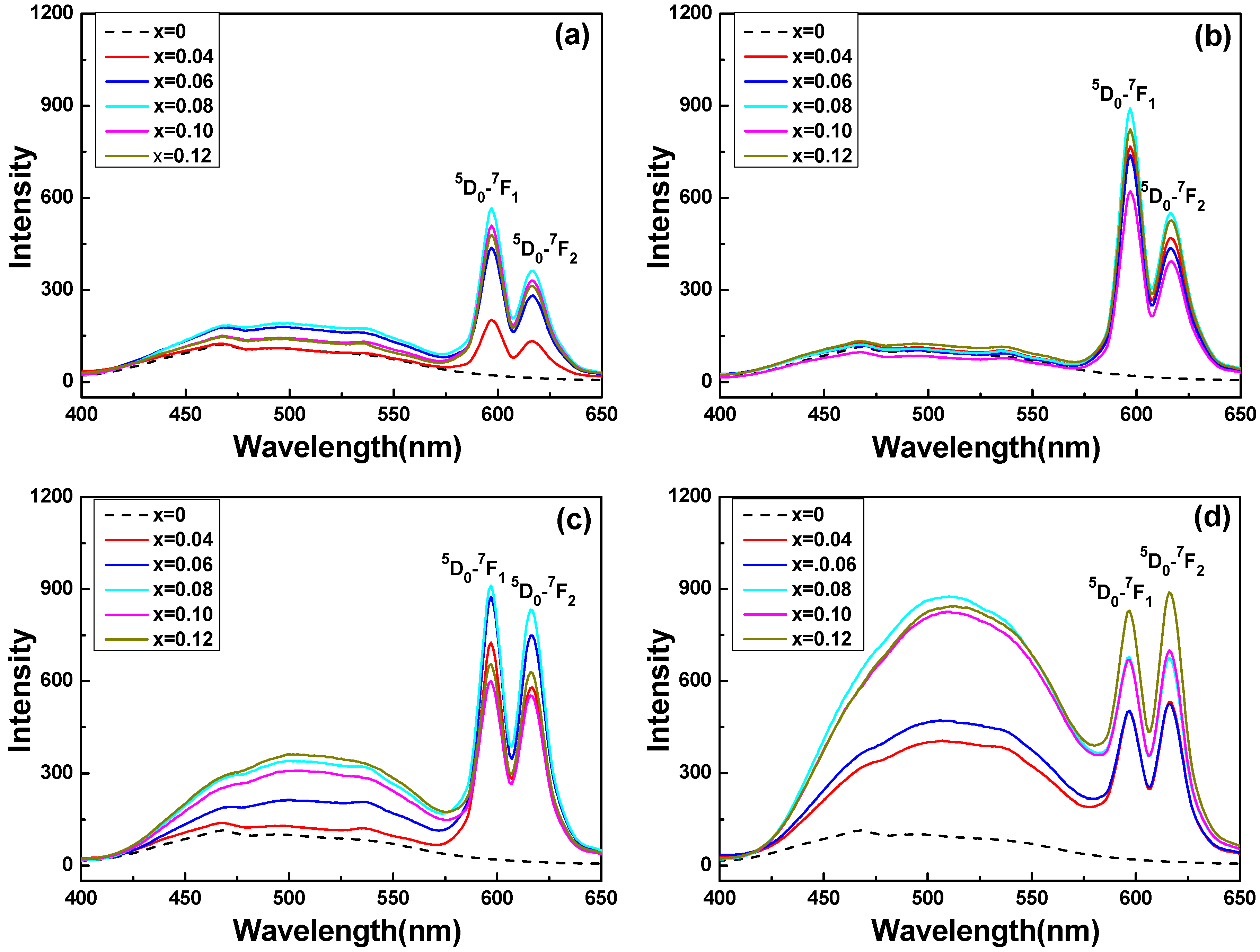
In order to find the best doping concentration of Eu
3+, we synthesized a series of Sr
2−xEu
xZnMoO
6 phosphors (
x = 0 to 0.12) and measured their emission spectra. The PL emission spectra of Sr
2−xEu
xZnMoO
6 powders excited at a wavelength of 350 nm are shown in
Figure 3 for the light wavelength range of 400–650 nm. The spectra in
Figure 3 show that that all the excitation spectra of Sr
2−xEu
xZnMoO
6 phosphors (except the Sr
2ZnMoO
6 powder) consisted of two parts: one broad band in the 400–575 nm region and two sharp peaks located at 597 nm and 616 nm. However, for the Sr
2ZnMoO
6 powder, even when the synthesizing temperature was 1200 °C, the two sharp peaks of Sr
2−xEu
xZnMoO
6 phosphors located at 597 nm and 616 nm were not found in the emission spectra. This means that no characteristic peaks were observed in Sr
2ZnMoO
6 powder even when the synthesizing temperature was 1200 °C. Obviously, the broad band in the 400–575 nm region is assignable to the well-known O
2–Mo
6+ charge transfer band (CTB) [
25]. As Eu
2O
3 was substituted for SrO, the Sr
2−xEu
xZnMoO
6 phosphors had the same spectral profile, but with a different concentration of Eu
3+ ions, the characteristic peaks were observed.
When the synthesizing temperature was changed from 900 to 1200 °C, the emission spectra of the Sr2−xEuxZnMoO6 phosphors consisted of sharp peaks in two strong bands at 597 and 616 nm, corresponding to the 5D0–7F1 (597 nm) and 5D0–7F2 (616 nm) transitions of Eu3+ ions. These results prove that the two emission peaks of the Sr2−xEuxZnMoO6 phosphors, 5D0–7F1 at 597 nm and 5D0–7F2 at 616 nm, were excited by the addition of Eu3+ ions. When the synthesizing temperature was raised to 1100 and 1200 °C, the intensity of the broadened emission went from 400 nm to 575 nm, increasing with the increase in the Eu3+ ion concentration. However, the emission intensities of the Sr2−xEuxZnMoO6 phosphors were influenced by the synthesizing temperature and the concentration of the Eu3+ ions.
In a previous study, the spectra of BaZrO
3 doped with Eu
3+ powders consisted of a series of resolved emission peaks located at 576 nm, 597 nm, 616 nm, 623 nm, 651 nm, 673 nm, 696 nm, and 704 nm, which are assignable to the
5D
0–
7F
J (
J = 0, 1, 2, 3, 4) transitions of Eu
3+ ions, namely,
5D
0–
7F
0 (576 nm),
5D
0–
7F
1 (597 nm),
5D
0–
7F
2 (616 nm, 623 nm),
5D
0–
7F
3 (651 nm), and
5D
0–
7F
4 (673 nm, 696 nm, and 704 nm) [
16]. Liu and Wang’s research also showed that the emission intensities of
5D
0–
7F
0 (576 nm),
5D
0–
7F
1 (597 nm), and
5D
0–
7F
2 (616, 623 nm) had almost the same values, even if the Eu concentration in the BaZr
1−xEu
xO
3 phosphor powders was different [
26]. These previous results [
6,
16], and the results this study, suggest that the transitions of Eu
3+ ions between different energy bands are affected by the host materials of the prepared phosphors.
As the synthesizing temperature was changed from 900 °C to 1100 °C, the intensities of the two emission peaks of the Sr
2−xEu
xZnMoO
6 phosphors was enhanced by increasing the Eu
3+ doping concentration and reached a maximum value at
x = 0.08. In contrast, the intensities of the two emission peaks of the Sr
2−xEu
xZnMoO
6 phosphors decreased when the Eu
3+ doping ratio was more than 0.08. This proves that the concentration-quenching effect of Eu
3+ doping happened in the Sr
2−xEu
xZnMoO
6 phosphors. When 1200 °C was used as the sintering temperature, the intensities of the two emission peaks of the Sr
2−xEu
xZnMoO
6 phosphors increased as the concentration of Eu
3+ ions rose. The results in
Figure 3 also show that when the temperature changed from 1100 °C to 1200 °C, the emission peak varied significantly. For the Sr
2−xEu
xZnMoO
6 phosphors, when the synthesizing temperature was changed from 1100 °C to 1300 °C, the emission peak with the maximum intensity shifted from
5D
0–
7F
1 (597 nm) to
5D
0–
7F
2 (616 nm). Nevertheless, the strong band at 576 nm corresponding to the
5D
0–
7F
0 transition was not observed in the emission spectra.
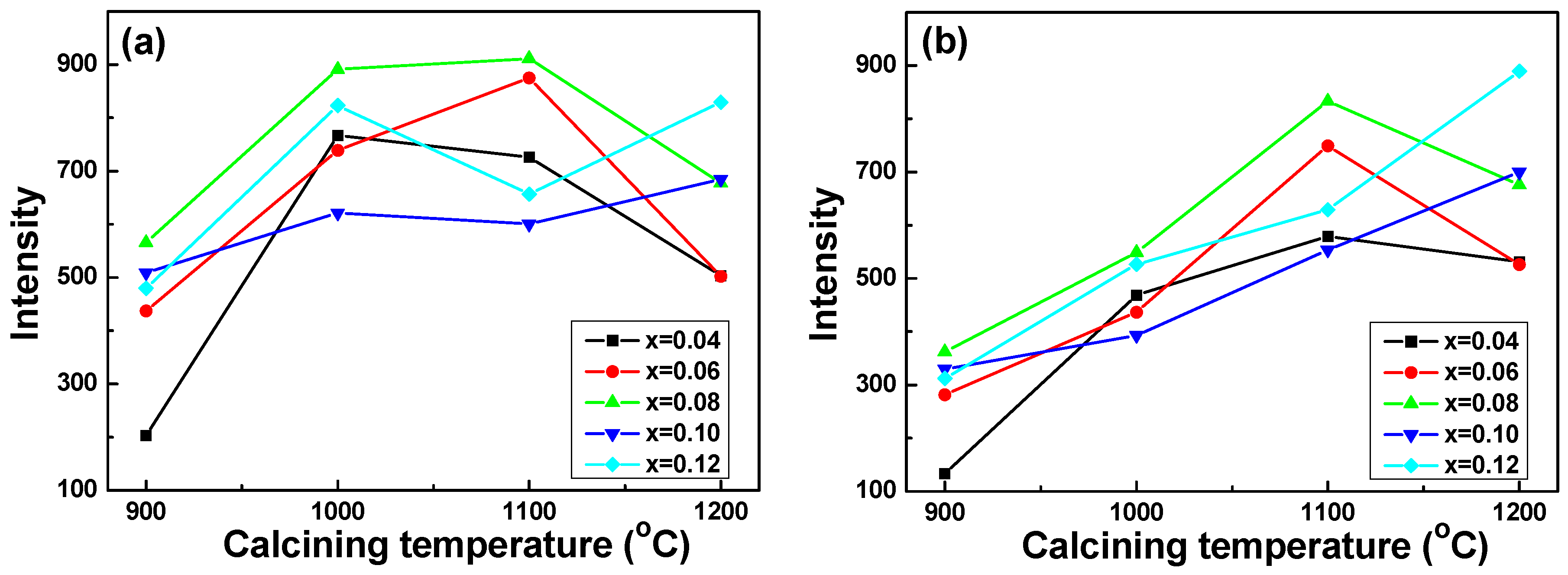
The maximum emission intensities (PL
max values) of the Sr
2−xEu
xZnMoO
6 phosphors in the transitions of the
5D
0–
7F
1 (597 nm) and
5D
0–
7F
2 (616 nm) peaks are presented in
Figure 4 as a function of the synthesizing temperature and Eu
3+ ion concentration. Those results suggest again that the PL characteristics of the Sr
2−xEu
xZnMoO
6 phosphors were strongly affected by the synthesizing temperature and the concentration of Eu
3+ ions. As the
x value of the Sr
2−xEu
xZnMoO
6 phosphors was smaller than 0.10, the emission intensities of the
5D
0–
7F
1 (597 nm) and
5D
0–
7F
2 (616 nm) peaks first increased, reached a maximum, and then decreased as the synthesizing temperature increased. When the
x values of Sr
2−xEu
xZnMoO
6 phosphors were 0.10 and 0.12, the emission intensity of the
5D
0–
7F
1 (597 nm) first increased, then decreased at 1100 °C, and then increased at 1200 °C. The emission intensity of the
5D
0–
7F
2 (616 nm) peaks increased with the increase in synthesizing temperature. These results suggest that 1100 °C is an important synthesizing temperature for Sr
2−xEu
xZnMoO
6 phosphors because the transition of PL properties happens at this temperature, but the reasons for this are not known.
The results in
Figure 3 and
Figure 4 present an important result regarding Sr
2−xEu
xZnMoO
6 phosphors: the PL
max values of the transition of the
5D
0–
7F
1 (597 nm) critically increased as the synthesizing temperature increased from 900 °C to 1000 °C, then they did not apparently increase as the synthesizing temperature increased from 1000 °C to 1100 °C, as
Figure 4a shows. However, for Sr
2−xEu
xZnMoO
6 phosphors with
x = 0.04, 0.06, and 0.08, the PL
max values of the transition of the
5D
0–
7F
2 (616 nm) linearly increased as the synthesizing temperature increased from 900 °C to 1100 °C. For Sr
2−xEu
xZnMoO
6 phosphors with
x = 0.10 and 0.12, the PL
max values of the transition of the
5D
0–
7F
2 (616 nm) linearly increased as the synthesizing temperature increased from 900 °C to 1200 °C, as
Figure 4b shows. When the synthesizing temperature was 1200 °C, the emission intensity of the transition of
5D
0–
7F
2 (616 nm) for Sr
1.9Eu
0.1ZnMoO
6 and Sr
1.88Eu
0.12ZnMoO
6 phosphors was higher than that of
5D
0–
7F
1 (597 nm), as
Figure 4 shows.
The transitions of
5D
0–
7F
1 (588 nm),
5D
0–
7F
2 (612 nm),
5D
0–
7F
3 (649 nm), and
5D
0–
7F
4 (695 nm) for the Eu
3+ ions can be simultaneously observed in the emission spectrum of the Eu
3+, Tb
3+-co-doped one-dimensional strontium germinate, full-color nano-phosphors [
24]. However, introduction of the Eu
3+ ions in the Sr
2−xEu
xZnMoO
6 lattice results in disorder. The disorder in the structure will cause transitions in
5D
0–
7F
2 and point defects in the lattice because of differences in the chemical valence and in the ionic radius between Eu
3+ ions and Sr
2+ ions. The Eu
3+ ions could occupy either of the sites of Sr
2+ or the sites of Zn
2+ (Mo
2+) in the Sr
2−xEu
xZnMoO
6 phosphor’s double-perovskite structure. If Eu
3+ ions occupy a lattice site with a strict center of symmetry, the odd terms of the static crystal field vanish. This will lead to electric dipole transitions being strictly forbidden for purely electric transitions and the transitions of the
5D
0–
7F
1 (597 nm) will predominantly produce the emission.
The Eu
3+ ions could occupy either the tetrahedral sites of Sr
2+ or the octahedral sites of Zn
2+ or Mo
6+ in the Sr
2−xEu
xZnMoO
6 double-perovskite crystal structure. Consequently, as both of these sites are centrosymmetric, electric dipole transitions (
5D
0–
7F
j=0,2, only the
5D
0–
7F
2 transition is observed in this study) are forbidden and, as such, should not appear in the spectra. As the synthesizing temperature is 900 °C, the fact that the
5D
0–
7F
2 electric dipole transition at 615 nm prevails over the
5D
0–
7F
1 magnetic dipole transition at 595 nm in the case of Sr
2−xEu
xZnMoO
6 phosphors leads us to the conclusion that the Eu
3+ ions are generally located in a disordered manner in the Sr
2−xEu
xZnMoO
6 powders [
27]. The charge-compensating oxygen vacancies surrounding the Eu
3+ ions will lead to the deviation from the point symmetry and relaxation of electric dipole transitions selection rules, with the appearance of the
5D
0–
7F
2 transition lines in the spectra [
27]. Additionally, the Eu
3+ luminescent centers contributing to the
5D
0–
7F
2 transition line are probably located at the Sr
2−xEu
xZnMoO
6 particle surface or subsurface and will dominate the emission of Sr
2−xEu
xZnMoO
6 phosphors when the synthesizing temperature is 1200 °C [
27]. If Eu
3+ ions occupy a non-centrosymmetric lattice site, both electric and magnetic transitions are possible, and the non-symmetric site occupancy will be a dominant reason for causing the emission of
5D
0–
7F
2 (612 nm) transition [
28].
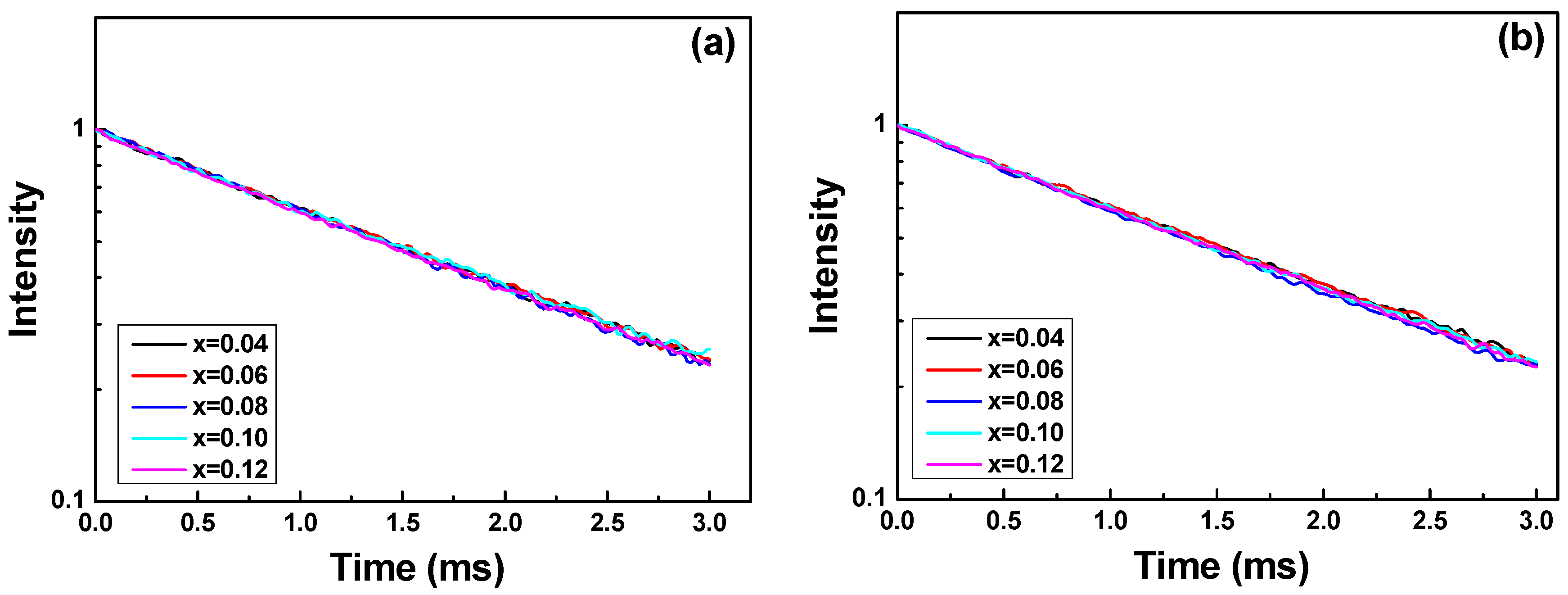
The definition of fluorescence (or phosphorescence) lifetime is the time for the intensity of a single emission peak to decrease to 1/e (approximately 37%) of its original intensity. The decay curves of Sr
2−xEu
xZnMoO
6 phosphors were obtained at different concentrations of Eu
3+ (
x = 0.04, 0.06, 0.08, 0.010, and 0.12), and
Figure 5 shows the intensity decay of the luminescence of Sr
2−xEu
xZnMoO
6 phosphors as a function of the synthesizing temperature, which can be used to calculate the lifetime. The wavelength of the exciting light was 271 nm, and the measured wavelength for the intensity decay was 597 nm (
5D
0–
7F
1), because it had the maximum emission intensity, as the synthesizing temperature was 900–1100 °C. Typically, the standard form of the single exponential decay function is:
where
A0 is the initial population, τ is constant for the decay time , and
t is time.
Figure 5 shows that the all curves of decay time can be fitted well by a single-exponential function.
Figure 5 also shows that if the same synthesizing temperature is used, the observed decay time is almost unchanged even the
x value in Sr
2−xEu
xZnMoO
6 phosphor increases from 0.04 to 0.12. Moreover, the decay time is a single exponential function for Sr
2−xEu
xZnMoO
6 phosphors with different concentrations of Eu
3+ ions and synthesized at different temperatures, because the activator lies in the same coordination environment [
29].
From the results shown in
Figure 5, the lifetimes of Sr
2−xEu
xZnMoO
6 phosphors, which are compared in
Table 1, were measured at 2.04–2.10 ms, 1.93–2.03 ms, 1.78–1.82 ms, and 1.65–1.67 ms, when the synthesizing temperature was 900 °C, 1000 °C, 1100 °C, and 1200 °C, respectively. The lifetimes of Sr
2−xEu
xZnMoO
6 phosphors apparently decreased with the increase in the synthesizing temperature. When the synthesizing temperature was 900 °C and 1000 °C, the lifetimes of the Sr
2−xEu
xZnMoO
6 phosphors varied more. When the synthesizing temperature was 1100 °C and 1200 °C, the Sr
2−xEu
xZnMoO
6 phosphors had less variation in their lifetimes. In
Figure 6 we use a fitting curve to find the relationship between lifetime and synthesizing temperature; the equation is:
where
t represents the emitting lifetime of the luminescent material and
T represents the synthesizing temperature (°C). In other words, the luminescence lifetime of Sr
2−xEu
xZnMoO
6 phosphors can be obtained by using Equation (2), as the synthesizing temperature is changed from 900 °C to 1200 °C. These results suggest that the luminescence lifetime of Sr
2−xEu
xZnMoO
6 phosphors can be a function of synthesizing temperature, and that the concentration of Eu
3+ ions not only can affect their emitting intensity but also can affect their luminescence lifetime.