High Electrocatalytic Performance of CuCoNi@CNTs Modified Glassy Carbon Electrode towards Methanol Oxidation in Alkaline Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurements
2.2. Electrodes Modification
3. Results
4. Discussion
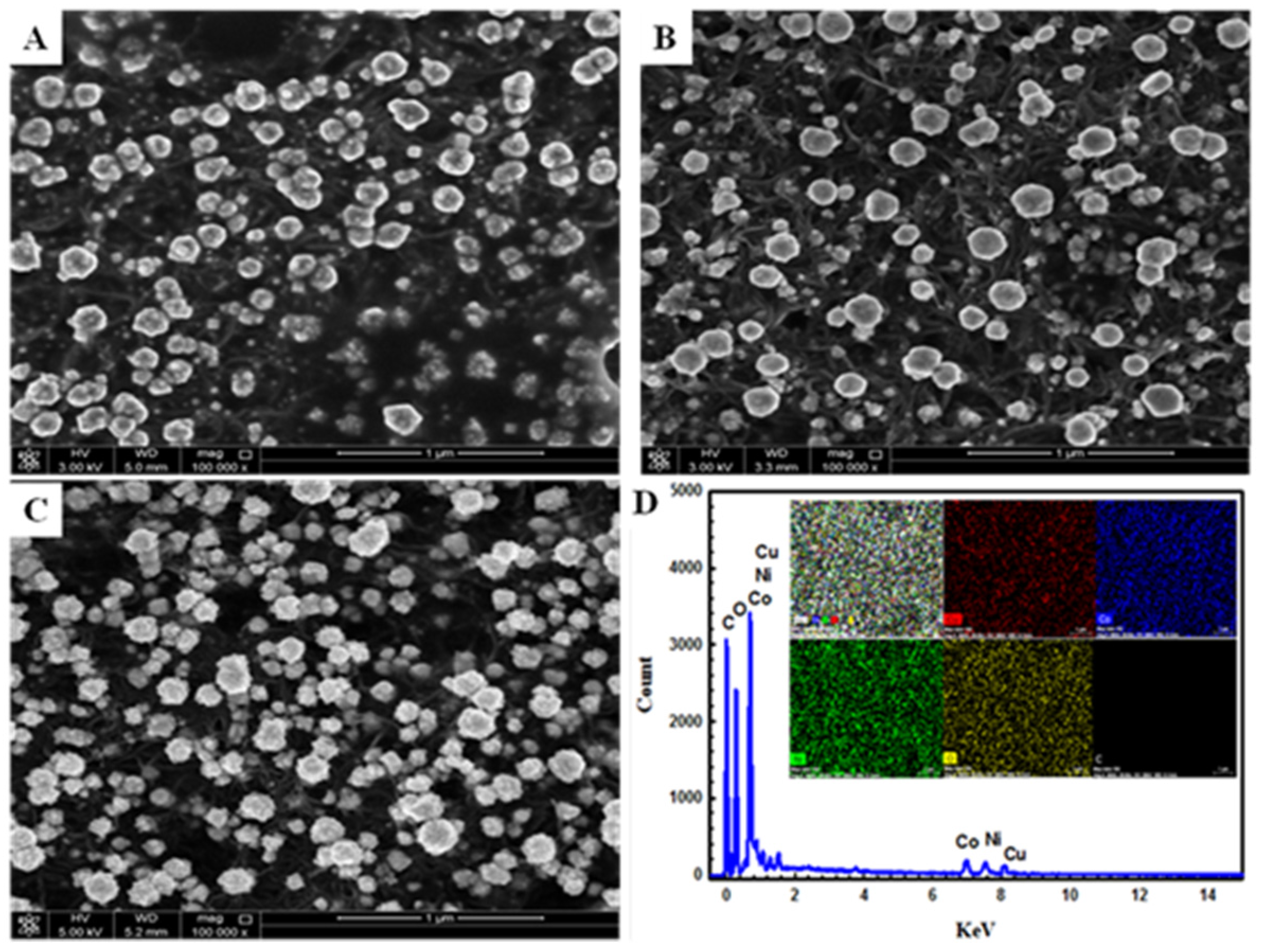
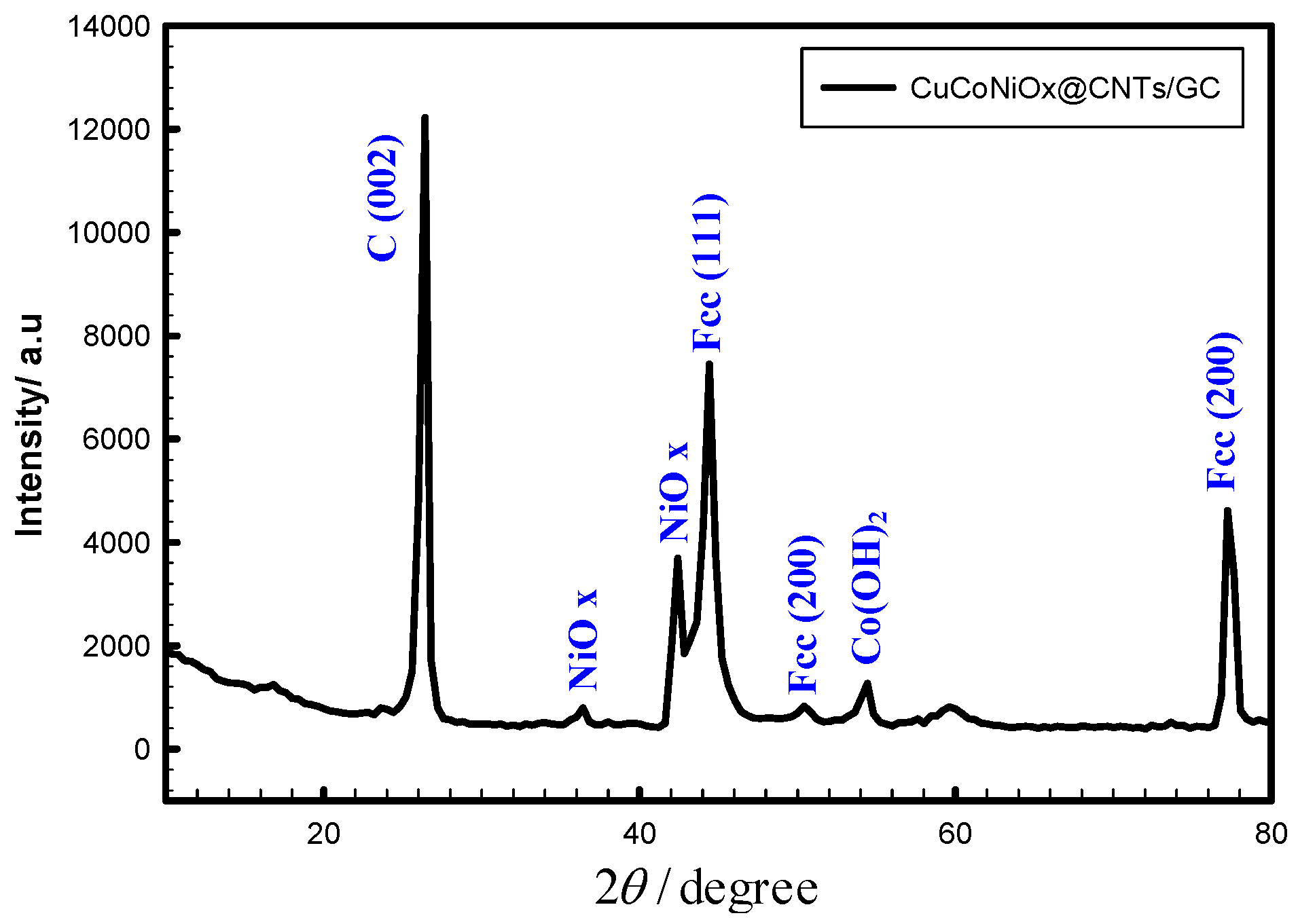
4.1. Materials and Electrochemical Characterization
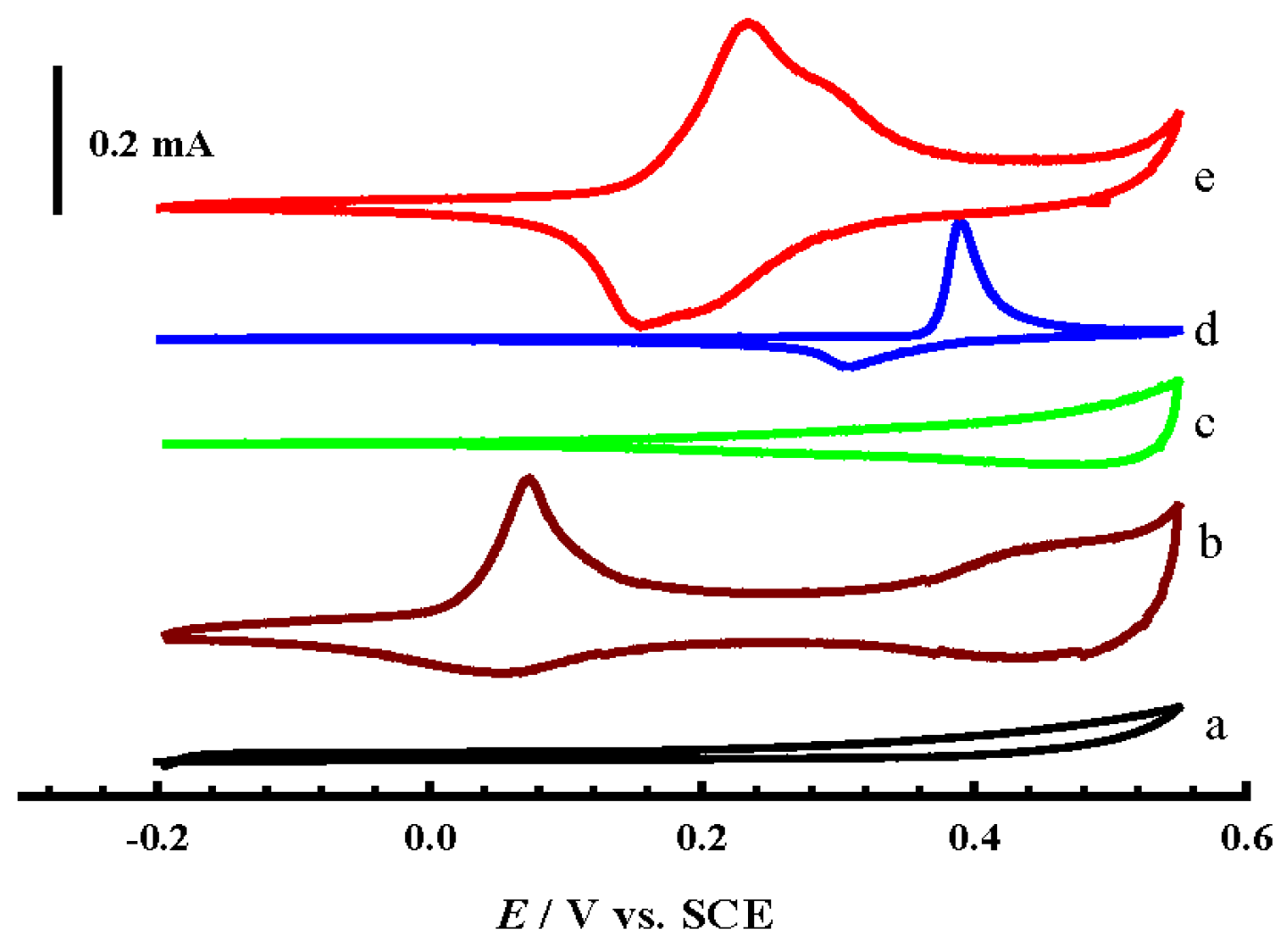
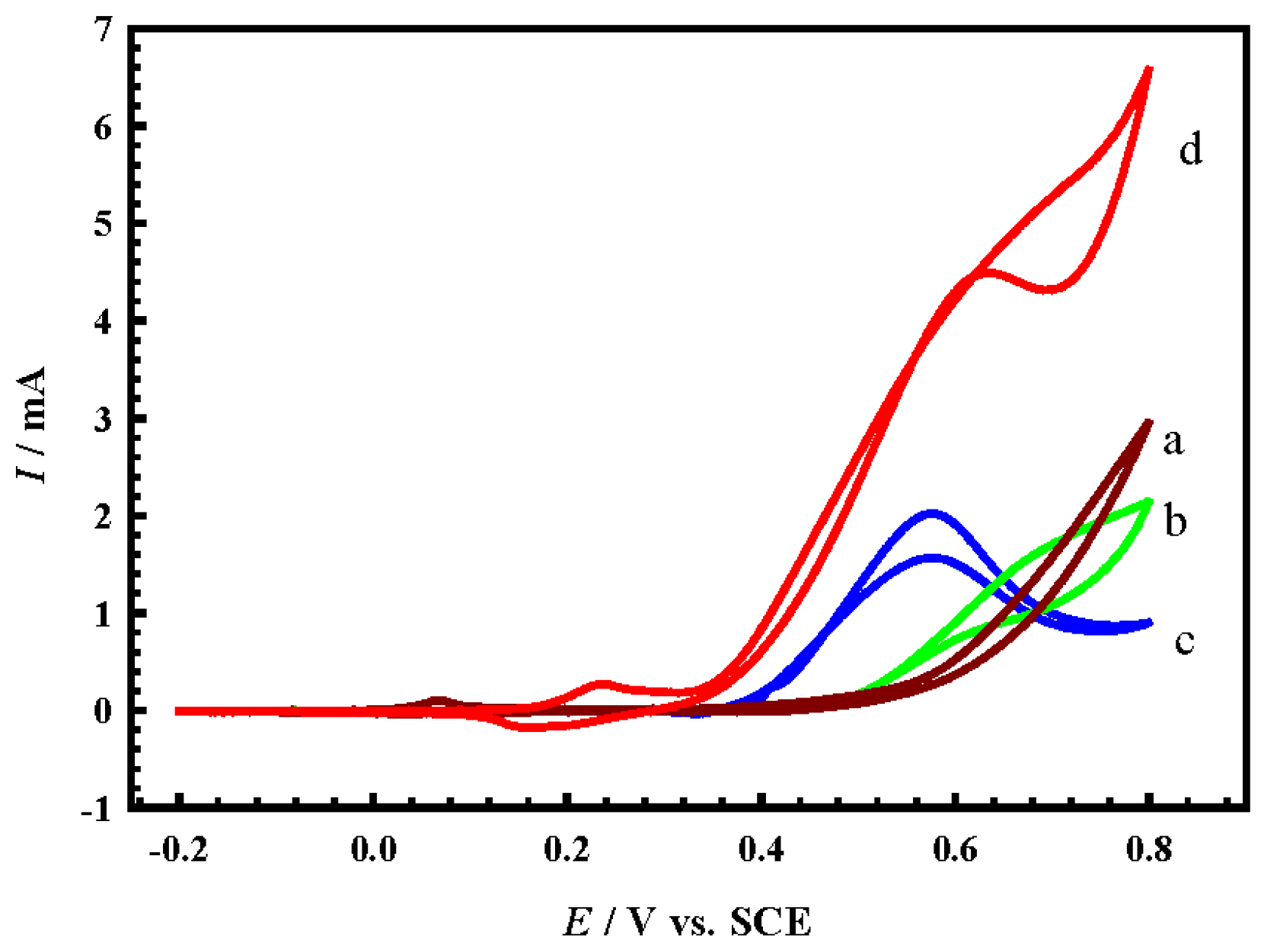
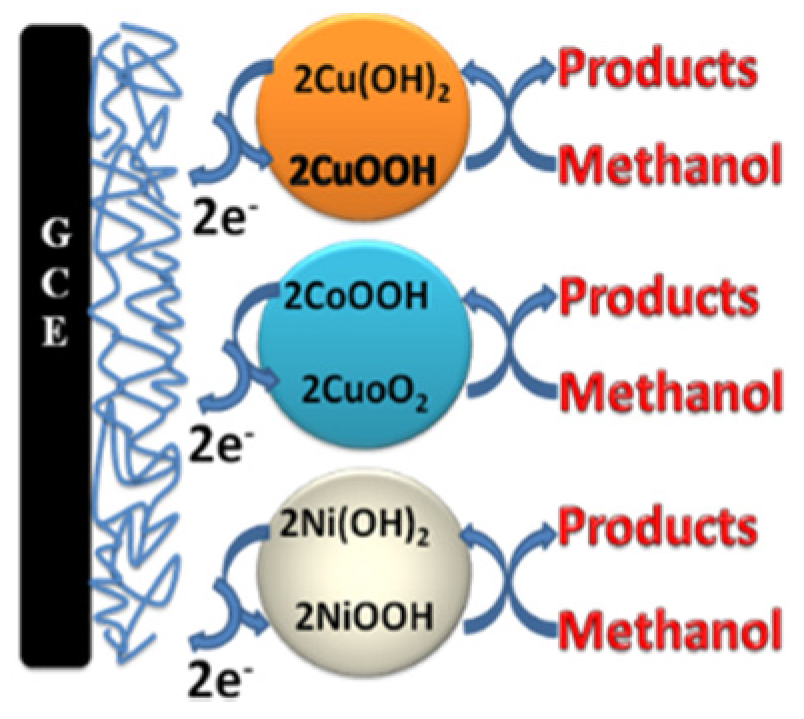
- Firstly, the Ni(II)/Ni(III) transformation potential is negatively shifted by about 0.2 V in comparison to NiOx@CNTs/GCE (d), and this is attributed to the influence of a partial alloy or alloy structure, where the peaks of Ni(II)/Ni(III) and Co(II)/Co(III) of the formed alloy shifted negatively and positively, respectively [19].
- Secondly, the charging current of the Ni(II)/Ni(III) transformation increased dramatically compared to the case of NiOx@CNTs/GCE, and this may be due to the synergism between NiOx and the other two oxides present at the same time that resulted in more transformation of Ni(II) to Ni(III). This enhanced transformation can be explained in the view of the results recorded in the literature previously, which assumed that mixing NiOx with other oxides to form binary alloys, for example MnOx, CoOx, or CuOx, causes an noticeable increase in the Ni(II)/Ni(III) transformation reaction [24,28,30]. This can be represented by Equation (6).
- Thirdly, the charging current for both CuOx and CoOx, observed in the potential range 0.3 V to 0.55 V in curve b and c, decreased slightly in the same potential range in the case of CuCoNiOx@CNTs/GCE; this is certainly due to the last-mentioned feature in point II. Where, the high charging current in the mentioned potential range for both CuOx and CoOx is attributed to the conversion of Cu(II) and Co(III) to the higher oxidation states mentioned in Equations (3) and (4), and immediately the produced higher oxidation states are consumed in the Ni(II)/Ni(III) transformation according to Equation (6), so that no high charging current is observed.
4.2. Methanol Electrocatalytic Oxidation at the Modified Electrodes
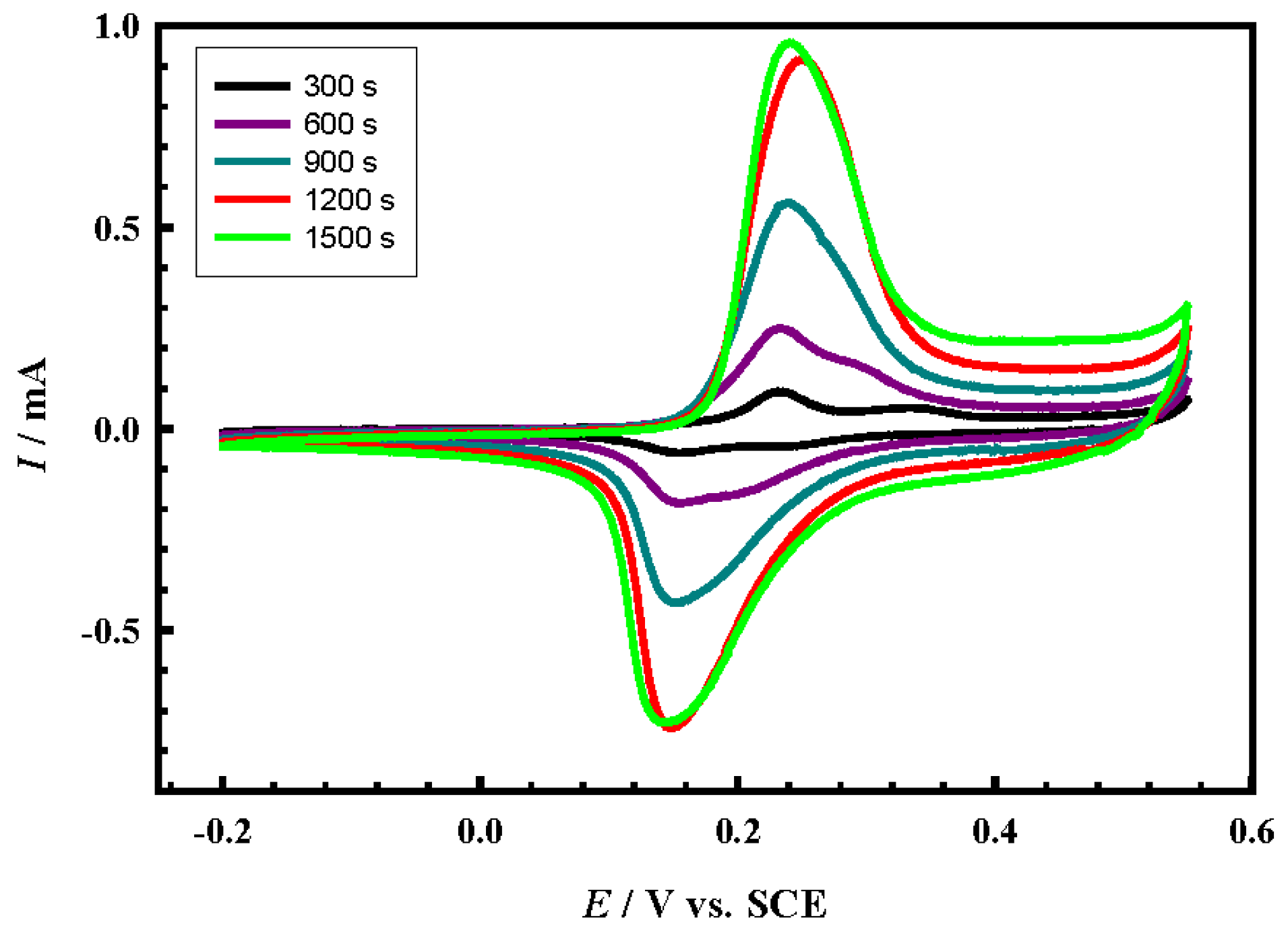
4.3. Effect of Loading Level
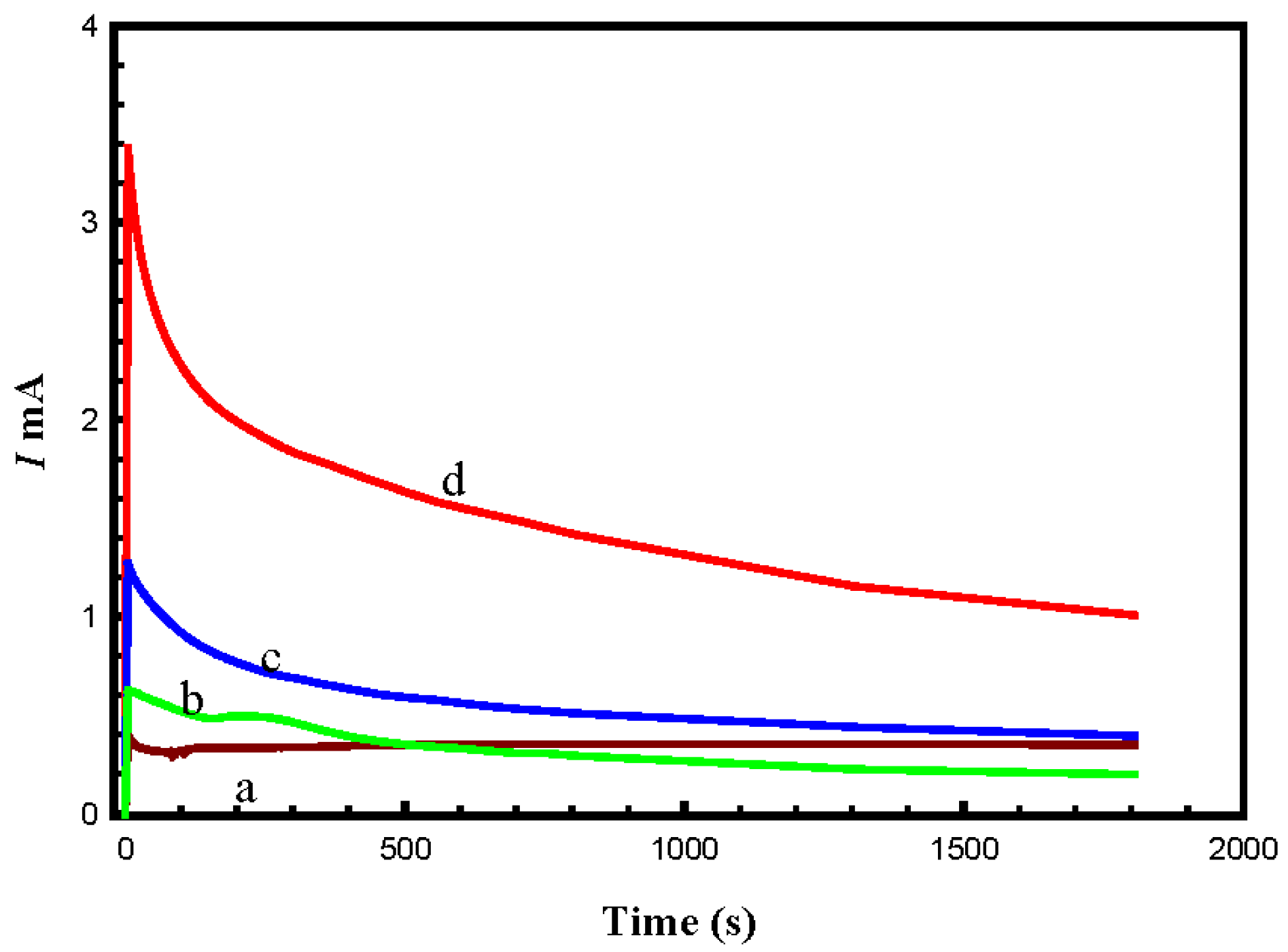
4.4. Long-Term Stability of the Prepared Electrocatalysts
5. Conclusions
- (I)
- CuCoNiOx@CNT/GCE has shown the highest electrocatalytic activity and stability towards methanol electrooxidation in comparison to the mono catalysts. For example, a minimum 2.5 times enhancement in both stability and activity were found.
- (II)
- The electrocatalytic performance of CuCoNiOx@CNT/GCE depends greatly on the loading level (deposition time).
- (III)
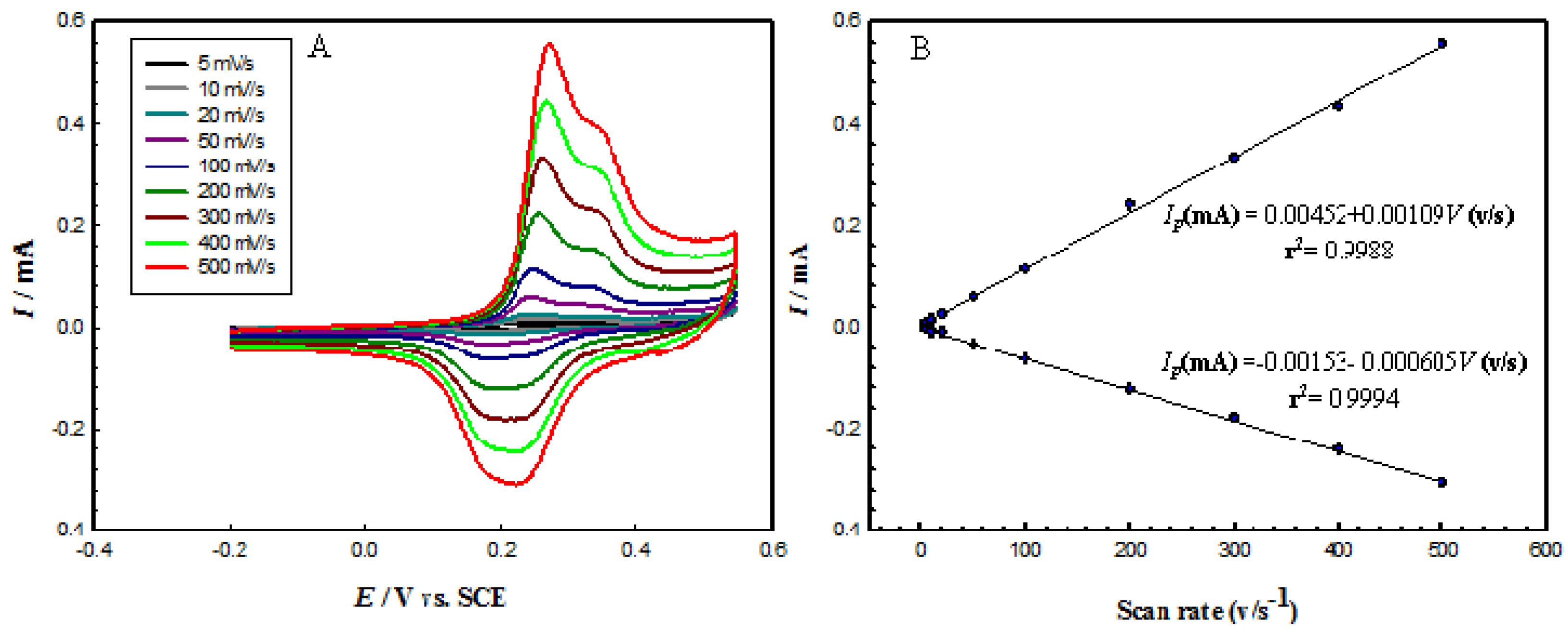
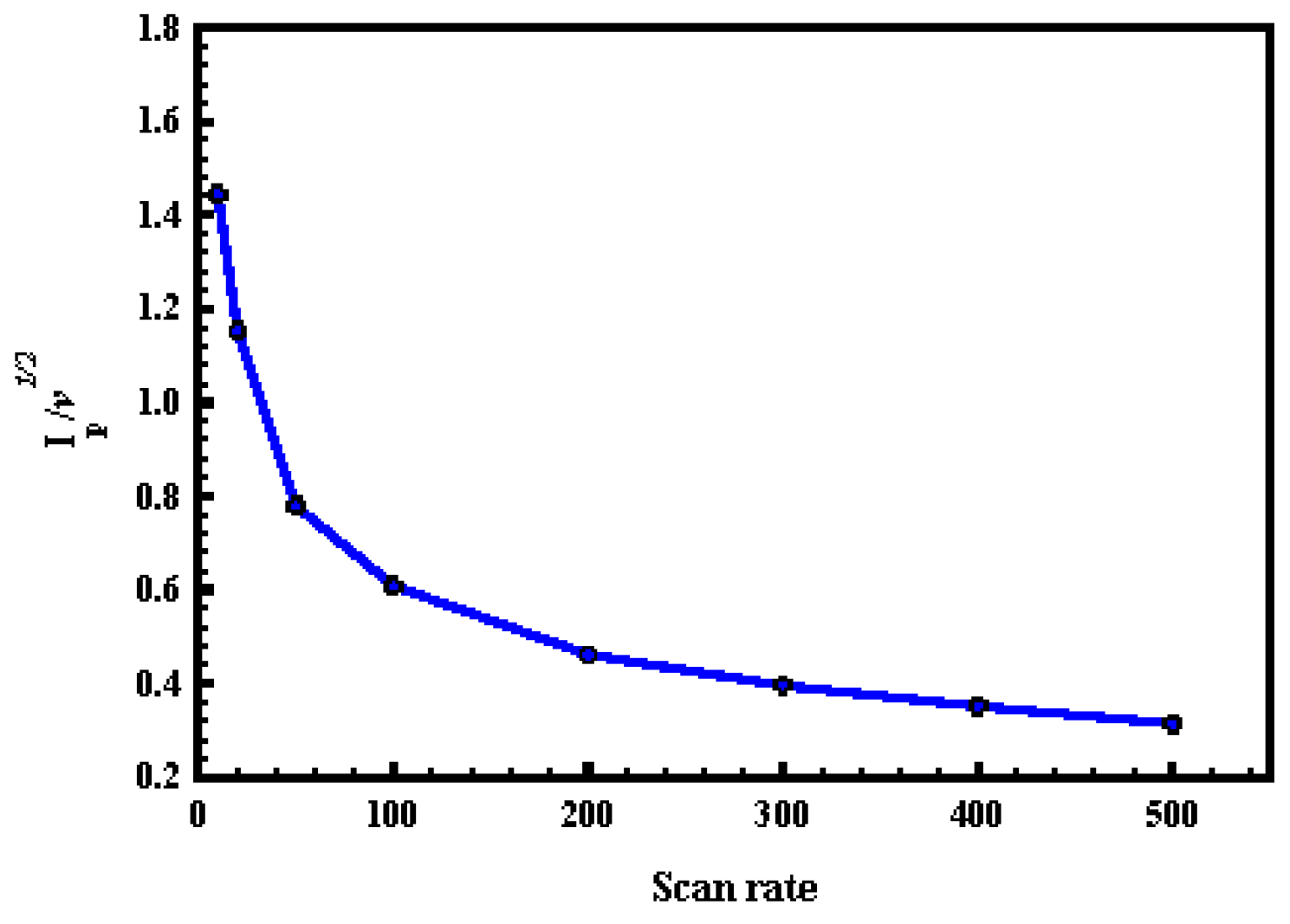
- At a scan rate of more than 50 mV·s−1, the (Ip/ν1/2) did not change significantly with scan rate, which is a characteristic feature of catalytic reactions.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamat, P.V. Energy Outlook for Planet Earth. J. Phys. Chem. Lett. 2013, 4, 1727–1729. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Faghri, A. Review and advances of direct methanol fuel cells (DMFCs) Part I: Design, fabrication, and testing with high concentration methanol solutions. J. Power Sources 2013, 230, 223–240. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Zhao, H.; Law, K.-Y.; Sambhy, V. Fabrication, Surface Properties, and Origin of Superoleophobicity for a Model Textured Surface. Langmuir 2011, 27, 5927–5935. [Google Scholar] [CrossRef] [PubMed]
- Danaee, I.; Jafarian, M.; Forouzandeh, F.; Gobal, F.; Mahjani, M.G. Electrocatalytic oxidation of methanol on Ni and NiCu alloy modified glassy carbon electrode. Int. J. Hydrog. Energy 2008, 33, 4367–4376. [Google Scholar] [CrossRef]
- Tong, X.; Qin, Y.; Guo, X.; Moutanabbir, O.; Ao, X.; Pippel, E.; Zhang, L.; Knez, M. Enhanced Catalytic Activity for Methanol Electro-oxidation of Uniformly Dispersed Nickel Oxide Nanoparticles—Carbon Nanotube Hybrid Materials. Small 2012, 8, 3390–3395. [Google Scholar] [CrossRef] [PubMed]
- Heli, H.; Jafarian, M.; Mahjani, M.G.; Gobal, F. Electro-oxidation of methanol on copper in alkaline solution. Electrochim. Acta 2004, 49, 4999–5006. [Google Scholar] [CrossRef]
- Jafarian, M.; Mahjani, M.G.; Heli, H.; Gobal, F.; Khajehsharifi, H.; Hamedi, M.H. A study of the electro-catalytic oxidation of methanol on a cobalt hydroxide modified glassy carbon electrode. Electrochim. Acta 2003, 48, 3423–3429. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Yuan, Q.-B.; Duan, D.-H.; Zhang, Z.-L.; Hao, X.-G.; Wei, G.-Q.; Liu, S.-B. Electrochemical activity and stability of core–shell Fe2O3/Pt nanoparticles for methanol oxidation. J. Power Sources 2013, 243, 622–629. [Google Scholar] [CrossRef]
- Orozco, G.; Pérez, M.C.; Rincón, A.; Gutiérrez, C. Electrooxidation of methanol on silver in alkaline medium. J. Electroanal. Chem. 2000, 495, 71–78. [Google Scholar] [CrossRef]
- Carugno, S.; Chassaing, E.; Rosso, M.; González, G.A. Enhanced electrochemical oxidation of methanol on copper electrodes modified by electrocorrosion and electrodeposition. Mater. Chem. Phys. 2014, 143, 1012–1017. [Google Scholar] [CrossRef]
- Hutton, L.A.; Vidotti, M.; Patel, A.N.; Newton, M.E.; Unwin, P.R.; Macpherson, J.V. Electrodeposition of Nickel Hydroxide Nanoparticles on Boron-Doped Diamond Electrodes for Oxidative Electrocatalysis. J. Phys. Chem. C 2011, 115, 1649–1658. [Google Scholar] [CrossRef]
- Xia, L.-P.; Guo, P.; Wang, Y.; Ding, S.-Q.; He, J.-B. Multi-laminated copper nanoparticles deposited on conductive substrates for electrocatalytic oxidation of methanol in alkaline electrolytes. J. Power Sources 2014, 262, 232–238. [Google Scholar] [CrossRef]
- Yang, M.; Jin, X.; Huang, Q. Electrochemical glucose oxidation on dendritic cuprous oxide film fabricated by PSS-assisted electrochemical deposition. Solid State Sci. 2011, 13, 427–433. [Google Scholar] [CrossRef]
- Jafarian, M.; Forouzandeh, F.; Danaee, I.; Gobal, F.; Mahjani, M.G. Electrocatalytic oxidation of glucose on Ni and NiCu alloy modified glassy carbon electrode. J. Solid State Electrochem. 2008, 13, 1171–1179. [Google Scholar] [CrossRef]
- Cui, X.; Guo, G.; Zhou, M.; Yang, Y.; Li, Y.; Xiao, P.; Zhang, Y.; Zhang, X. Promoting Effect of Co inNimCon (m + n = 4) Bimetallic Electrocatalysts for Methanol Oxidation Reaction. ACS Appl. Mater. Interfaces 2015, 7, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Rostami, T.; Jafarian, M.; Miandari, S.; Mahjani, M.G.; Gobal, F. Synergistic effect of cobalt and copper on a nickel-based modified graphite electrode during methanol electro-oxidation in NaOH solution. Chin. J. Catal. 2015, 36, 1867–1874. [Google Scholar] [CrossRef]
- Linares, N.; Silvestre-Albero, A.M.; Serrano, E.; Silvestre-Albero, J.; Garcia-Martinez, J. Mesoporous materials for clean energy technologies. Chem. Soc. Rev. 2014, 43, 7681–7717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.W.; Tan, K.H.; Ong, Y.T.; Mohamed, A.R.; Zein, S.H.S.; Tan, S.H. Carbon Nanotubes Applications: Solar and Fuel Cells, Hydrogen Storage, Lithium Batteries, Supercapacitors, Nanocomposites, Gas, Pathogens, Dyes, Heavy Metals and Pesticides. In Environmental Chemistry for a Sustainable World: Volume 1: Nanotechnology and Health Risk; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 3–46. [Google Scholar]
- Al-Enizi, A.M.; Elzatahry, A.A.; Abdullah, A.M.; AlMaadeed, M.A.; Wang, J.; Zhao, D.; Al-Deyab, S. Synthesis and electrochemical properties of nickel oxide/carbon nanofiber composites. Carbon 2014, 71, 276–283. [Google Scholar] [CrossRef]
- El-Refaei, S.M.; Awad, M.I.; El-Anadouli, B.E.; Saleh, M. Electrocatalytic glucose oxidation at binary catalyst of nickel and manganese oxides nanoparticles modified glassy carbon electrode: Optimization of the loading level and order of deposition. Electrochim. Acta 2013, 92, 460–467. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Y.; Lu, X.; Li, Z.; Sun, L.; Song, Y. Dendritic copper-cobalt nanostructures/reduced graphene oxide-chitosan modified glassy carbon electrode for glucose sensing. Sens. Actuator B 2014, 195, 1–7. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Lu, X.; Xie, S.; Xiao, F.; Liu, P.; Tong, Y. Facile synthesis of porous 3D CoNiCunano-network structure and their activity towards hydrogen evolution reaction. Int. J. Hydrog. Energy 2012, 37, 18688–18693. [Google Scholar] [CrossRef]
- Garcia-Cerada, L.A.; Bernal-Ramos, K.M.; Montemayor, S.M.; Quevedo-Lopez, M.A.; Betancourt-Galindo, R.; Bueno-Baques, D. Preparation of hcp and fcc Ni and Ni/NiO Nanoparticles Using a Citric Acid Assisted Pechini-Type Method. J. Nanomater. 2011, 2011. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, H.; Li, G.; Wu, Z. Nickel-cobalt bimetallic anode catalysts for direct urea fuel cell. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Berchmans, S. CuO microspheres modified glassy carbon electrode as sensors materials and fuel cell catalysts. J. Electrochem. Soc. 2012, 159, F73–F80. [Google Scholar] [CrossRef]
- Ahn, S.H.; Park, H.-Y.; Choi, I.; Yoo, S.J.; Hwang, S.J.; Kim, H.-J.; Cho, E.; Yoon, C.W.; Park, H.; Son, H.; et al. Electrochemically fabricated NiCu alloy catalysts for hydrogen production in alkaline water electrolysis. Int. J. Hydrog. Energy 2013, 38, 13493–13501. [Google Scholar] [CrossRef]
- Fleischmann, M.; Korinek, K.; Pletcher, D. The oxidation of organic compounds at a nickel anode in alkaline solution. J. Electroanal. Chem. Interface Electrochem. 1971, 31, 39–49. [Google Scholar] [CrossRef]
- Fleischmann, M.; Korinek, K.; Pletcher, D. The kinetics and mechanism of the oxidation of amines and alcohols at oxide-covered nickel, silver, copper, and cobalt electrodes. J. Chem. Soc. Perkin Trans. 1972, 2, 1396–1403. [Google Scholar] [CrossRef]
- Taraszewska, J.; Rosłonek, G. Electrocatalytic oxidation of methanol on a glassy carbon electrode modified by nickel hydroxide formed by ex situ chemical precipitation. J. Electroanal. Chem. 1994, 364, 209–213. [Google Scholar] [CrossRef]
- Allen, J.R.; Florido, A.; Young, S.D.; Daunert, S.; Bachas, L.G. Nitrite-selective electrode based on an electropolymerized cobalt phthalocyanine. Electroanalysis 1995, 7, 710–713. [Google Scholar] [CrossRef]
- El-Refaei, S.M.; Saleh, M.M.; Awad, M.I. Enhanced glucose electrooxidation at a binary catalyst of manganese and nickel oxides modified glassy carbon electrode. J. Power Sources 2013, 223, 125–128. [Google Scholar] [CrossRef]
- Albo, J.; Sáez, A.; Solla-Gullón, J.; Montiel, V.; Irabien, A. Production of methanol from CO2 electroreduction at Cu2O and Cu2O/ZnO-based electrodes in aqueous solution. Appl. Catal. B Environ. 2015, 176–177, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Albo, J.; Irabien, A. Cu2O-loaded gas diffusion electrodes for the continuous electrochemical reduction of CO2 to methanol. J. Catal. 2016, 343, 232–239. [Google Scholar] [CrossRef]

© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, A.A.; El-Refaei, S.M.; Elzatahry, A.A.; Abdullah, A.M. High Electrocatalytic Performance of CuCoNi@CNTs Modified Glassy Carbon Electrode towards Methanol Oxidation in Alkaline Medium. Appl. Sci. 2017, 7, 64. https://doi.org/10.3390/app7010064
Hamza AA, El-Refaei SM, Elzatahry AA, Abdullah AM. High Electrocatalytic Performance of CuCoNi@CNTs Modified Glassy Carbon Electrode towards Methanol Oxidation in Alkaline Medium. Applied Sciences. 2017; 7(1):64. https://doi.org/10.3390/app7010064
Chicago/Turabian StyleHamza, Amina A., Sayed M. El-Refaei, Ahmed A. Elzatahry, and Aboubakr M. Abdullah. 2017. "High Electrocatalytic Performance of CuCoNi@CNTs Modified Glassy Carbon Electrode towards Methanol Oxidation in Alkaline Medium" Applied Sciences 7, no. 1: 64. https://doi.org/10.3390/app7010064
APA StyleHamza, A. A., El-Refaei, S. M., Elzatahry, A. A., & Abdullah, A. M. (2017). High Electrocatalytic Performance of CuCoNi@CNTs Modified Glassy Carbon Electrode towards Methanol Oxidation in Alkaline Medium. Applied Sciences, 7(1), 64. https://doi.org/10.3390/app7010064





