Comparative Research on RC Equivalent Circuit Models for Lithium-Ion Batteries of Electric Vehicles
Abstract
:1. Introduction
2. Experiments and Methods
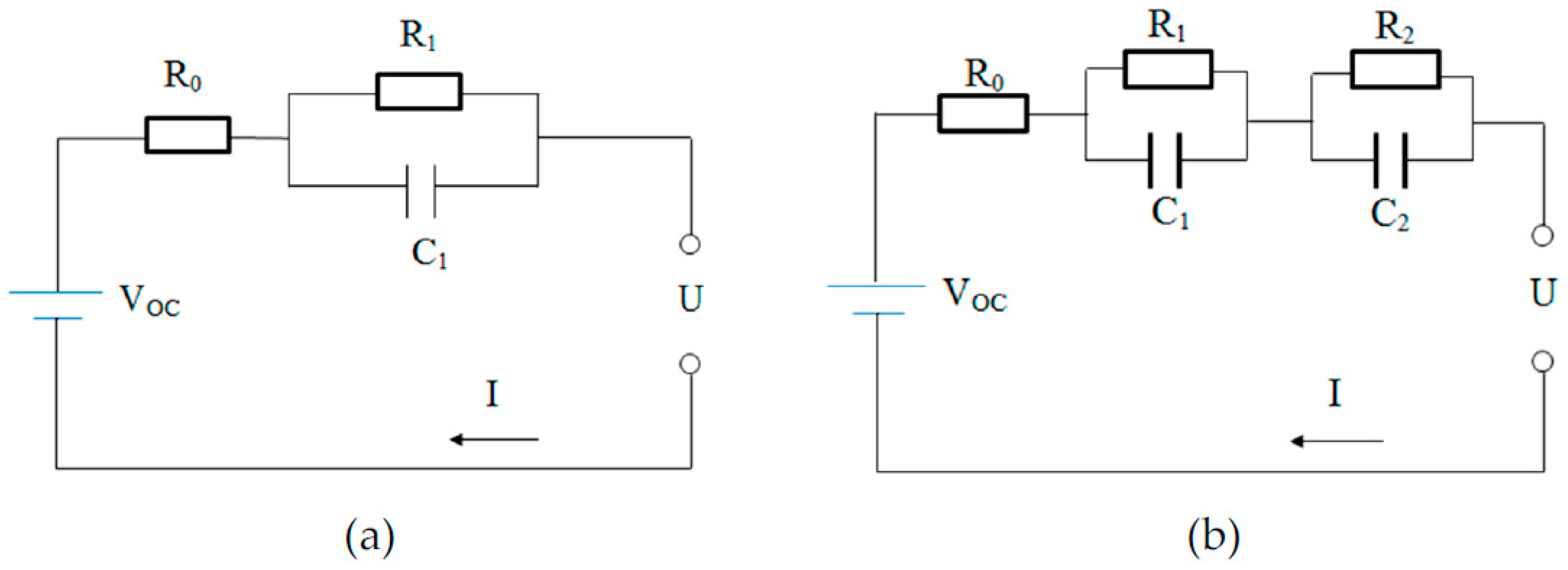
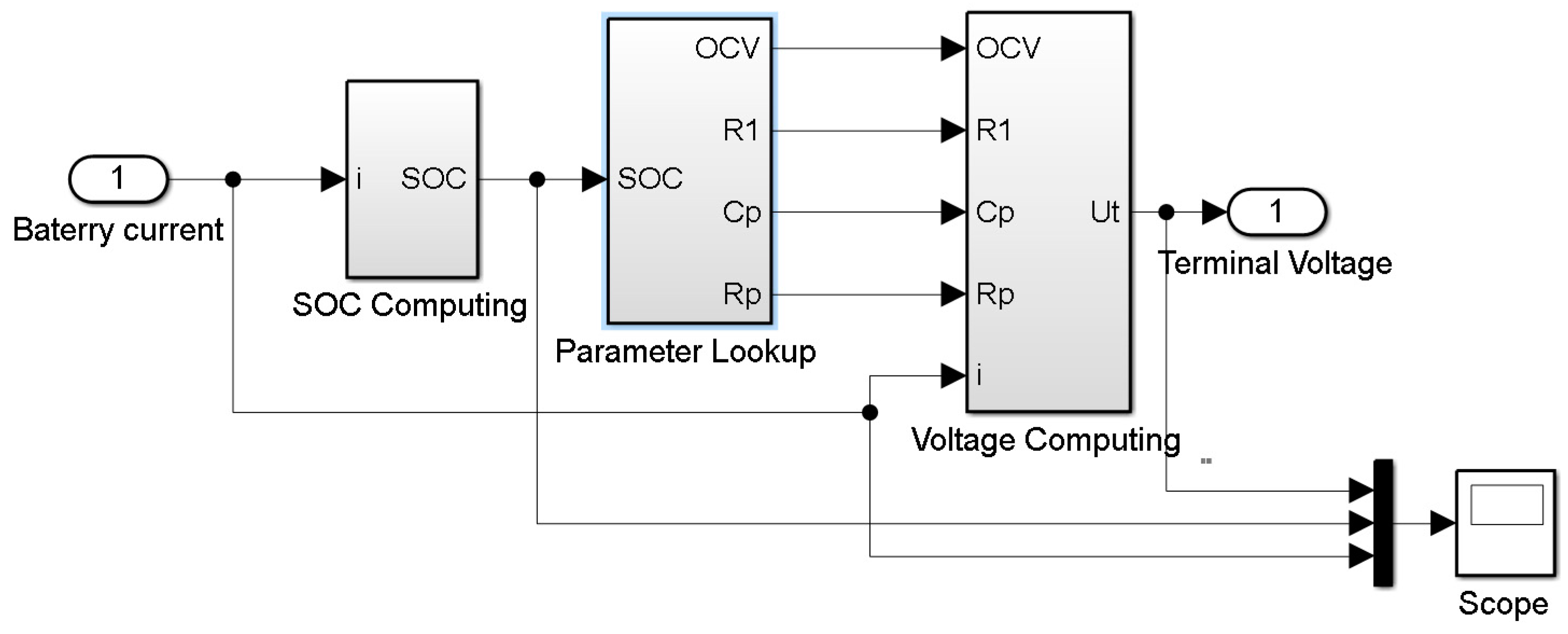
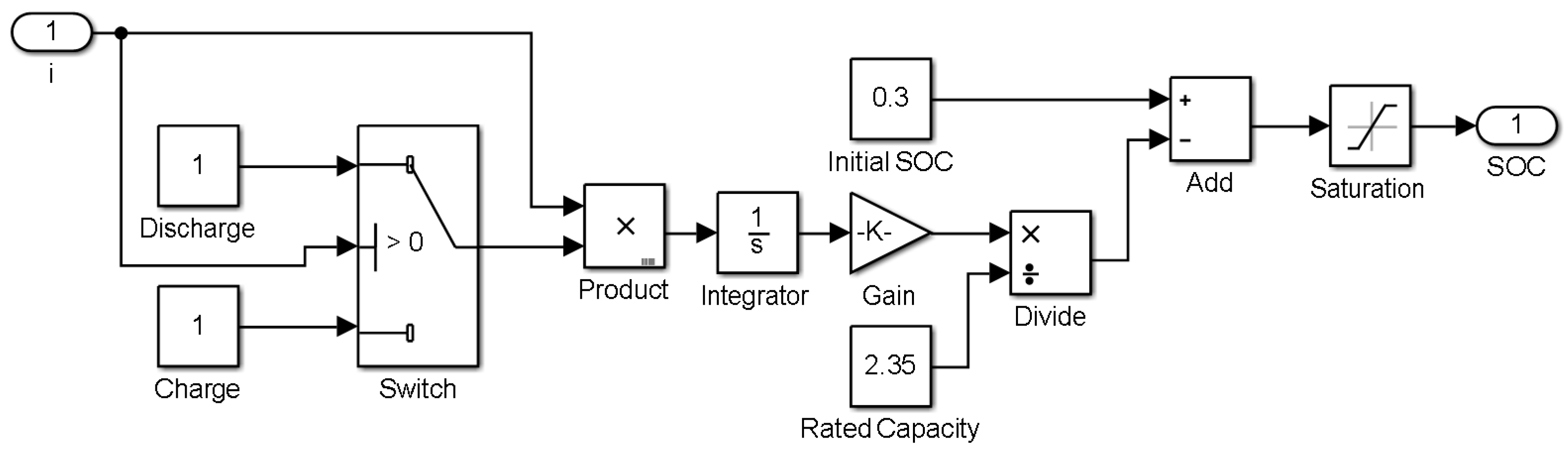
2.1. ECM for Lithium-Ion Batteries
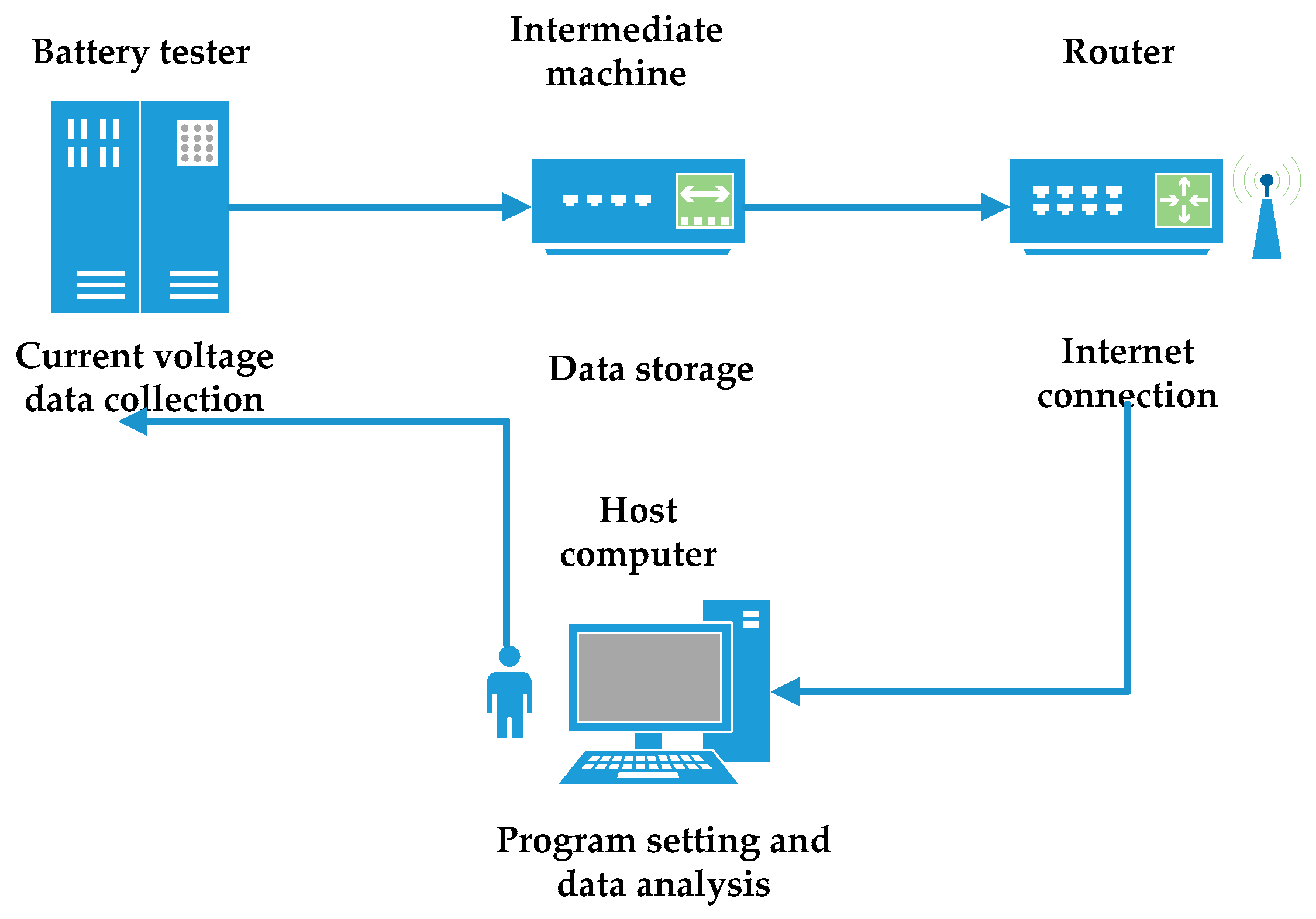
2.2. Battery Testing Bench
2.3. Model Parameter Identification
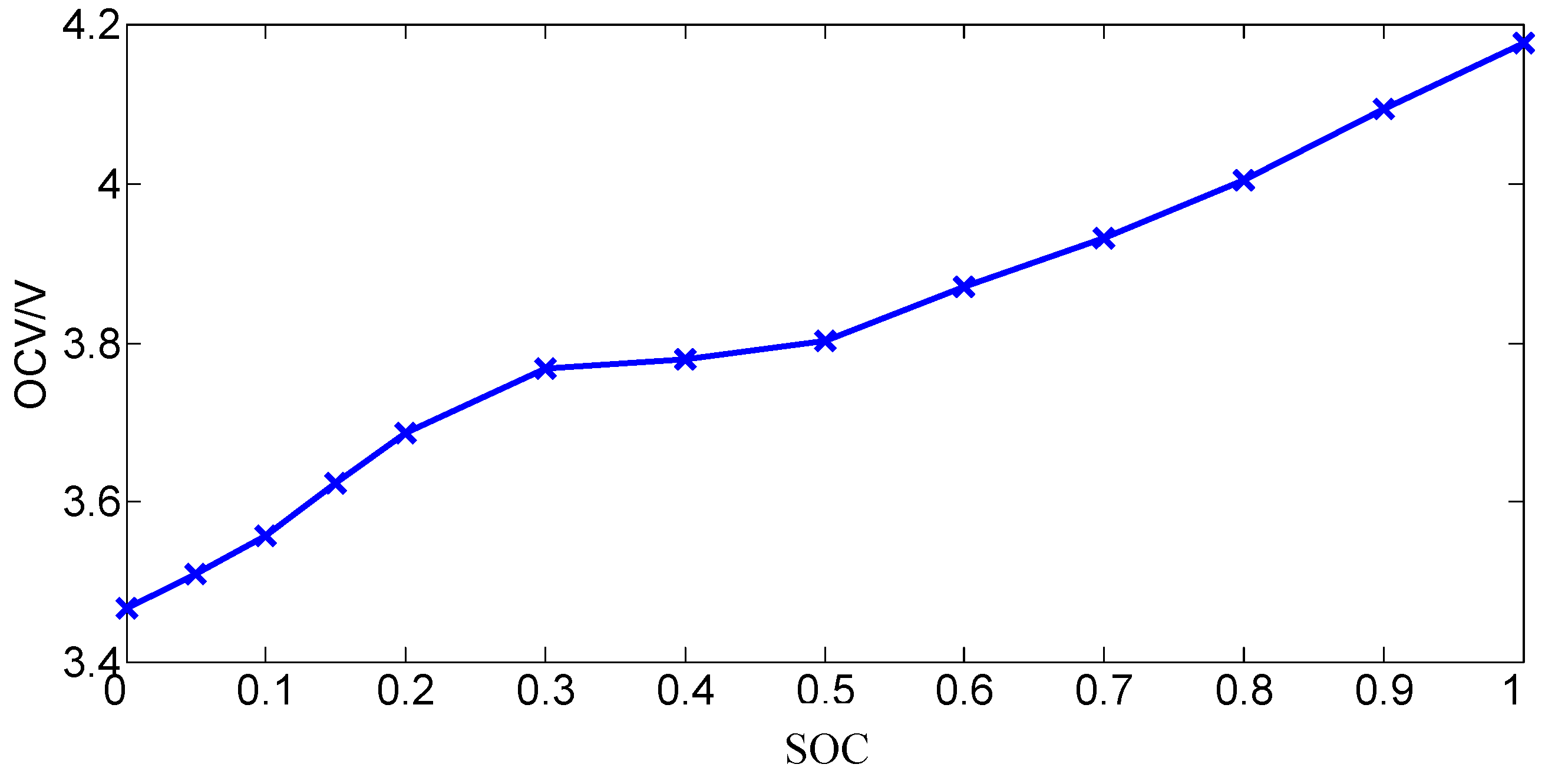
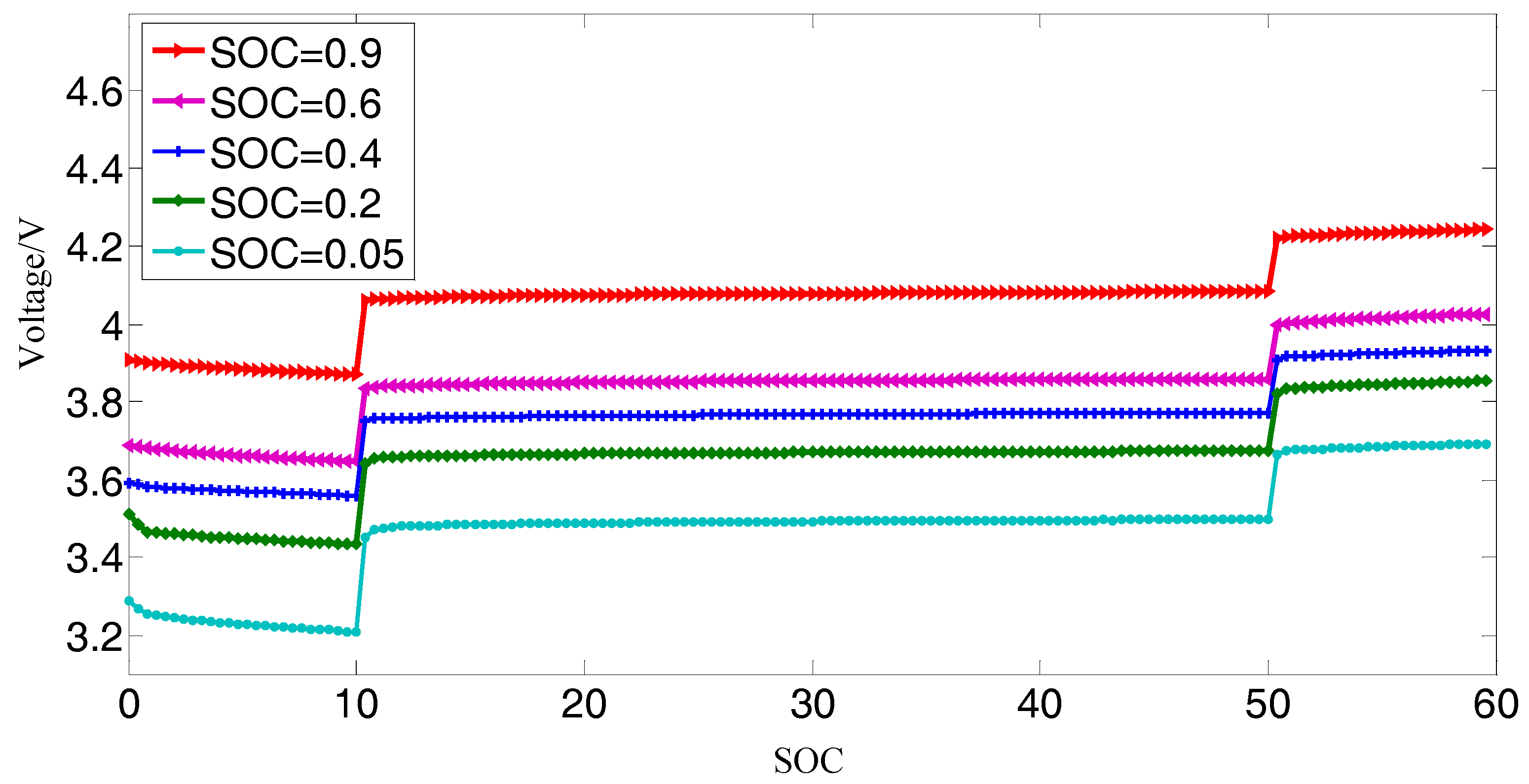
2.3.1. Open Circuit Voltage
- (1)
- Calibrate the battery capacity. The battery is fully charged with the standard charging method, in which the battery is charged using a constant current phase of 2.35 A (1C) to 4.2 V followed by a constant voltage phase of 4.2 V until the current is reduced to 0.02 A. Then, the battery is discharged with a constant current phase of 2.35 A to its discharge cut-off voltage 2.7 V. The experiment is repeated until the difference between the discharge capacity of each measurement does not exceed 2%, and then the measured capacity is deemed to be the actual capacity of the battery.
- (2)
- The battery is fully charged with the standard charging method described by Step (1) and then the battery is left in the open-circuit condition to rest for 4 h to achieve electrochemical and heat equilibrium [9].
- (3)
- The battery is discharged with a constant current 2.35 A for 6 minutes (i.e., discharging by 10% of the capacity) and then the battery is left in the open-circuit condition to rest for 4 h to achieve electrochemical and heat equilibrium.
- (4)
- Step (3) is repeated eight times.
- (5)
- The battery is discharged with a constant current 2.35 A by 3 min (i.e., discharging by 10% of the capacity) and then the battery is left in the open-circuit condition for 4 h to achieve electrochemical and heat equilibrium.
- (6)
- Step (5) is repeated four times.
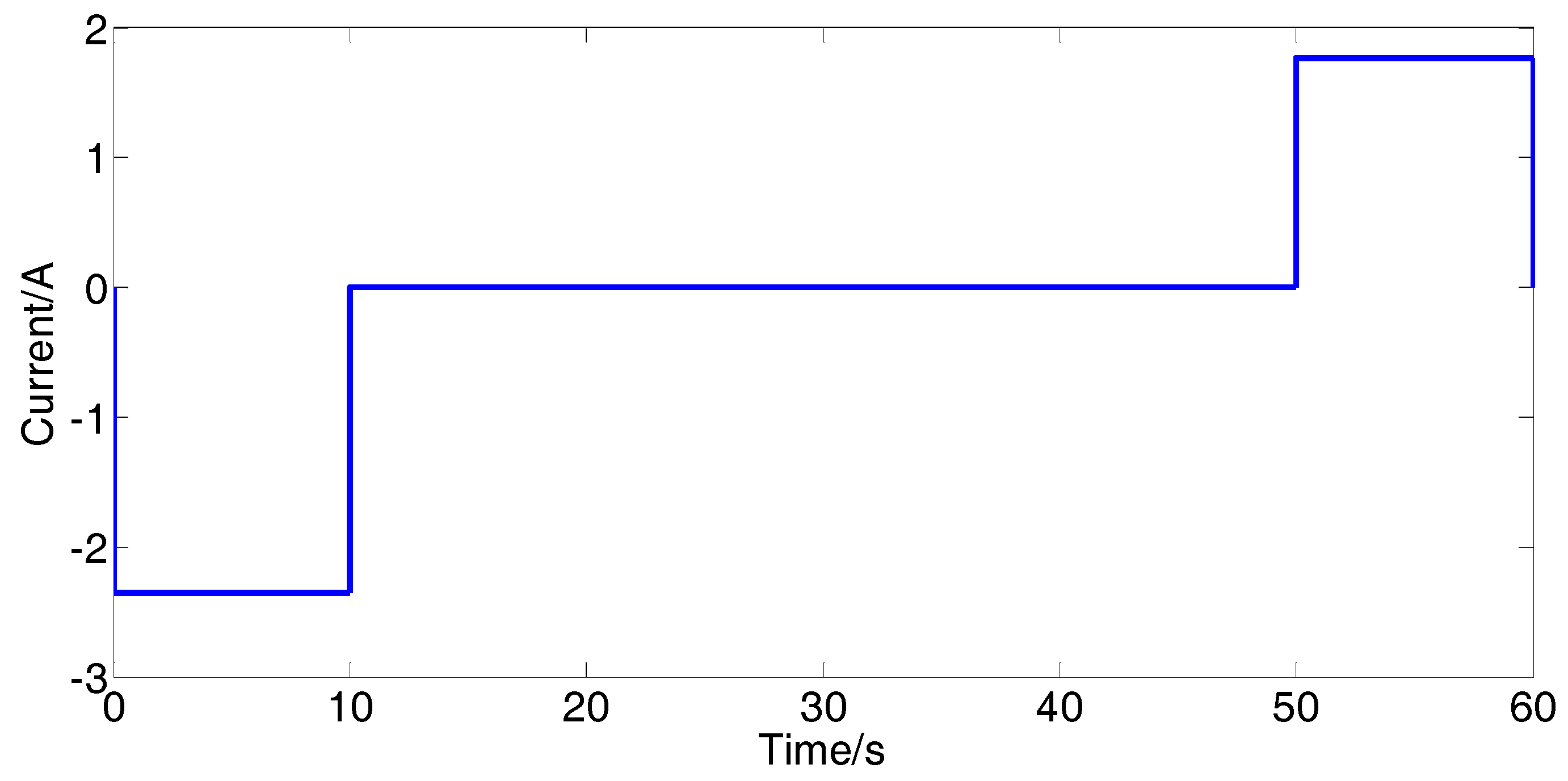
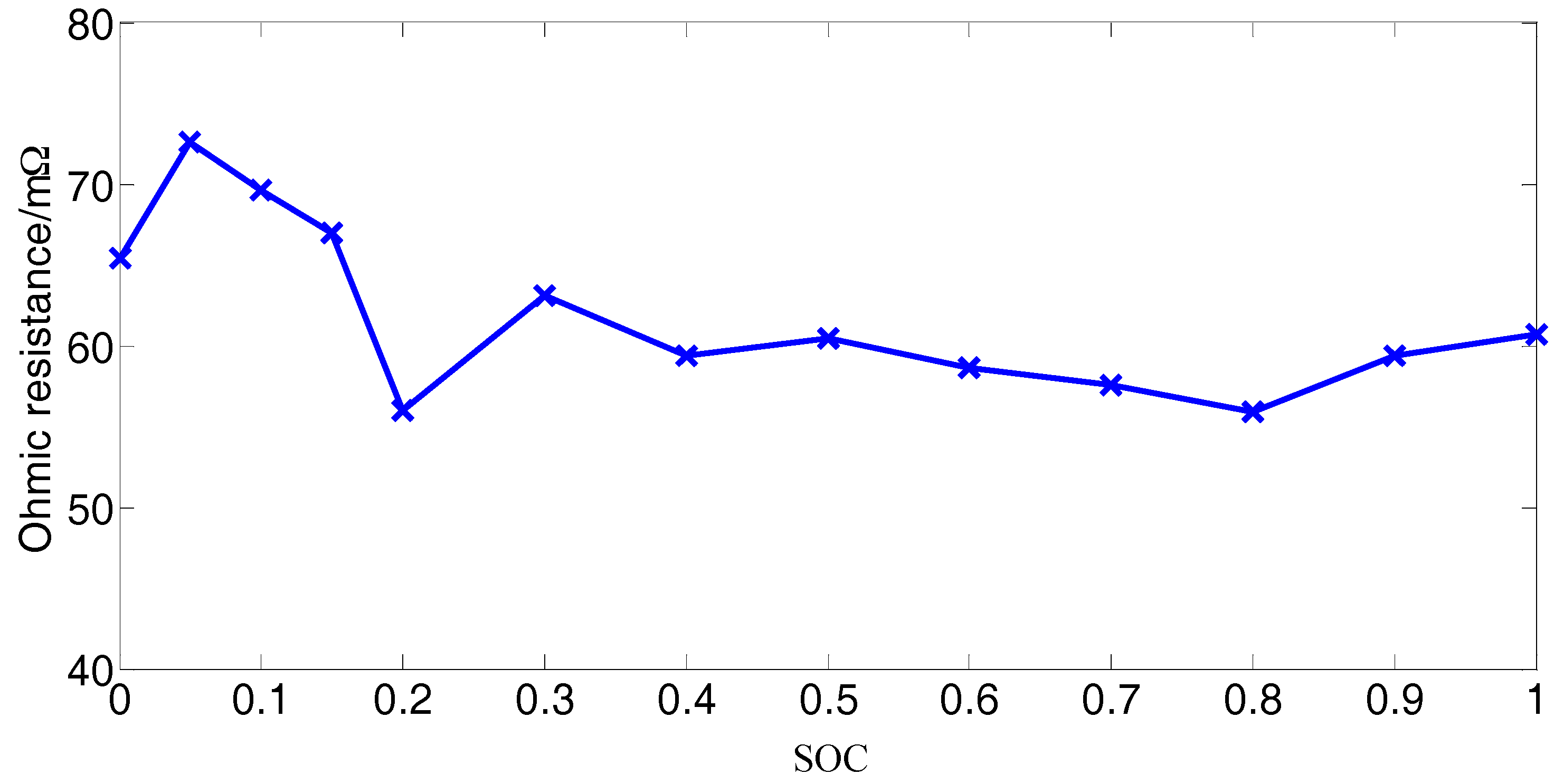
2.3.2. Ohmic Resistance
- (1)
- Calibrate the battery capacity as described in Section 2.3.1.
- (2)
- Charge the battery fully with the standard charging method the same as described in Section 2.2.
- (3)
- The HPPC test is performed and then the battery is left in the open-circuit condition to rest for 4 h to achieve electrochemical and heat equilibrium [9].
- (4)
- The battery is discharged with a constant current 2.35 A for 6 minutes and then the battery is left in the open-circuit condition to rest for 4 h to achieve electrochemical and heat equilibrium.
- (5)
- Steps (3) and (4) are respectively repeated eight times alternately.
- (6)
- The battery is discharged with a constant current 2.35 A for 3 min and then the battery is left in the open-circuit condition to rest for 4 h to achieve electrochemical and heat equilibrium.
- (7)
- The HPPC test is performed and then the battery is left in the open-circuit condition to rest for 4 h to achieve electrochemical and heat equilibrium.
- (8)
- Steps (6) and (7) are respectively repeated four times alternately.
2.3.3. Polarization Resistance and Polarization Capacitance
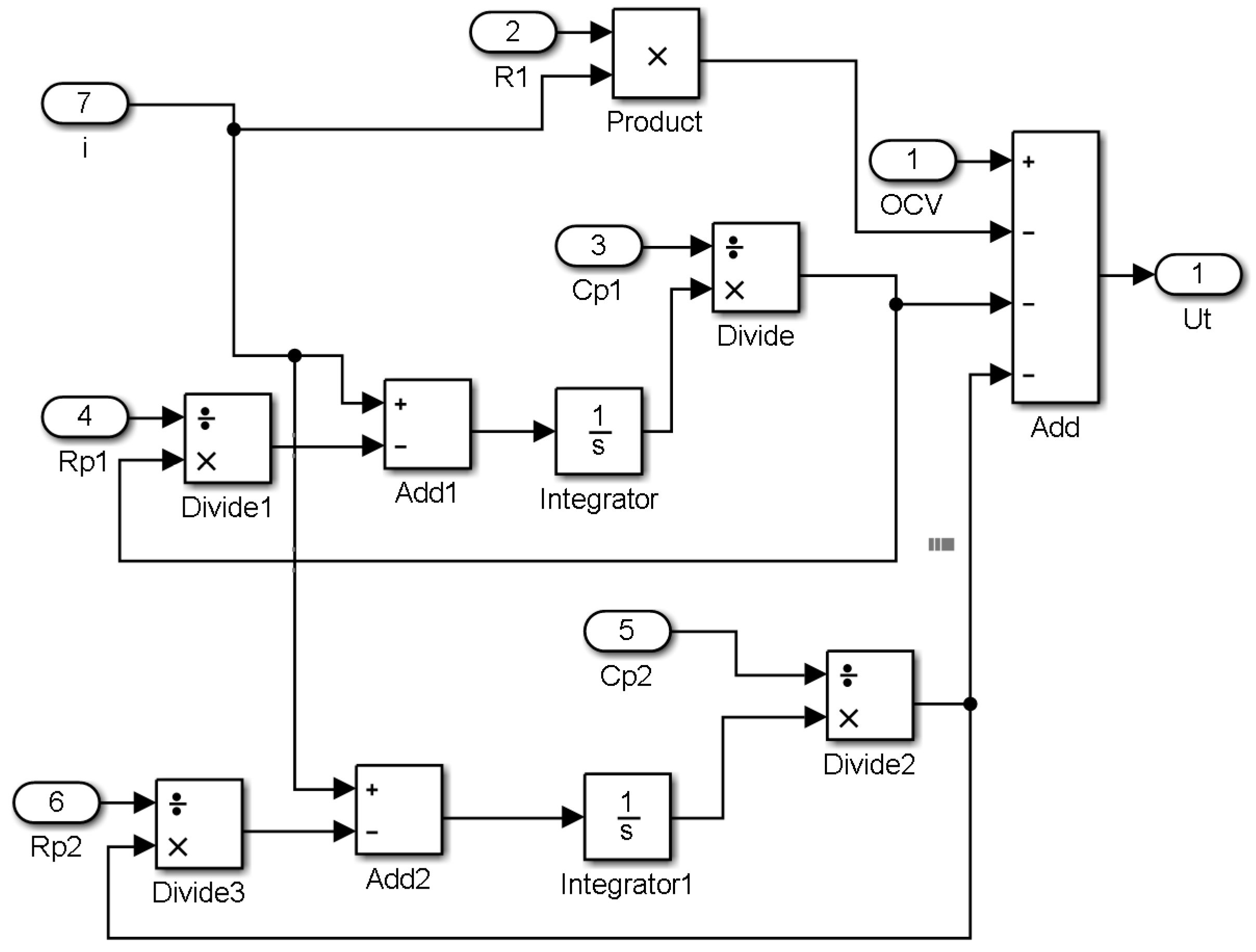
3. Model Simulation
4. Results and Discussion
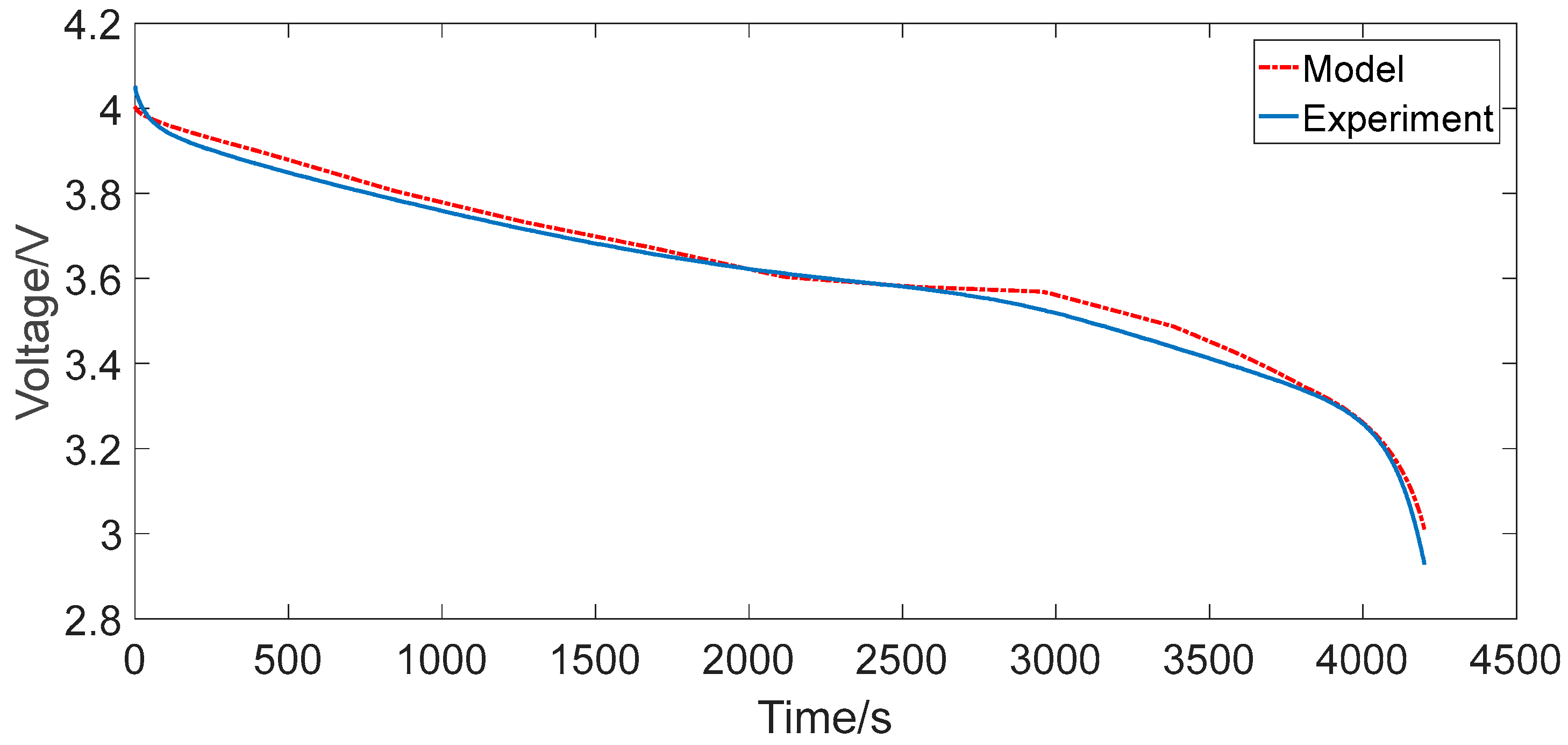
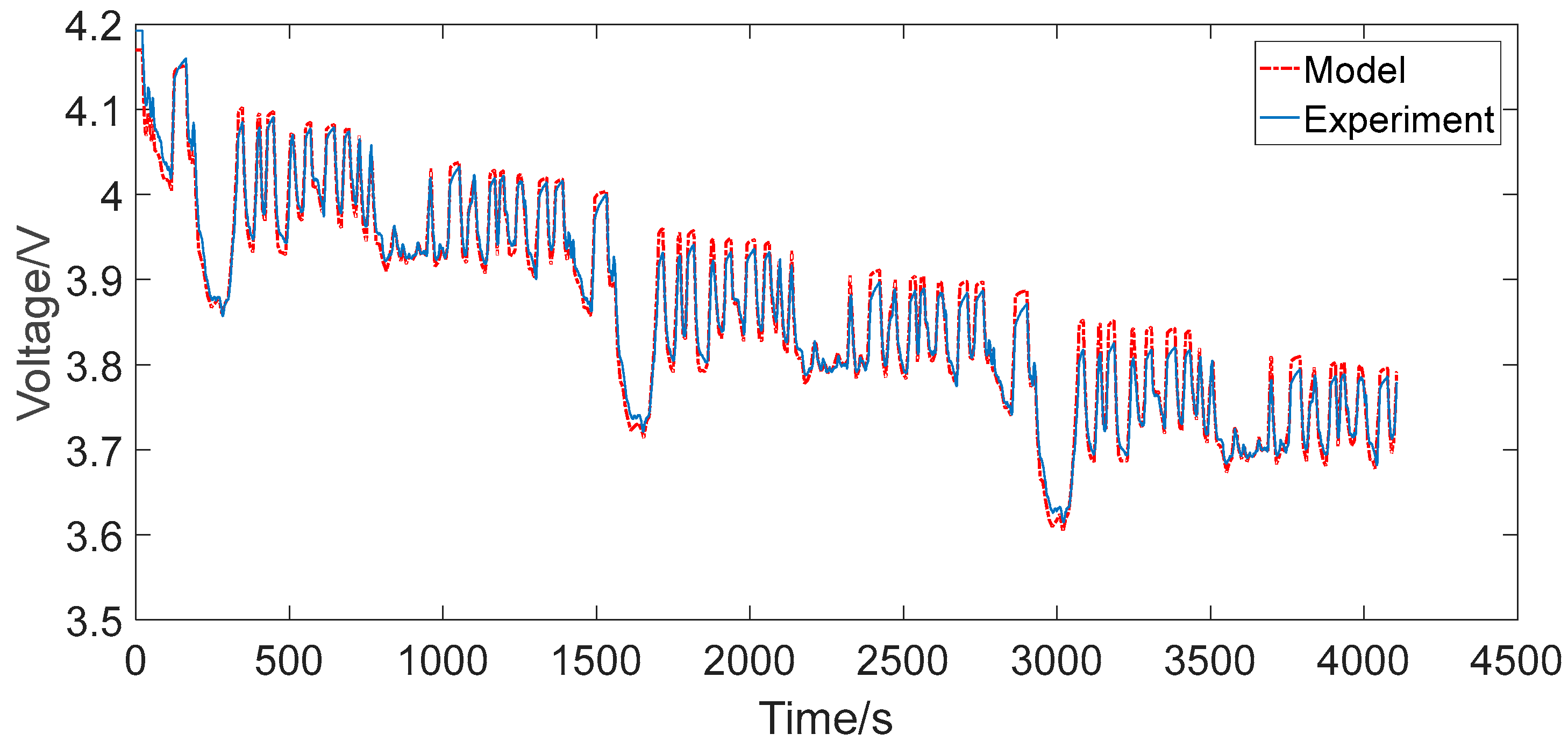
4.1. Constant Current Discharging
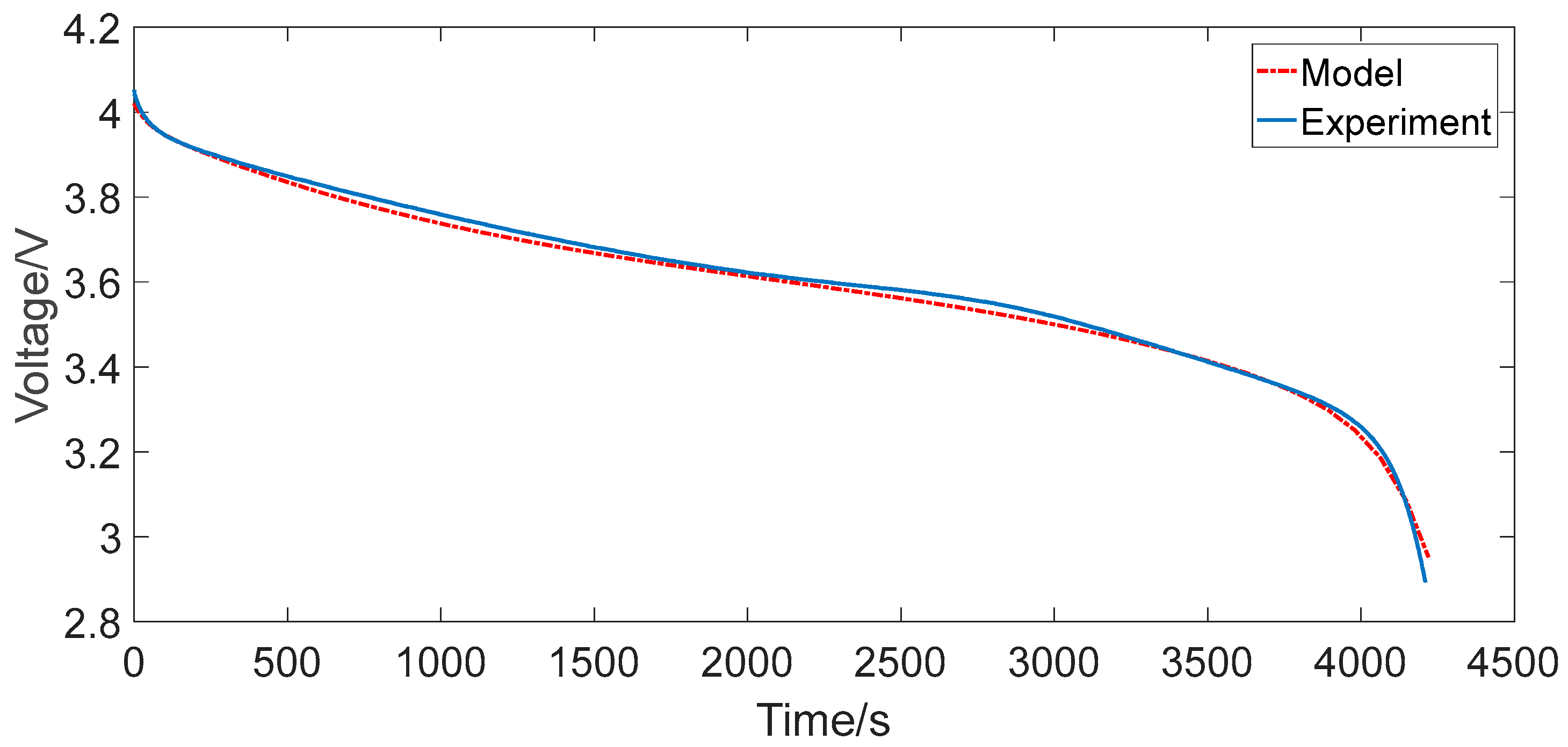
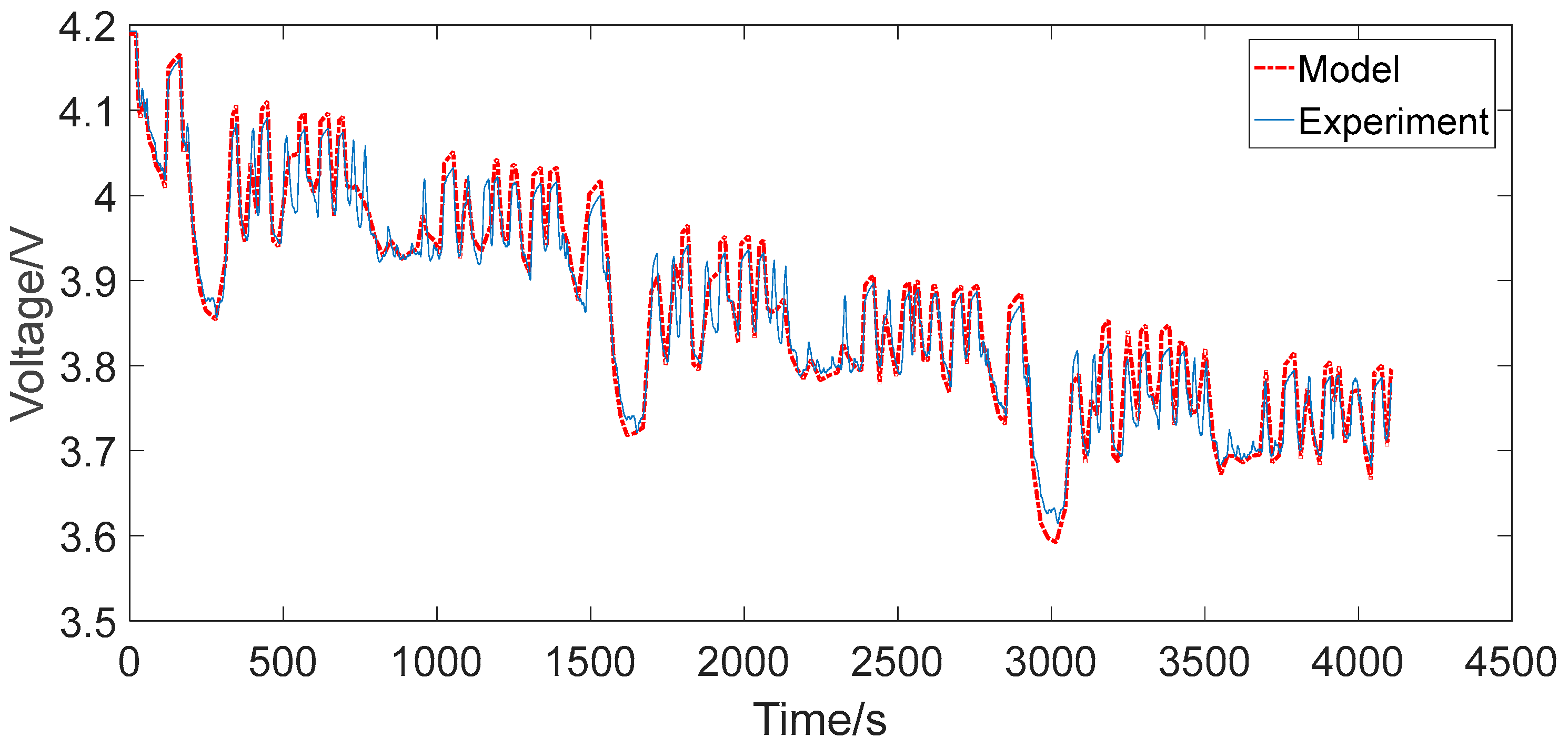
4.2. Urban Dynamometer Driving Schedule Cycle
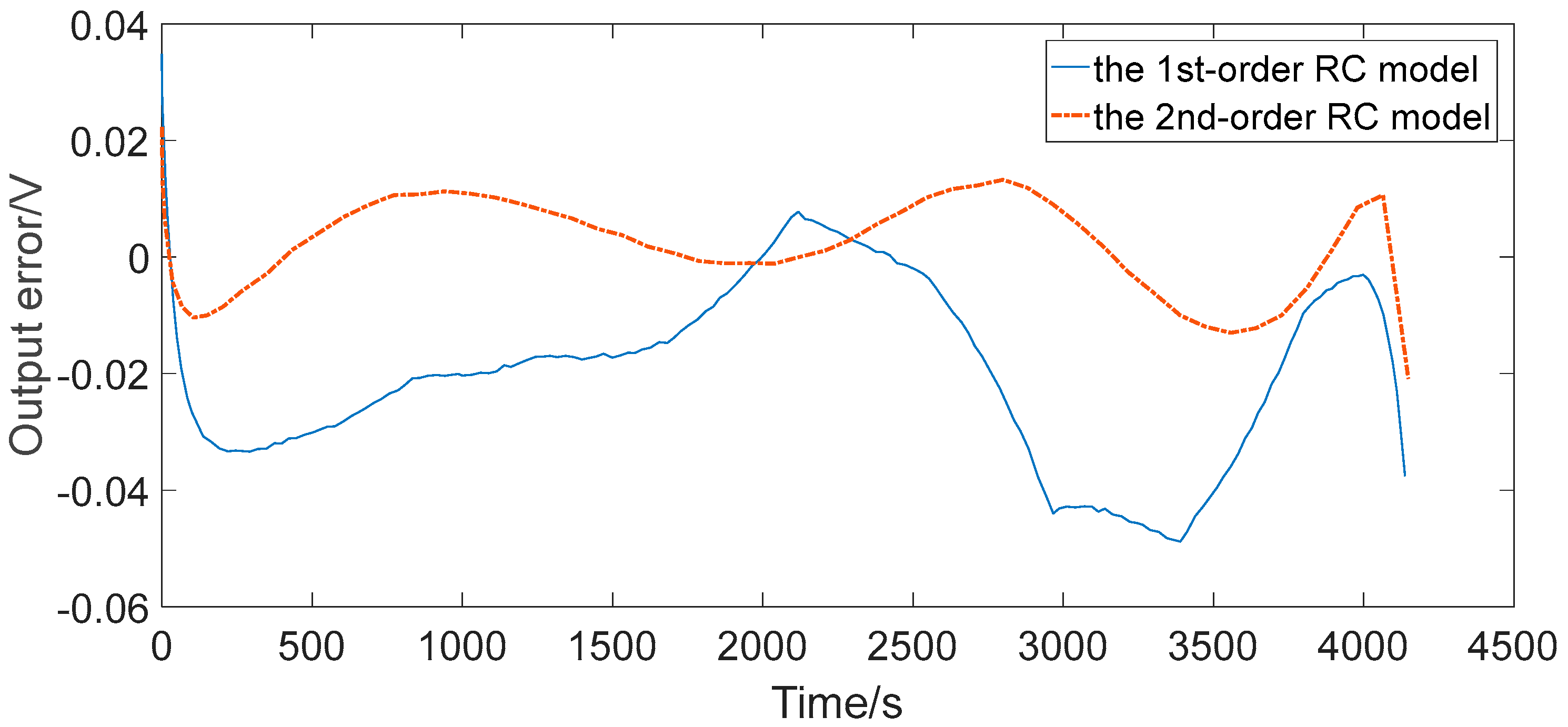
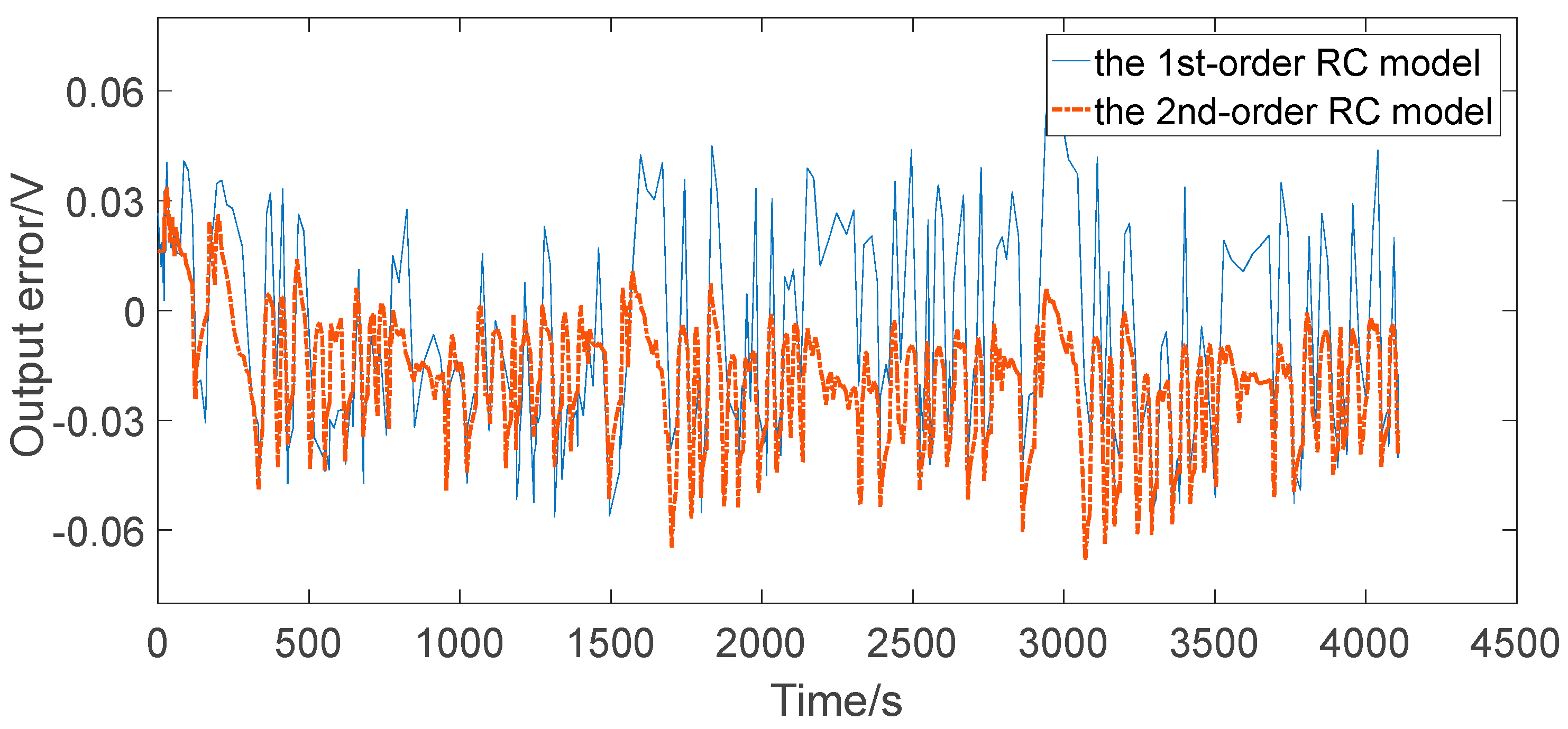
4.3. Comparative Analysis
5. Conclusions
- (1)
- The ECM has a very large advantage in computation time in contrast to electrochemical models; the simulation time based on the electrochemical model was as long as 9600 s [13], while the simulation time based on equivalent circuit model in this paper was negligible.
- (2)
- The maximum relative errors of the two RC models under the two representative operating conditions were all less than 2%, which can generally satisfy the precision requirements for the practical engineering calculation, such as algorithms based on ECM for advanced BMSs.
- (3)
- The second-order RC model improved the output error in contrast to the first-order RC model in both simple and complex discharging conditions, but did not improve much. Therefore, for ordinary applications such as portable consumer electronics, the first-order RC model could be the preferred choice. However, for stringent applications such as automotive and aerospace, the second-order RC model could be the preferred choice. The results are of great instructional significance to the application in practical control systems for the equivalent circuit modeling of lithium-ion batteries.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| VOC | open circuit voltage (V) |
| R0 | ohmic resistance (Ω) |
| R1 | polarization resistance (Ω) |
| C1 | polarization capacitance (F) |
| I | current (A) |
| U | the terminal voltage (V) |
| U0 | the voltage before the current changes (V) |
| U1 | the voltage after the moment the current changes (V) |
| the time constant | |
| Ω | ohmic |
| AC | alternating current |
| Ah | ampere-hour |
| BMS | battery management system |
| ECM | equivalent circuit model |
| EIS | electrochemical impedance spectroscopy |
| HEV | hybrid electric vehicle |
| HPPC | hybrid pulse power characterization |
| LiFePO4 | lithium iron phosphate |
| MATLAB | MATrix LABoratory |
| OCV | open circuit voltage |
| RC | resistance capacitance |
| RMS | root mean square |
| SOC | state-of-charge |
| SOH | state-of-health |
| SOF | state-of-function |
| UDDS | urban dynamometer driving schedule |
References
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Wu, L.; Fu, X.; Guan, Y. Review of the remaining useful life prognostics of vehicle lithium-ion batteries using data-driven methodologies. Appl. Sci. 2016, 6, 166. [Google Scholar] [CrossRef]
- Sbarufatti, C.; Corbetta, M.; Giglio, M.; Cadini, F. Adaptive prognosis of lithium-ion batteries based on the combination of particle filters and radial basis function neural networks. J. Power Sources 2017, 344, 128–140. [Google Scholar] [CrossRef]
- Chaturvedi, N.A.; Klein, R.; Christensen, J. Algorithms for advanced battery-management systems. IEEE Control Syst. 2010, 30, 49–68. [Google Scholar] [CrossRef]
- Worwood, D.; Kellner, Q.; Wojtala, M.; Widanage, W.D.; McGlen, R.; Greenwood, D.; Marco, J. A new approach to the internal thermal management of cylindrical battery cells for automotive applications. J. Power Sources 2017, 346, 151–166. [Google Scholar] [CrossRef]
- Brand, M.J.; Schuster, S.F.; Bach, T. Effects of vibrations and shocks on lithium-ion cells. J. Power Sources 2015, 288, 62–69. [Google Scholar] [CrossRef]
- Liu, X.; He, Y.; Zheng, X.; Zhang, J.; Zeng, G. A new state-of-charge estimation method for electric vehicle lithium-ion batteries based on multiple input parameter fitting model. Int. J. Energy Res. 2017, 41, 1265–1276. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.M.; Mohamed, A.; Ayob, A. Review of energy storage systems for electric vehicle applications: Issues and challenges. Renew. Sustain. Energy Rev. 2017, 69, 771–789. [Google Scholar] [CrossRef]
- Barai, A.; Chouchelamane, G.H.; Guo, Y.; McGordon, A.; Jennings, P. A study on the impact of lithium-ion cell relaxation on electrochemical impedance spectroscopy. J. Power Sources 2015, 280, 74–80. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Y.; Yang, F.; Tsui, K.-L. Nonlinear-drifted Brownian motion with multiple hidden states for remaining useful life prediction of rechargeable batteries. Mech. Syst. Signal Process. 2017, 93, 531–544. [Google Scholar] [CrossRef]
- Nikolian, A.; Firouz, Y.; Gopalakrishnan, R.; Timmermans, J.M.; Omar, N.; van den Bossche, P.; van Mierlo, J. Lithium ion batteries—Development of advanced electrical equivalent circuit models for nickel manganese cobalt lithium-ion. Energies 2016, 9, 360. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, X.; Lu, L.; Li, J.; Han, X.; Ouyang, M. Non-destructive fast charging algorithm of lithium-ion batteries based on the control-oriented electrochemical model. Appl. Energy 2017, in press. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, J.; Yuan, S.; Yang, J.; Zhou, X. A novel method for identification of lithium-ion battery equivalent circuit model parameters considering electrochemical properties. J. Power Sources 2017, 345, 21–29. [Google Scholar] [CrossRef]
- Wang, Q.K.; He, Y.J.; Shen, J.N.; Ma, Z.F.; Zhong, G.B. A unified modeling framework for lithium-ion batteries: An artificial neural network based thermal coupled equivalent circuit model approach. Energy 2017, 138, 118–132. [Google Scholar] [CrossRef]
- Feng, X.; Weng, C.; Ouyang, M.; Sun, J. Online internal short circuit detection for a large format lithium ion battery. Appl. Energy 2016, 161, 168–180. [Google Scholar] [CrossRef]
- Chiang, Y.H.; Sean, W.Y.; Ke, J.C. Online estimation of internal resistance and open-circuit voltage of lithium-ion batteries in electric vehicles. J. Power Sources 2011, 196, 3921–3932. [Google Scholar] [CrossRef]
- Mathew, M.; Kong, Q.H.; McGrory, J.; Fowler, M. Simulation of lithium ion battery replacement in a battery pack for application in electric vehicles. J. Power Sources 2017, 349, 94–104. [Google Scholar] [CrossRef]
- Panchal, S.; Dincer, I.; Agelin-Chaab, M.; Fraser, R.; Fowler, M. Experimental and simulated temperature variations in a LiFePO 4–20 Ah battery during discharge process. Appl. Energy 2016, 180, 504–515. [Google Scholar] [CrossRef]
- Panchal, S.; Mcgrory, J.; Kong, J.; Fraser, R.; Fowler, M.; Dincer, I.; Agelin-Chaab, M. Cycling degradation testing and analysis of a LiFePO4 battery at actual conditions. Int. J. Energy Res. 2017. [Google Scholar] [CrossRef]
- Mu, H.; Xiong, R.; Zheng, H.; Chang, Y.; Chen, Z. A novel fractional order model based state-of-charge estimation method for lithium-ion battery. Appl. Energy 2017, in press. [Google Scholar] [CrossRef]
- Dang, X.; Yan, L.; Xu, K.; Wu, X.; Jiang, H.; Sun, H. Open-circuit voltage-based state of charge estimation of lithium-ion battery using dual neural network fusion battery model. Electrochim. Acta 2016, 188, 356–366. [Google Scholar] [CrossRef]
- Dang, X.; Yan, L.; Jiang, H.; Wu, X.; Sun, H. Open-circuit voltage-based state of charge estimation of lithium-ion power battery by combining controlled auto-regressive and moving average modeling with feedforward-feedback compensation method. Int. J. Electr. Power Energy Syst. 2017, 90, 27–36. [Google Scholar] [CrossRef]
- Tian, Y.; Li, D.; Tian, J.; Xia, B. State of charge estimation of lithium-ion batteries using an optimal adaptive gain nonlinear observer. Electrochim. Acta 2017, 225, 225–234. [Google Scholar] [CrossRef]
- Li, J.; Barillas, J.K.; Guenther, C.; Danzer, M.A. A comparative study of state of charge estimation algorithms for LiFePO4 batteries used in electric vehicles. J. Power Sources 2013, 230, 244–250. [Google Scholar] [CrossRef]
- Du, J.; Liu, Z.; Wang, Y.; Wen, C. An adaptive sliding mode observer for lithium-ion battery state of charge and state of health estimation in electric vehicles. Control Eng. Pract. 2016, 54, 81–90. [Google Scholar] [CrossRef]
- Tong, S.; Klein, M.P.; Park, J.W. On-line optimization of battery open circuit voltage for improved state-of-charge and state-of-health estimation. J. Power Sources 2015, 293, 416–428. [Google Scholar] [CrossRef]
- Remmlinger, J.; Buchholz, M.; Meiler, M.; Bernreuter, P.; Dietmayer, K. State-of-health monitoring of lithium-ion batteries in electric vehicles by on-board internal resistance estimation. J. Power Sources 2011, 196, 5357–5363. [Google Scholar] [CrossRef]
- Xia, B.; Sun, Z.; Zhang, R.; Lao, Z. A Cubature Particle Filter Algorithm to Estimate the State of the Charge of Lithium-Ion Batteries Based on a Second-Order Equivalent Circuit Model. Energies 2017, 10, 457. [Google Scholar] [CrossRef]
- He, W.; Williard, N.; Chen, C.; Pecht, M. State of charge estimation for electric vehicle batteries using unscented kalman filtering. Microelectron. Reliab. 2013, 53, 840–847. [Google Scholar] [CrossRef]
- Schweiger, H.G.; Obeidi, O.; Komesker, O.; Raschke, A.; Schiemann, M.; Zehner, C.; Birke, P. Comparison of several methods for determining the internal resistance of lithium ion cells. Sensors 2010, 10, 5604–5625. [Google Scholar] [CrossRef] [PubMed]

| BTS4000 Voltage Range (V) | BTS4000 Current Range (A) | BTS4000 Measuring Errors | Cell Nominal Capacity (mAh) | Cell Maximum Continuous Discharging Current (A) | Cell Limit Voltage (V) | Sampling Frequency | |
|---|---|---|---|---|---|---|---|
| Constant Current Discharge (Hz) | HPPC Test (Hz) | ||||||
| 0–10 | −6 to 6 | 0.5% | 2350 | 10 | 2.7–4.2 | 1 | 10 |
| SOC | τ1/s | R1/Ω | C1/103 F |
|---|---|---|---|
| 0 | 56.2430 | 0.0382 | 1.4743 |
| 0.05 | 42.8082 | 0.0263 | 1.6265 |
| 0.1 | 51.3875 | 0.0226 | 2.2758 |
| 0.15 | 47.5964 | 0.0244 | 1.9491 |
| 0.2 | 55.9597 | 0.0237 | 2.3622 |
| 0.3 | 34.7826 | 0.0203 | 1.7126 |
| 0.4 | 35.8938 | 0.0204 | 1.7612 |
| 0.5 | 41.9287 | 0.0211 | 1.9919 |
| 0.6 | 37.5657 | 0.0267 | 1.4049 |
| 0.7 | 40.6504 | 0.0242 | 1.6798 |
| 0.8 | 38.5654 | 0.0272 | 1.4178 |
| 0.9 | 37.3134 | 0.0235 | 1.5878 |
| 1 | 46.2321 | 0.0240 | 1.9287 |
| SOC | τ1/s | R1/Ω | C1/103 F | τ2/s | R2/Ω | C2/104 F |
|---|---|---|---|---|---|---|
| 0 | 1.4769 | 0.0334 | 0.0442 | 51.5996 | 0.0169 | 0.3044 |
| 0.05 | 5.5741 | 0.0051 | 1.0871 | 62.0732 | 0.0091 | 0.6801 |
| 0.1 | 5.2938 | 0.0041 | 1.2881 | 66.0502 | 0.0085 | 0.7804 |
| 0.15 | 7.8370 | 0.0043 | 1.8020 | 81.9001 | 0.0079 | 1.0314 |
| 0.2 | 5.3763 | 0.0040 | 1.3375 | 69.9301 | 0.0091 | 0.7719 |
| 0.3 | 18.7512 | 0.0072 | 2.6151 | 86.1204 | 0.0023 | 3.5084 |
| 0.4 | 15.5497 | 0.0045 | 3.4769 | 69.7350 | 0.0048 | 1.4490 |
| 0.5 | 2.9833 | 0.0025 | 1.1805 | 45.3309 | 0.0086 | 0.5300 |
| 0.6 | 7.1276 | 0.0047 | 1.5090 | 56.7859 | 0.0087 | 0.6500 |
| 0.7 | 6.7249 | 0.0052 | 1.2954 | 60.4230 | 0.0102 | 0.5897 |
| 0.8 | 4.6404 | 0.0047 | 0.9950 | 47.1254 | 0.0102 | 0.4634 |
| 0.9 | 5.3220 | 0.0049 | 1.0819 | 89.7116 | 0.0433 | 0.8196 |
| 1 | 10.8684 | 0.0043 | 2.5064 | 93.8967 | 0.0070 | 1.3357 |
| Model Type | Maximum Absolute Error (V) | Maximum Relative Error | RMS Error (V) |
|---|---|---|---|
| The first-order RC model | 0.0610 | 1.65% | 0.0221 |
| The second-order RC model | 0.0452 | 1.22% | 0.0156 |
| Model Type | Maximum Absolute Error (V) | Maximum Relative Error | RMS Error (V) |
|---|---|---|---|
| The first-order RC model | 0.0695 | 1.88% | 0.0298 |
| The second-order RC model | −0.0628 | −1.69% | 0.0282 |
| Model Type | The Area Surrounded by the Discharge Curve (Vs) |
|---|---|
| The first-order RC model | 15,896 |
| The second-order RC model | 15,875 |
| Experimental data curve | 15,873 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Peng, H.; Ning, Z.; Mu, Z.; Sun, C. Comparative Research on RC Equivalent Circuit Models for Lithium-Ion Batteries of Electric Vehicles. Appl. Sci. 2017, 7, 1002. https://doi.org/10.3390/app7101002
Zhang L, Peng H, Ning Z, Mu Z, Sun C. Comparative Research on RC Equivalent Circuit Models for Lithium-Ion Batteries of Electric Vehicles. Applied Sciences. 2017; 7(10):1002. https://doi.org/10.3390/app7101002
Chicago/Turabian StyleZhang, Lijun, Hui Peng, Zhansheng Ning, Zhongqiang Mu, and Changyan Sun. 2017. "Comparative Research on RC Equivalent Circuit Models for Lithium-Ion Batteries of Electric Vehicles" Applied Sciences 7, no. 10: 1002. https://doi.org/10.3390/app7101002






