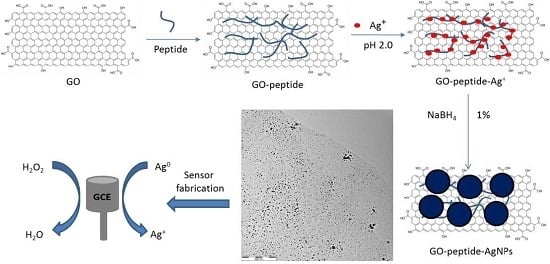
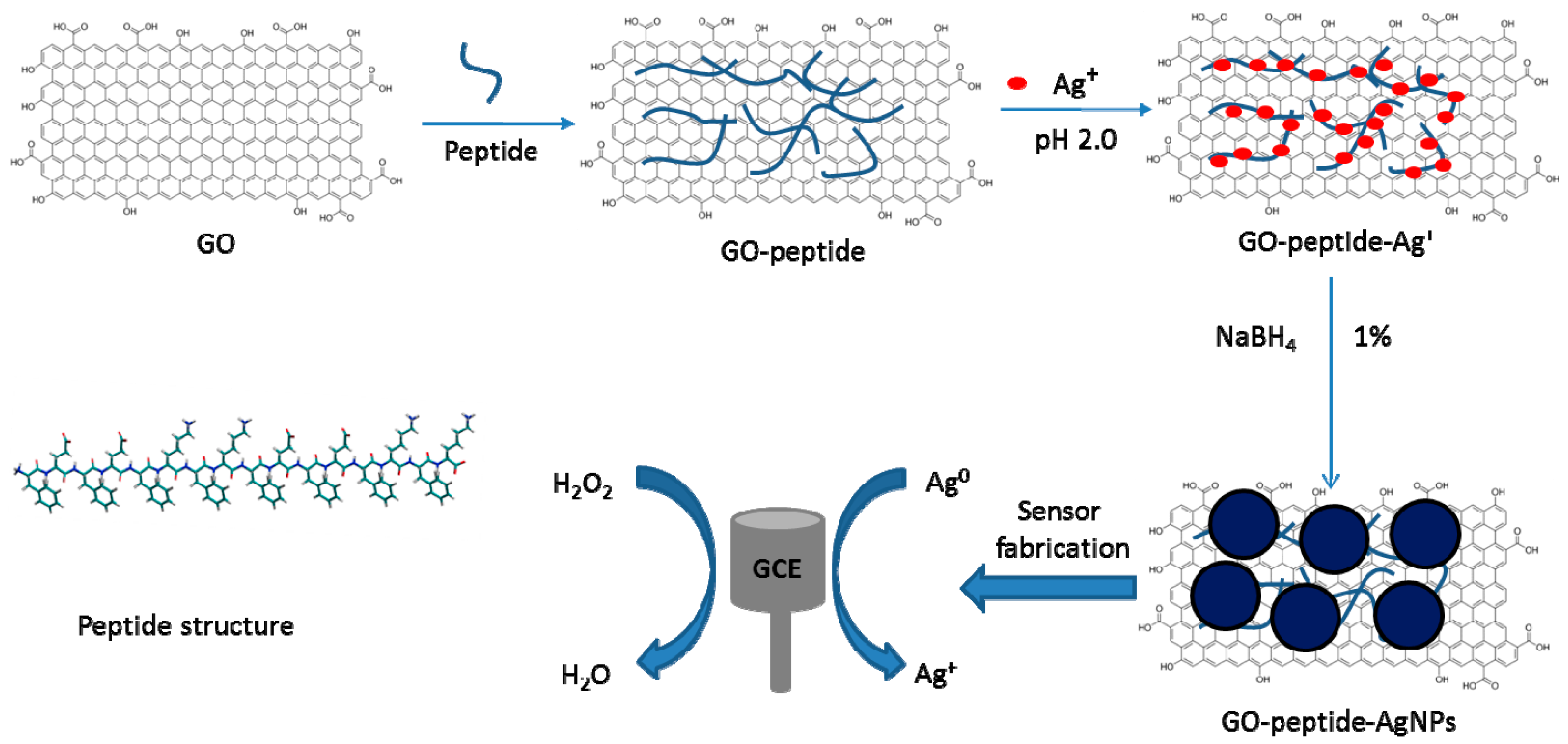
Phenylalanine-Rich Peptide Mediated Binding with Graphene Oxide and Bioinspired Synthesis of Silver Nanoparticles for Electrochemical Sensing
Abstract
:1. Introduction
2. Materials and Methods
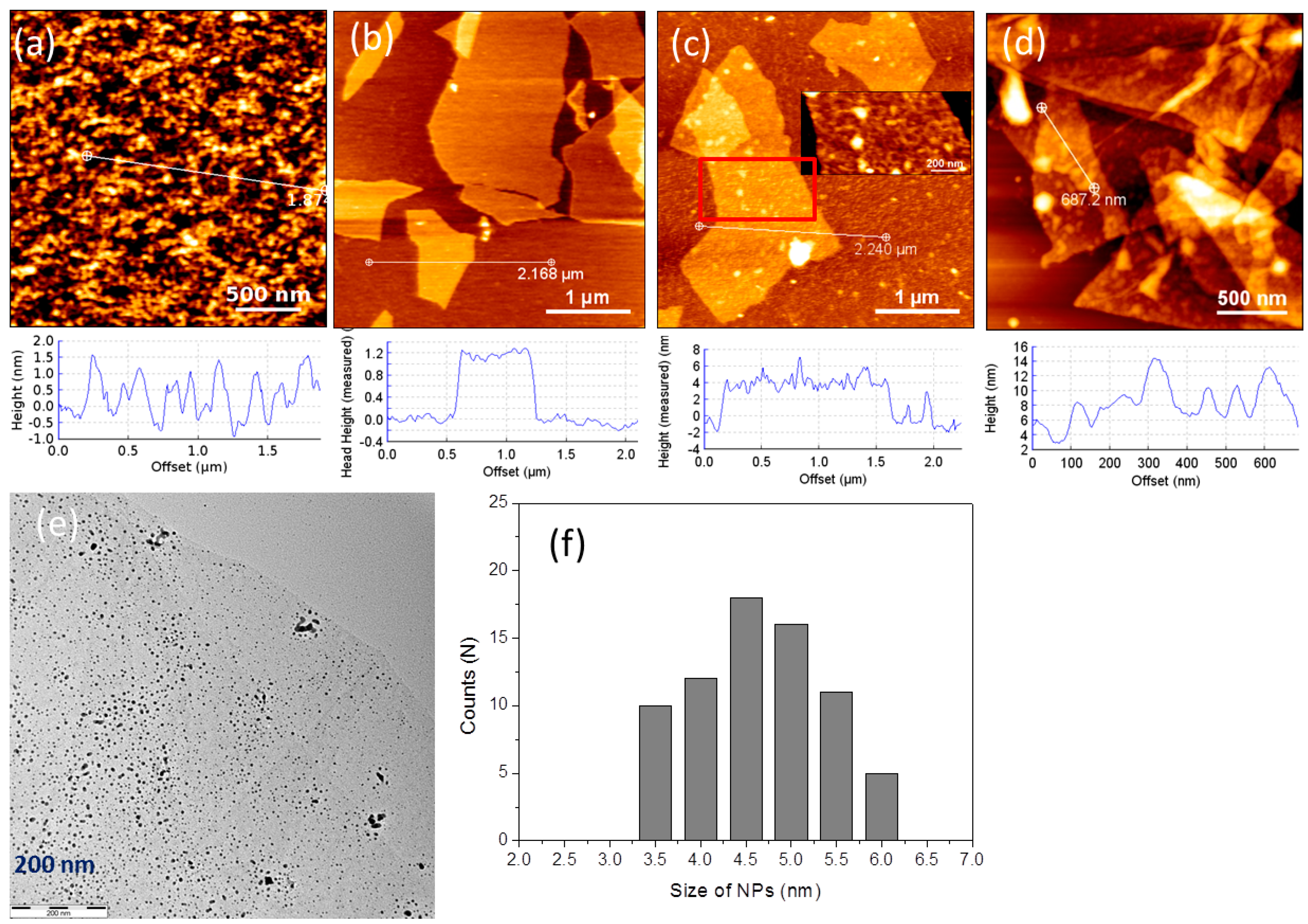
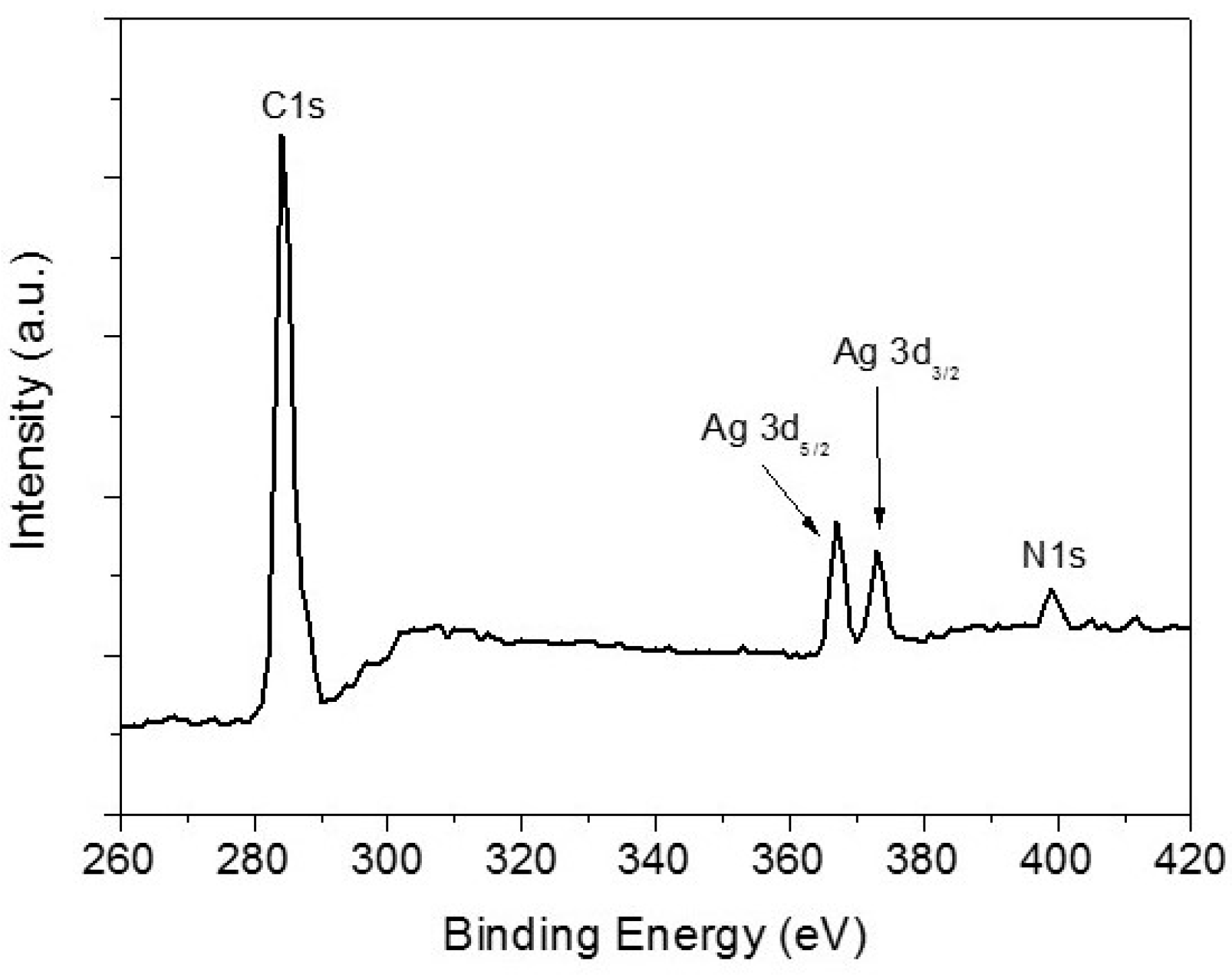
3. Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, M.; Dong, Y.Z.; Zhang, G.; Qu, J.H.; Li, J.H. Alpha-Fe2O3 spherical nanocrystals supported on CNTs as efficient non-noble electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 13635–13640. [Google Scholar]
- Sun, M.; Liu, H.J.; Liu, Y.; Qu, J.H.; Li, J.H. Graphene-based transition metal oxide nanocomposites for the oxygen reduction reaction. Nanoscale 2015, 7, 1250–1269. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, H.J.; Qu, J.H.; Li, J.H. Earth-rich transition metal phosphide for energy conversion and storage. Adv. Energy Mater. 2016, 6, 1600087. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Vij, V.; Kemp, K.C.; Kim, K.S. Engineered carbon-nanomaterial-based electrochemical sensors for biomolecules. ACS Nano 2016, 10, 46–80. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Zhang, J.T.; Xie, L.; Jandt, K.D. Biomimetic growth of hydroxyapatite on super water-soluble carbon nanotube-protein hybrid nanofibers. Carbon 2011, 49, 2216–2226. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.N.; Zhang, P.P.; Chen, Y.T.; Su, Z.Q.; Wei, G. Recent advances in the fabrication and structure-specific applications of graphene-based inorganic hybrid membranes. Nanoscale 2015, 7, 5080–5093. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Tang, L.H.; Li, J.H. Graphene-based materials in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.W.; Zhu, S.Y.; Zhu, T.; Sun, W.; Li, Q.; Wei, G.; Su, Z.Q. Hydrothermal synthesis of zinc oxide-reduced graphene oxide nanocomposites for an electrochemical hydrazine sensor. RSC Adv. 2015, 5, 22935–22942. [Google Scholar] [CrossRef]
- Wang, M.Y.; Shen, T.; Wang, M.; Zhang, D.E.; Chen, J. One-pot green synthesis of Ag nanoparticles-decorated reduced graphene oxide for efficient nonenzymatic H2O2 biosensor. Mater. Lett. 2013, 107, 311–314. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.P.; Ouyang, Z.F.; Zhang, M.F.; Lin, Z.J.; Li, J.F.; Su, Z.Q.; Wei, G. Nanoscale graphene doped with highly dispersed silver nanoparticles: Quick synthesis, facile fabrication of 3D membrane-modified electrode, and super performance for electrochemical sensing. Adv. Funct. Mater. 2016, 26, 2122–2134. [Google Scholar] [CrossRef]
- Zhang, P.P.; Zhao, X.N.; Ji, Y.C.; Ouyang, Z.F.; Wen, X.; Li, J.F.; Su, Z.Q.; Wei, G. Electrospinning graphene quantum dots into a nanofibrous membrane for dual-purpose fluorescent and electrochemical biosensors. J. Mater. Chem. B 2015, 3, 2487–2496. [Google Scholar] [CrossRef]
- Dong, H.F.; Gao, W.C.; Yan, F.; Ji, H.X.; Ju, H.X. Fluorescence resonance energy transfer between quantum dots and graphene oxide for sensing biomolecules. Anal. Chem. 2010, 82, 5511–5517. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.H.; Wang, J.; Li, J.H.; Lin, Y.H. Graphene and graphene oxide: Biofunctionalization and applications in biotechnology. Trends Biotechnol. 2011, 29, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.S.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Zhang, M.F.; Li, Y.; Su, Z.Q.; Wei, G. Recent advances in the synthesis and applications of graphene-polymer nanocomposites. Polym. Chem. 2015, 6, 6107–6124. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perrnan, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, H.B.; Li, J.H. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, K.S.; Ghosh, A.; Gomathi, A.; Govindaraj, A.; Rao, C.N.R. Covalent and noncovalent functionalization and solubilization of graphene. Nanosci. Nanotechnol. Lett. 2009, 1, 28–31. [Google Scholar] [CrossRef]
- Wang, J.H.; Ouyang, Z.F.; Ren, Z.W.; Li, J.F.; Zhang, P.P.; Wei, G.; Su, Z.Q. Self-assembled peptide nanofibers on graphene oxide as a novel nanohybrid for biomimetic mineralization of hydroxyapatite. Carbon 2015, 89, 20–30. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhao, X.J.; Li, J.F.; Kuang, X.; Fan, Y.Q.; Wei, G.; Su, Z.Q. Electrostatic assembly of peptide nanofiber-biomimetic silver nanowires onto graphene for electrochemical sensors. ACS Macro Lett. 2014, 3, 529–533. [Google Scholar] [CrossRef]
- Yang, H.; Fung, S.Y.; Sun, W.; Mikkelsen, S.; Pritzker, M.; Chen, P. Ionic-complementary peptide-modified highly ordered pyrolytic graphite electrode for biosensor application. Biotechnol. Prog. 2008, 24, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Kuang, Z.F.; Slocik, J.M.; Jones, S.E.; Cui, Y.; Farmer, B.L.; McAlpine, M.C.; Naik, R.R. Preferential binding of peptides to graphene edges and planes. J. Am. Chem. Soc. 2011, 133, 14480–14483. [Google Scholar] [CrossRef] [PubMed]
- Katoch, J.; Kim, S.N.; Kuang, Z.F.; Farmer, B.L.; Nalk, R.R.; Tatulian, S.A.; Ishigami, M. Structure of a peptide adsorbed on graphene and graphite. Nano Lett. 2012, 12, 2342–2346. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Kim, S.N.; Jones, S.E.; Wissler, L.L.; Naik, R.R.; McAlpine, M.C. Chemical functionalization of graphene enabled by phage displayed peptides. Nano Lett. 2010, 10, 4559–4565. [Google Scholar] [CrossRef] [PubMed]
- Li, P.P.; Chen, X.; Yang, W.S. Graphene-induced self-assembly of peptides into macroscopic-scale organized nanowire arrays for electrochemical nadh sensing. Langmuir 2013, 29, 8629–8635. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.J.; Wang, J.Q.; Wang, Z.F.; Li, Z.P.; Qiu, Y.N.; Wang, H.G.; Xu, Y.; Niu, L.Y.; Gong, P.W.; Yang, S.R. Casein phosphopeptide-biofunctionalized graphene biocomposite for hydroxyapatite biomimetic mineralization. J. Phys. Chem. C 2013, 117, 10375–10382. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, Y.; Steckbeck, S.; Su, Z.Q.; Li, Z. Biomimetic graphene-fept nanohybrids with high solubility, ferromagnetism, fluorescence, and enhanced electrocatalytic activity. J. Mater. Chem. 2012, 22, 17190–17195. [Google Scholar] [CrossRef]
- Li, S.K.; Shen, Y.H.; Xie, A.J.; Yu, X.R.; Qiu, L.G.; Zhang, L.; Zhang, Q.F. Green synthesis of silver nanoparticles using capsicum annuum l. Extract. Green Chem. 2007, 9, 852–858. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.Q.; Wang, L.; Li, H.L.; Zhang, Y.W.; Sun, X.P. Stable aqueous dispersion of graphene nanosheets: Noncovalent functionalization by a polymeric reducing agent and their subsequent decoration with ag nanoparticles for enzymeless hydrogen peroxide detection. Macromolecules 2010, 43, 10078–10083. [Google Scholar] [CrossRef]
- Fang, Y.X.; Guo, S.J.; Zhu, C.Z.; Zhai, Y.M.; Wang, E.K. Self-assembly of cationic polyelectrolyte-functionalized graphene nanosheets and gold nanoparticles: A two-dimensional heterostructure for hydrogen peroxide sensing. Langmuir 2010, 26, 11277–11282. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.Q.; Wang, L.; Sun, X.P. A method for the production of reduced graphene oxide using benzylamine as a reducing and stabilizing agent and its subsequent decoration with ag nanoparticles for enzymeless hydrogen peroxide detection. Carbon 2011, 49, 3158–3164. [Google Scholar] [CrossRef]
- Zhou, K.F.; Zhu, Y.H.; Yang, X.L.; Luo, J.; Li, C.Z.; Luan, S.R. A novel hydrogen peroxide biosensor based on au-graphene-hrp-chitosan biocomposites. Electrochim. Acta 2010, 55, 3055–3060. [Google Scholar] [CrossRef]
- Xie, L.L.; Xu, Y.D.; Cao, X.Y. Hydrogen peroxide biosensor based on hemoglobin immobilized at graphene, flower-like zinc oxide, and gold nanoparticles nanocomposite modified glassy carbon electrode. Colloids Surf. B 2013, 107, 245–250. [Google Scholar] [CrossRef] [PubMed]
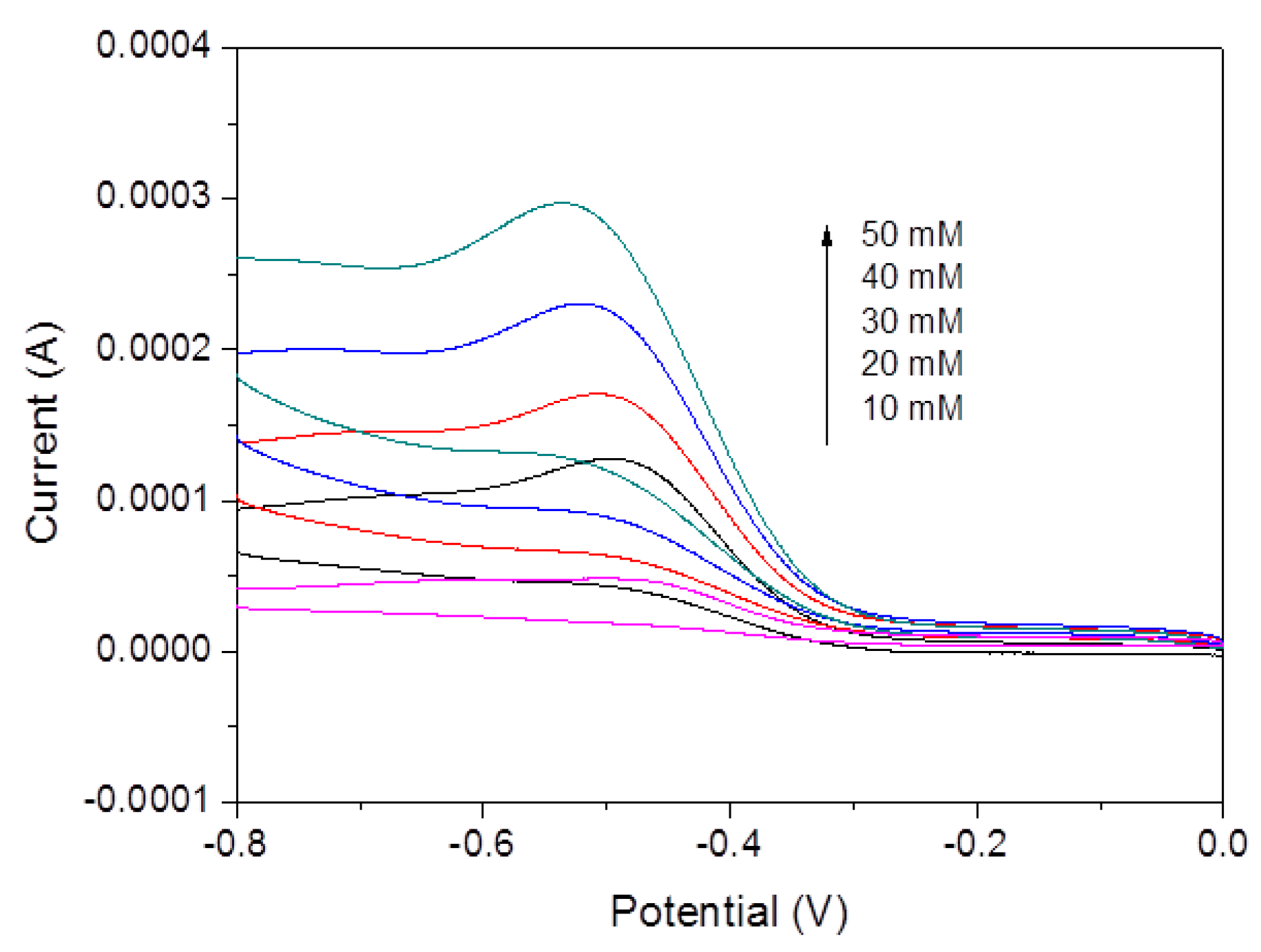
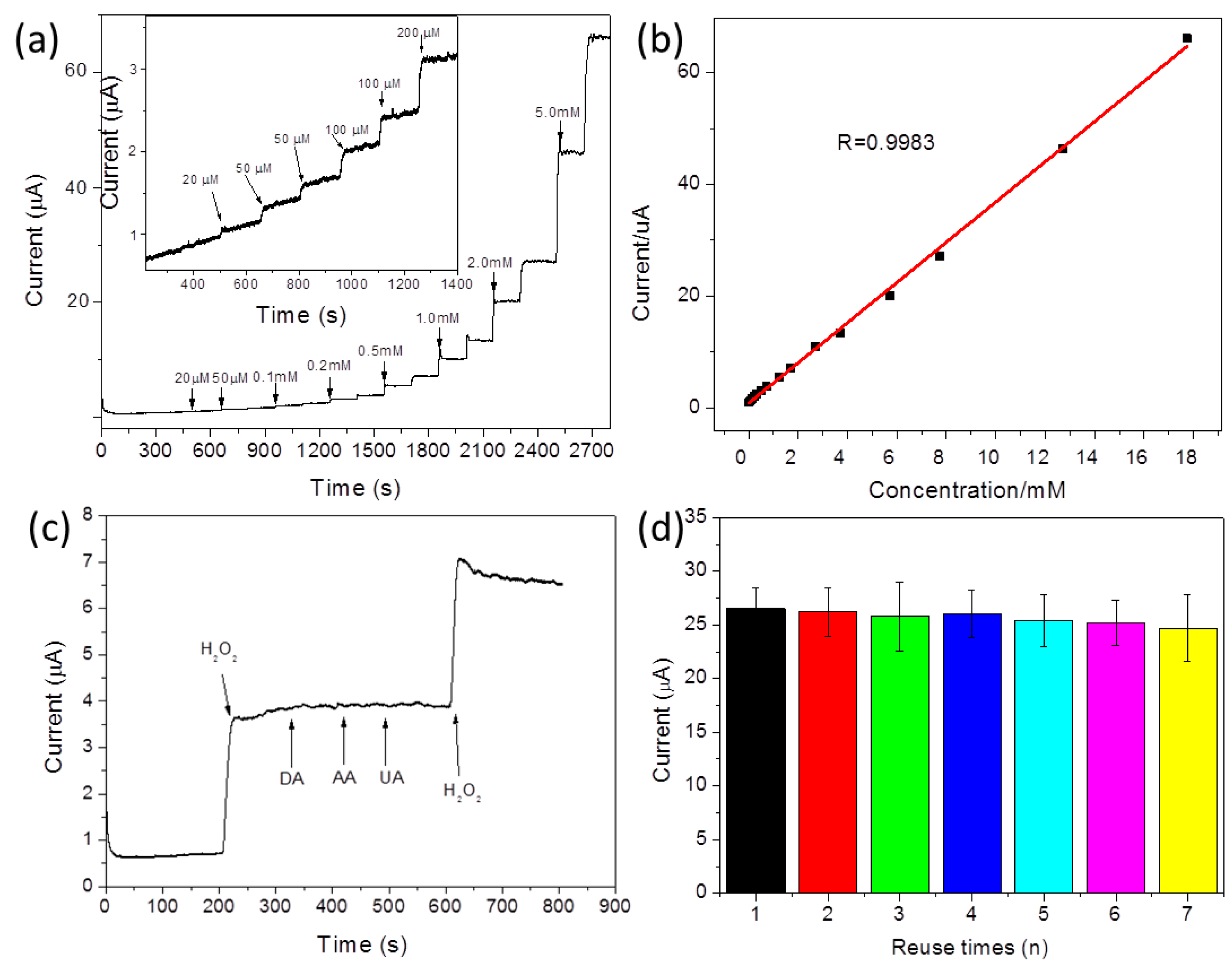
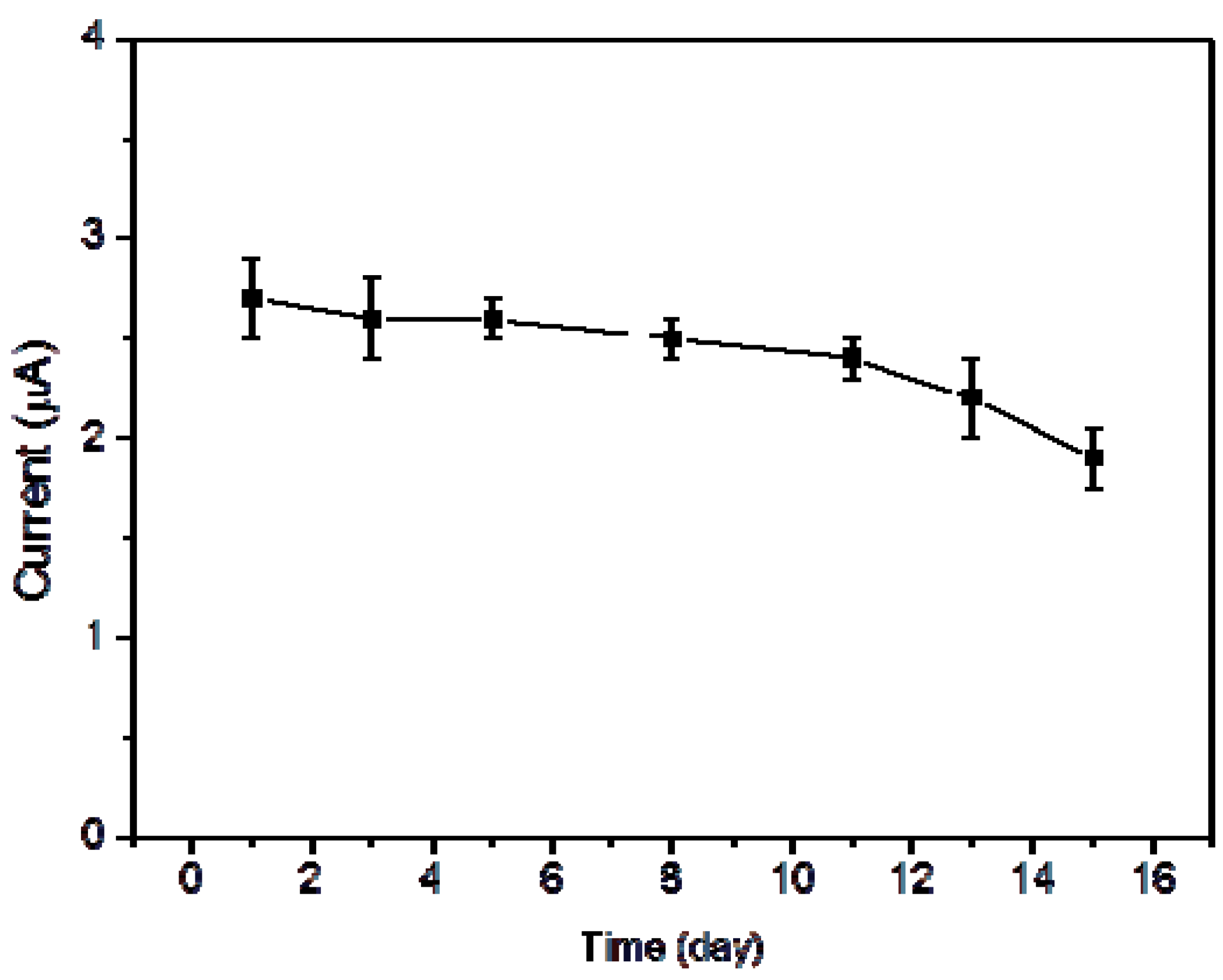
| Materials | Linear Range | Detection Limit | Reference |
|---|---|---|---|
| RGO–peptide nanofiber–AgNPs | 0.05–5 mM | 10.4 μM | [23] |
| RGO–Polymer–AgNPs | 0.1–40 mM | 28 μM | [32] |
| RGO–AuNPs | 0.5 μM–0.5 mM | 0.22 μM | [33] |
| RGO–AgNPs | 0.1–100 mM | 31.3 μM | [34] |
| Graphene/Au/horseradish peroxidase/chitosan | 0.005–5.13 mM | 1.7 μM | [35] |
| Hb/AuNPs/ZnO/RGO | 0.006–1.13 mM | 0.8 μM | [36] |
| GO–peptide–AgNPs | 0.02–18 mM | 0.13 μM | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Lin, J. Phenylalanine-Rich Peptide Mediated Binding with Graphene Oxide and Bioinspired Synthesis of Silver Nanoparticles for Electrochemical Sensing. Appl. Sci. 2017, 7, 160. https://doi.org/10.3390/app7020160
Wang L, Lin J. Phenylalanine-Rich Peptide Mediated Binding with Graphene Oxide and Bioinspired Synthesis of Silver Nanoparticles for Electrochemical Sensing. Applied Sciences. 2017; 7(2):160. https://doi.org/10.3390/app7020160
Chicago/Turabian StyleWang, Li, and Jing Lin. 2017. "Phenylalanine-Rich Peptide Mediated Binding with Graphene Oxide and Bioinspired Synthesis of Silver Nanoparticles for Electrochemical Sensing" Applied Sciences 7, no. 2: 160. https://doi.org/10.3390/app7020160
APA StyleWang, L., & Lin, J. (2017). Phenylalanine-Rich Peptide Mediated Binding with Graphene Oxide and Bioinspired Synthesis of Silver Nanoparticles for Electrochemical Sensing. Applied Sciences, 7(2), 160. https://doi.org/10.3390/app7020160