Fouling in Membrane Distillation, Osmotic Distillation and Osmotic Membrane Distillation
Abstract
:1. Introduction
2. Membrane Characteristics
3. Fouling in MD, OD and OMD
3.1. Fouling in MD
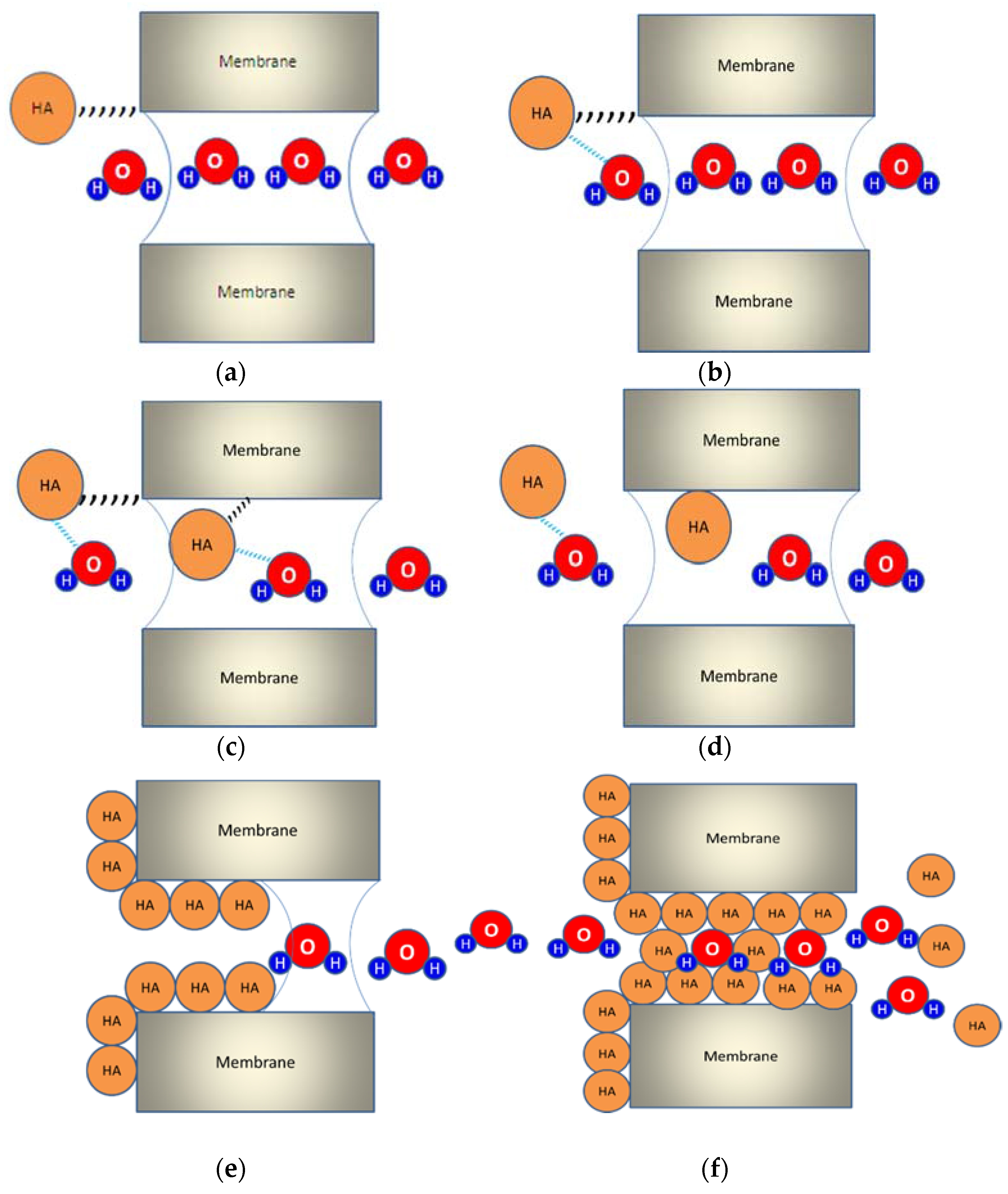
3.1.1. Organic Fouling
3.1.2. Inorganic Fouling
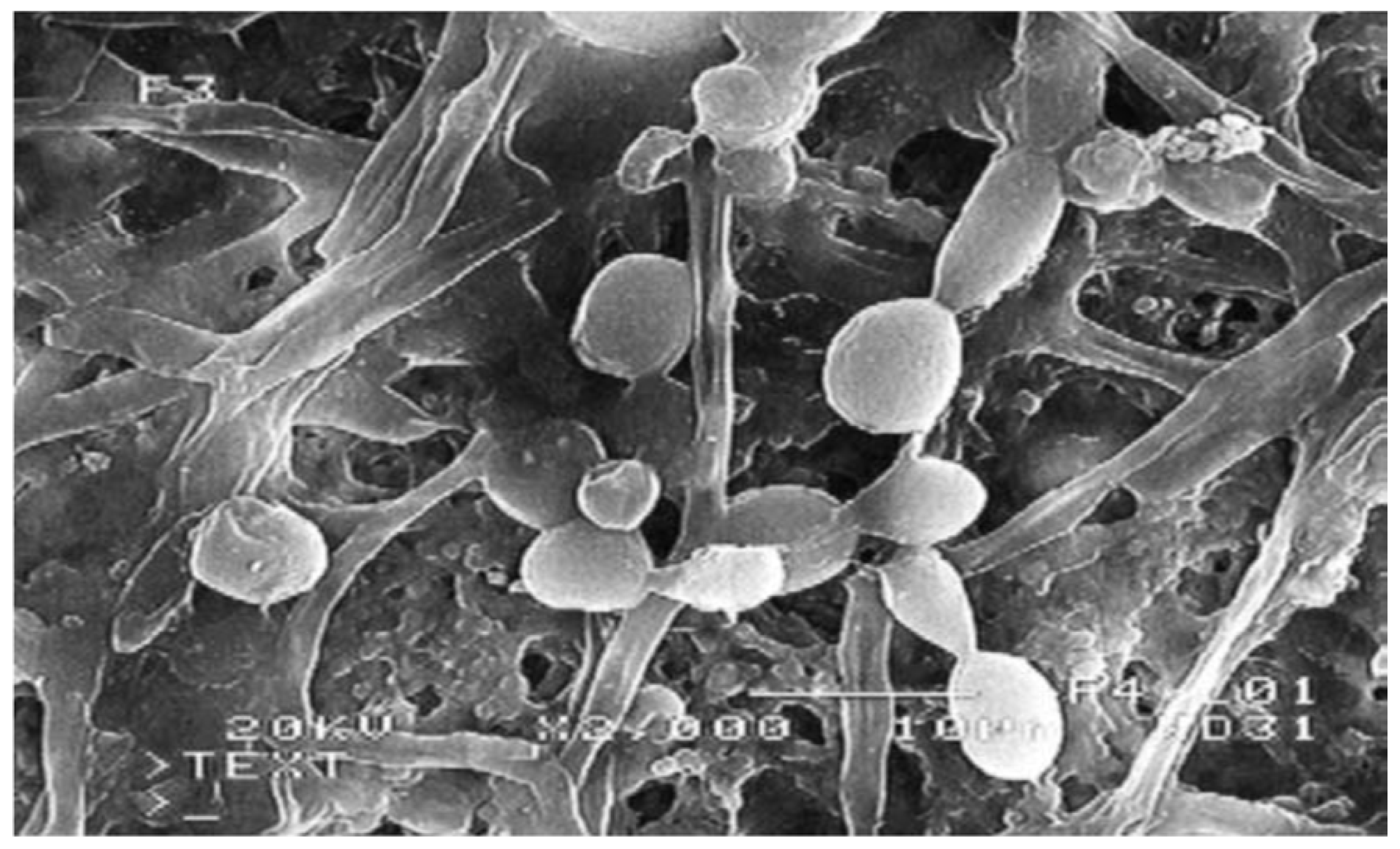
3.1.3. Biofouling
3.2. Fouling in OD
3.3. Fouling in OMD
4. Characterization Techniques for Fouling Analysis
4.1. Visual Analysis
4.2. Microscopy Techniques
4.2.1. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
4.2.2. Transmission Electron Microscopy (TEM)
4.2.3. Atomic Force Microscopy (AFM)
4.2.4. Confocal Laser Scanning Microscopy (CLSM)
4.3. Contact Angle
4.4. Infrared Thermography Technique (IRT)
4.5. Ultrasonic Time Domain Reflectometry (UTDR)
4.6. Zeta Potential
4.7. X-ray Diffraction (XRD)
4.8. X-ray Fluorescence (XRF)
4.9. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
4.10. Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES)
4.11. Atomic Absorption Spectroscopy (AAS) Analysis
4.12. Excitation Emission-Matrix Fluorescence Spectroscopy (EEM)
4.13. Field Flow Fractionation (FFF)
5. Methodologies for Membrane Fouling Prevention and Reduction
5.1. Pretreatment
5.2. Use of Antiscalants
5.3. Membrane Modification
5.4. Membrane Cleaning
6. Conclusions and Future Perspectives
Author Contributions
Conflicts of Interest
References
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Membrane Distillation: Principles and Aplication; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Chew, J.W.; Krantz, W.B.; Fane, A.G. Effect of a macromolecular- or bio-fouling layer on membrane distillation. J. Membr. Sci. 2014, 456, 66–76. [Google Scholar] [CrossRef]
- Bui, A.V.; Nguyen, H.M.; Muller, J. A laboratory study on glucose concentration by OD in hollow fibre module. J. Food Eng. 2004, 63, 237–245. [Google Scholar] [CrossRef]
- Zambra, C.; Romero, J.; Pino, L.; Saavedra, A.; Sanchez, J. Concentration of cranberry juice by osmotic distillation process. J. Food Eng. 2015, 144, 58–65. [Google Scholar] [CrossRef]
- Cassano, A.; Drioli, E. Concentration of clarified kiwifruit juice by osmotic distillation. J. Food Eng. 2007, 79, 1397–1404. [Google Scholar] [CrossRef]
- Gryta, M. Osmotic MD and other membrane distillation variants. J. Membr. Sci. 2005, 246, 145–156. [Google Scholar] [CrossRef]
- Wang, L.; Min, J. Modeling and analyses of membrane osmotic distillation using non-equilibrium thermodynamics. J. Membr. Sci. 2011, 378, 462–470. [Google Scholar] [CrossRef]
- Ravindra Babu, B.; Rastogi, N.K.; Raghavarao, K.S.M.S. Concentration and temperature polarization effects during osmotic membrane distillation. J. Membr. Sci. 2008, 322, 146–153. [Google Scholar] [CrossRef]
- Raghavarao, K.S.M.S.; Madhusudhan, M.C.; Tavanandi, T.H.; Niranjan, K. Athermal Membrane Processes for the Concentration of Liquid Foods and Natural Colors. Chapter 12 BOOK Da-Wen Sun-Emerging Technologies for Food Processing, 2nd ed.; Academic Press, Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Khayet, M.; Mengual, J.I. Effect of salt concentration during the treatment of humic acid solutions by membrane distillation. Desalination 2004, 168, 373–381. [Google Scholar] [CrossRef]
- Hausmann, A.; Sanciolo, P.; Vasiljevic, T.; Weeks, M.; Schroën, K.; Gray, S.; Duke, M. Fouling mechanisms of dairy streams during membrane distillation. J. Membr. Sci. 2013, 441, 102–111. [Google Scholar] [CrossRef]
- Nguyen, Q.M.; Lee, S. Fouling analysis and control in a DCMD process for SWRO brine. Desalination 2015, 367, 21–27. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard V, J.H. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.S.; Lee, S.; Kim, S.H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Alklaibi, A.M.; Lior, N. Membrane-distillation desalination: Status and potential. Desalination 2005, 171, 111–131. [Google Scholar] [CrossRef]
- Lee, H.; Amy, G.; Cho, J.; Yoon, Y.; Moon, S.H.; Kim, I.S. Cleaning strategies for flux recovery of an ultrafiltration membrane fouled by natural organic matter. Water Res. 2001, 35, 3301–3308. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. Recent advances in membrane distillation processes: Membrane development, configuration design and application exploring. J. Membr. Sci. 2015, 474, 39–56. [Google Scholar] [CrossRef]
- Khayet, M. Membranes and theoretical modeling of membrane distillation, a review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef] [PubMed]
- Nane, S.; Patil, G.; Raghavarao, K.S.M.S. Chapter 19: Membrane Distillation in Food Processing. In Handbook of Membrane Separations: Chemical, Pharmaceutical, Food, and Biotechnological Applications; Pabby, A.K., Rizvi, S.S.H., Sastre, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation: A review. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. How to Optimize the Membrane Properties for Membrane Distillation: A Review. Ind. Eng. Chem. Res. 2016, 55, 9333–9343. [Google Scholar] [CrossRef]
- Hilal, N.; Khayet, M.; Wright, C.J. Membrane Modification: Technology and Application; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Mansouri, J.; Fane, A.G. Membrane development for processing of oily feeds in IMD, osmotic distillation: Developments in technology and modelling. In Proceedings of the Workshop on “Membrane Distillation, Osmotic Distillation and Membrane Contactors” (CNRIRMERC), Cetraro, Italy, 2–4 July 1998; pp. 43–46. [Google Scholar]
- Cheng, D.Y.; Wiersma, S.J. Composite Membrane for Membrane Distillation System. U.S. Patent 4,419,242, 23 February 1982. [Google Scholar]
- Boo, C.; Lee, J.; Elimelech, M. Engineering Surface Energy and Nanostructure of Microporous Films for Expanded Membrane Distillation Applications. Environ. Sci. Technol. 2016, 50, 8112–8119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Boo, C.; Ryu, W.H.; Taylor, A.D.; Elimelech, M. Development of Omniphobic Desalination Membranes Using a Charged Electrospun Nanofiber Scaffold. ACS Appl. Mater. Interfaces 2016, 8, 11154–11161. [Google Scholar] [CrossRef] [PubMed]
- An, A.K.; Guo, J.; Jeong, S.; Lee, E.J.; Tabatabai, S.A.; Leiknes, T. High flux and antifouling properties of negatively charged membrane for dyeing wastewater treatment by membrane distillation. Water Res. 2016, 103, 362–371. [Google Scholar] [CrossRef] [PubMed]
- An, A.K.; Guo, J.; Lee, E.J.; Jeong, S.; Zhao, Y.; Wang, Z.; Leiknes, T. PDMS/PVDF hybrid electrospun membrane with superhydrophobic property and drop impact dynamics for dyeing wastewater treatment using membrane distillation. J. Membr. Sci. 2017, 525, 57–67. [Google Scholar] [CrossRef]
- Khayet, M.; Velázquez, A.; Mengual, J.I. Direct contact membrane distillation of humic acid solutions. J. Membr. Sci. 2004, 240, 123–128. [Google Scholar] [CrossRef]
- Narayan, A.V.; Nagaraj, N.; Hebbar, H.U.; Chakkaravarthi, A.; Raghavarao, K.S.M.S.; Nene, S. Acoustic field-assisted osmotic membrane distillation. Desalination 2002, 147, 149–156. [Google Scholar] [CrossRef]
- Gryta, M. Fouling in direct contact membrane distillation process. J. Membr. Sci. 2008, 325, 383–394. [Google Scholar] [CrossRef]
- He, F.; Gilron, J.; Lee, S.; Sirkar, K.K. Potential for scaling by sparingly soluble salts in cross flow DCMD. J. Membr. Sci. 2008, 311, 68–80. [Google Scholar] [CrossRef]
- He, F.; Sirkar, K.K.; Gilron, J. Studies on scaling of membranes in desalination by direct contact membrane distillation: CaCO3 and mixed CaCO3/CaSO4 systems. Chem. Eng. Sci. 2009, 64, 1844–1859. [Google Scholar] [CrossRef]
- Jacob, P.; Phungsai, P.; Fukushi, K.; Visvanathan, C. Direct contact membrane distillation for anaerobic effluent treatment. J. Membr. Sci. 2015, 475, 330–339. [Google Scholar] [CrossRef]
- Hausmann, A.; Sanciolo, P.; Vasiljevic, T.; Weeks, M.; Schroën, K.; Gray, S.; Duke, M. Fouling of dairy components on hydrophobic polytetrafluoroethylene (PTFE) membranes for membrane distillation. J. Membr. Sci. 2013, 442, 149–159. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, L.; Liu, Z.; Ma, R. Fouling resistance in concentrating TCM extract by direct contact membrane distillation. J. Membr. Sci. 2010, 362, 317–325. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Vigneswaran, S. Interaction of humic substances on fouling in membrane distillation for seawater desalination. Chem. Eng. J. 2015, 262, 946–957. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Kim, S.J.; Kim, I.S.; Vigneswaran, S. Organic fouling behavior in direct contact membrane distillation. Desalination 2014, 347, 230–239. [Google Scholar] [CrossRef]
- Guillen-Burrieza, E.; Thomas, R.; Mansoor, B.; Johnson, D.; Hilal, N.; Arafat, H. Effect of dry-out on the fouling of PVDF and PTFE membranes under conditions simulating intermittent seawater membrane distillation (SWMD). J. Membr. Sci. 2013, 438, 126–139. [Google Scholar] [CrossRef]
- Srisurichan, S.; Jiraratananon, R.; Fane, A.G. Humic acid fouling in the membrane distillation process. Desalination 2005, 174, 63–72. [Google Scholar] [CrossRef]
- Ge, J.; Peng, Y.; Li, Z.; Chen, P.; Wang, S. Membrane fouling and wetting in a DCMD process for RO brine concentration. Desalination 2014, 344, 97–107. [Google Scholar] [CrossRef]
- Mokhtar, N.M.; Lau, W.J.; Ismail, A.F.; Veerasamy, D. Membrane Distillation Technology for Treatment of Wastewater from Rubber Industry in Malaysia. Procedia CIRP 2015, 26, 792–796. [Google Scholar] [CrossRef]
- Yu, X.; Yang, H.; Lei, H.; Shapiro, A. Experimental evaluationon concentrating cooling tower blow down water by direct contact membrane distillation. Desalination 2013, 323, 134–141. [Google Scholar] [CrossRef]
- Gryta, M. Polyphosphates used for membrane scaling inhibition during water desalination by membrane distillation. Desalination 2012, 285, 170–176. [Google Scholar] [CrossRef]
- Gryta, M. Influence of polypropylene membrane surface porosity on the performance of membrane distillation process. J. Membr. Sci. 2007, 287, 67–78. [Google Scholar] [CrossRef]
- Gryta, M.; Tomaszewska, M.; Grzechulska, J.; Morawski, A.W. Membrane distillation of NaCl solution containing natural organic matter. J. Membr. Sci. 2001, 181, 279–287. [Google Scholar] [CrossRef]
- Gryta, M. Alkaline scaling in the membrane distillation process. Desalination 2008, 228, 128–134. [Google Scholar] [CrossRef]
- Gryta, M.; Grzechulska-Damszel, J.; Markowska, A.; Karakulski, K. The influence of polypropylene degradation on the membrane wettability during membrane distillation. J. Membr. Sci. 2009, 326, 493–502. [Google Scholar] [CrossRef]
- Tian, R.; Gao, H.; Yang, X.H.; Yan, S.Y.; Li, S. A new enhancement technique on air gap membrane distillation. Desalination 2014, 332, 52–59. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Treatment of high salinity solutions: Application of air gap membrane distillation. Desalination 2012, 287, 55–60. [Google Scholar] [CrossRef]
- Mericq, J.P.; Laborie, S.; Cabassud, C. Vacuum membrane distillation of seawater reverse osmosis brines. Water Res. 2010, 44, 5260–5273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.P.; Zhu, C.Y.; Liu, D.Z.; Liu, W.F. Concentration of ginseng extracts aqueous solution by vacuum membrane distillation 2. Theory analysis of critical operating conditions and experimental confirmation. Desalination 2011, 267, 147–153. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, J.; Hou, D.; Yin, Z.; Luan, Z. Effect of microwave irradiation on vacuum membrane distillation. J. Membr. Sci. 2013, 429, 473–479. [Google Scholar] [CrossRef]
- Zhiqing, Y.; Xiaolong, L.; Chunrui, W.; Xuan, W. Effect of pretreatment on membrane fouling and VMD performance in the treatment of RO- concentrated wastewater. Desalin. Water Treat. 2013, 51, 6994–7003. [Google Scholar] [CrossRef]
- Banat, F.; Al-Asheh, S.; Qtaishat, M. Treatment of waters colored with methylene blue dye by vacuum membrane distillation. Desalination 2005, 174, 87–96. [Google Scholar] [CrossRef]
- Zarebska, A.; Nieto, D.R.; Christensen, K.V.; Norddahl, B. Ammonia recovery from agricultural wastes by membrane distillation: Fouling characterization and mechanism. Water Res. 2014, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Criscuoli, A.; Zhong, J.; Figoli, A.; Carnevale, M.C.; Huang, R.; Drioli, E. Treatment of dye solutions by vacuum membrane distillation. Water Res. 2008, 42, 5031–5037. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zydney, A.L. Humic acid fouling during microfiltration. J. Membr. Sci. 1999, 157, 1–12. [Google Scholar] [CrossRef]
- Jermann, D.; Pronk, W.; Meylan, S.; Boller, M. Interplay of different NOM fouling mechanisms during ultrafiltration for drinking water production. Water Res. 2007, 41, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Nilson, J.A.; Digiano, F.A. Influence of NOM composition on nanofiltration. J. Am. Water Works Assoc. 1996, 88, 53–66. [Google Scholar]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Gryta, M. Concentration of saline wastewater from the production of heparin. Desalination 2000, 129, 35–44. [Google Scholar] [CrossRef]
- Hamrouni, B.; Dhahbi, M. Calco-carbonic equilibrium calculation. Desalination 2002, 152, 167–174. [Google Scholar] [CrossRef]
- Wirth, D.; Cabassud, C. Water desalination using membrane distillation: Comparison between inside/out and outside/in permeation. Desalination 2002, 147, 139–145. [Google Scholar] [CrossRef]
- Safavi, M.; Mohammadi, T. High-salinity water desalination using VMD. Chem. Eng. J. 2009, 149, 191–195. [Google Scholar] [CrossRef]
- Gryta, M.; Tomaszewska, M.; Karakulski, K. Wastewater treatment by membrane distillation. Desalination 2006, 198, 67–73. [Google Scholar] [CrossRef]
- Gryta, M. Long-term performance of membrane distillation process. J. Membr. Sci. 2005, 265, 153–159. [Google Scholar] [CrossRef]
- Tun, C.M.; Fane, A.G.; Matheickal, J.T.; Sheikholeslami, R. Membranes distillation crystallization of concentrated salts-flux and crystal formation. J. Membr. Sci. 2005, 257, 144–155. [Google Scholar] [CrossRef]
- Yun, Y.; Ma, R.; Zhang, W.; Fane, A.G.; Li, J. Direct contact membrane distillation mechanism for high concentration NaCl solutions. Desalination 2006, 188, 251–262. [Google Scholar] [CrossRef]
- Mariah, L.; Buckley, C.A.; Brouckaert, C.J.; Curcio, E.; Drioli, E.; Jaganyi, D.; Ramjugernath, D. Membrane distillation of concentrated brines-Role of water activities in the evaluation of driving force. J. Membr. Sci. 2006, 280, 937–947. [Google Scholar] [CrossRef]
- Drioli, E.; Criscuoli, A.; Curcio, E. Integrated membrane operation for seawater desalination. Desalination 2002, 147, 77–81. [Google Scholar] [CrossRef]
- Curcio, E.; Profio, G.D.; Drioli, E. Membrane crystallization of macromolecular solutions. Desalination 2002, 145, 173–177. [Google Scholar] [CrossRef]
- Bouguecha, S.; Dhabi, M. Fluidized bed crystallizer and air gap membrane distillation as a solution to geothermal water desalination. Desalination 2002, 152, 237–244. [Google Scholar] [CrossRef]
- Gryta, M. The assessment of microorganism growth in the membrane distillation system. Desalination 2002, 142, 79–88. [Google Scholar] [CrossRef]
- Vogt, M.; Flemming, H.; Veeman, W. Diffusion in Pseudomonas aeruginosa biofilms: A pulsed field gradient NMR study. J. Biotechnol. 2000, 77, 137–146. [Google Scholar] [CrossRef]
- Flemming, H.C.; Schaule, G.; McDonogh, R.; Ridgway, H.F. Effects and extent of biofilm accumulation in membrane systems. In Biofouling and Biocorrosionin Industrial Water Systems; Geesey, G.G., Lewandowsky, Z., Flemming, H.C., Eds.; CRC Press/Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 63–89. [Google Scholar]
- Goh, S.; Zhang, J.; Liu, Y.; Fane, A.G. Fouling and wetting in membrane distillation (MD) and MD-bioreactor (MDBR) for wastewater reclamation. Desalination 2013, 323, 39–47. [Google Scholar] [CrossRef]
- Goh, S.; Zhang, Q.; Zhang, J.; McDougald, D.; Krantz, W.B.; Liu, Y.; Fane, A.G. Impact of a biofouling layer on the vapor pressure driving force and performance of a membrane distillation process. J. Membr. Sci. 2013, 438, 140–152. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Khayet, M.; Kiai, H.; Hafidi, A.; García-Payo, M.C. Treatment of crude olive mill wastewaters by osmotic distillation and osmotic membrane distillation. Sep. Purif. Technol. 2013, 104, 327–332. [Google Scholar] [CrossRef]
- Durham, R.J.; Nguyen, H.M. Hydrophobic membrane evaluation and cleaning for osmotic distillation of tomato puree. J. Membr. Sci. 1994, 87, 181–189. [Google Scholar] [CrossRef]
- Kujawski, W.; Sobolewska, A.; Jarzynka, K.; Güell, C.; Ferrando, M.; Warczok, J. Application of osmotic membrane distillation process in red grape juice concentration. J. Food Eng. 2013, 116, 801–808. [Google Scholar] [CrossRef]
- Bui, A.V.; Nguyen, H.M. Scaling up of osmotic distillation from laboratory to pilot plant for concentration of fruit juices. Int. J. Food Eng. 2005, 1, 1556–3758. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Hafidi, A.; Khayet, M.; García-Payo, M.C. Integrated direct contact membrane distillation for olive mill wastewater treatment. Desalination 2013, 323, 31–38. [Google Scholar] [CrossRef]
- Ruiz Salmón, I.; Janssens, R.; Luis, P. Mass and heat transfer study in osmotic membrane distillation-crystallization for CO2 valorization as sodium carbonate. Sep. Purif. Technol. 2017, 176, 173–183. [Google Scholar] [CrossRef]
- Bui, A.V.; Nguyen, H.M.; Joachim, M. Characterisation of the polarisations in osmotic distillation of glucose solutions in hollow fibre module. J. Food Eng. 2005, 68, 391–402. [Google Scholar] [CrossRef]
- Karlsson, E.; Luh, B.S. Vegetable juices, sauces and soups. In Commercial Vegetable Processing; Luh, B.S., Woodroof, J.G., Eds.; Medtech: New York, NY, USA, 1988. [Google Scholar]
- Wills, R.B.H.; Lim, J.S.K.; Greenfield, H. Composition of Australian foods. Tomato Food Thechnol. Aust. 1984, 36, 78–80. [Google Scholar]
- Charfi, A.; Jang, H.; Kim, J. Membrane fouling by sodium alginate in high salinity conditions to simulate biofouling during seawater desalination. Bioresour. Technol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Lokare, O.R.; Tavakkoli, S.; Wadekar, S.; Khanna, V.; Vidic, R.D. Fouling in direct contact membrane distillation of produced water from unconventional gas extraction. J. Membr. Sci. 2017, 524, 493–501. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S. Membrane fouling and wetting in membrane distillation and their mitigation by novel membranes with special wettability. Water Res. 2017, 112, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Chu, H.; Yu, Z.; Jiang, S.; Zhao, X.; Zhou, X.; Zhang, Y. The filtration and fouling performance of membranes with different pore sizes in algae harvesting. Sci. Total Environ. 2017. [Google Scholar] [CrossRef] [PubMed]
- Girão, A.V.; Caputo, G.; Ferro, M.C. Application of Scanning Electron Microscopy–Energy Dispersive X-Ray Spectroscopy (SEM-EDS). Compr. Anal. Chem. 2017, 75. [Google Scholar] [CrossRef]
- Jafarzadeh, Y.; Yegani, R.; Sedaghat, M. Preparation, characterization and fouling analysis of ZnO/polyethylene hybrid membranes for collagen separation. Chem. Eng. Res. Des. 2015, 94, 417–427. [Google Scholar] [CrossRef]
- Shirazi, S.; Lin, C.J.; Chen, D. Inorganic fouling of pressure-driven membrane processes—A critical review. Desalination 2010, 250, 236–248. [Google Scholar] [CrossRef]
- Liu, Z.; Ohsuna, T.; Sato, K.; Mizuno, T.; Kyotani, T.; Nakane, T.; Terasaki, O. Transmission electron microscopy observation on fine structure of zeolite NaA membrane. Chem. Mater. 2006, 18, 922–927. [Google Scholar] [CrossRef]
- Heinzl, C.; Ossiander, T.; Gleich, S.; Scheu, C. Transmission electron microscopy study of silica reinforced polybenzimidazole membranes. J. Membr. Sci. 2015, 478, 65–74. [Google Scholar] [CrossRef]
- Taheri, M.L.; Stach, E.A.; Arslan, I.; Crozier, P.A.; Kabius, B.C.; LaGrange, T.; Minor, A.M.; Takeda, S.; Tanase, M.; Wagner, J.B.; et al. Current status and future directions for in situ transmission electron microscopy. Ultramicroscopy 2016, 170, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; McLoughlin, P.; Cathcart, H. Characterisation of dry powder inhaler formulations using atomic force microscopy. A Review. Int. J. Pharm. 2015, 494, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Khulbe, K.C.; Feng, C.Y.; Matsuura, T. Synthetic Polymeric Membranes: Characterization by Atomic Force Microscopy; Springer: Heidelberg, Germany, 2008. [Google Scholar]
- Bowen, W.R.; Hilal, N. Atomic Force Microscopy in Process Engineering: An Introduction to AFM for Improved Processes and Products; Elsevier: Oxford, UK, 2009. [Google Scholar]
- Zarebska, A.; Amor, Á.C.; Ciurkot, K.; Karring, H.; Thygesen, O.; Andersen, T.P.; Hägg, M.-B.; Christensen, K.V.; Norddahl, B. Fouling mitigation in membrane distillation processes during ammonia stripping from pig manure. J. Membr. Sci. 2015, 484, 119–132. [Google Scholar] [CrossRef]
- Yun, M.A.; Yeon, K.M.; Park, J.S.; Lee, C.H.; Chun, J.; Lim, D.J. Characterization of biofilm structure and its effect on membrane permeability in MBR for dye wastewater treatment. Water Res. 2006, 40, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Canette, A.; Briandet, R. Confocal Laser Scanning Microscopy. Agro ParisTech 2014, 2, 1389–1396. [Google Scholar]
- Yuan, B.; Wang, X.; Tang, C.; Li, X.; Yu, G. In situ observation of the growth of biofouling layer in osmotic membrane bioreactors by multiple fluorescence labeling and confocal laser scanning microscopy. Water Res. 2015, 75, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, M.; Rrzek, A.; Zator, M.; Lopez, F.; Guell, C. An approach to membrane fouling characterization by confocal scanning laser microscopy. J. Membr. Sci. 2005, 250, 283–293. [Google Scholar] [CrossRef]
- West, S.; Horn, H.; Hijnen, W.A.M.; Castillo, C.; Wagner, M. Confocal laser scanning microscopy as a tool to validate the efficiency of membrane cleaning procedures to remove biofilms. Sep. Purif. Technol. 2014, 122, 402–411. [Google Scholar] [CrossRef]
- Spettmann, D.; Eppmann, S.; Flemming, H.-C.; Wingender, J. Visualization of membrane cleaning using confocal laser scanning microscopy. Desalination 2008, 224, 195–200. [Google Scholar] [CrossRef]
- Courel, M.; Tronel-Peyroz, E.; Rios, G.M.; Dornier, M.; Reynes, M. The problem of membrane characterization for the process of osmotic distillation. Desalination 2001, 140, 15–25. [Google Scholar] [CrossRef]
- Guillen-Burrieza, E.; Ruiz-Aguirre, A.; Zaragoza, G.; Arafat, H.A. Membrane fouling and cleaning in long term plant-scale membrane distillation operations. J. Membr. Sci. 2014, 468, 360–372. [Google Scholar] [CrossRef]
- Sanmartino, J.A.; Khayet, M.; García-Payo, M.C.; El Bakouri, H.; Riaza, A. Desalination and concentration of saline aqueous solutions up to supersaturation by air gap membrane distillation and crystallization fouling. Desalination 2016, 393, 39–51. [Google Scholar] [CrossRef]
- Bagavathiappan, S.; Lahiri, B.B.; Saravanan, T.; Philip, J.; Jayakumar, T. Infrared thermography for condition monitoring—A review. Infrared Phys. Technol. 2013, 60, 35–55. [Google Scholar] [CrossRef]
- Marinetti, S.; Cesaratto, P.G. Emissivity estimation for accurate quantitative thermography. NDT & E Int. 2012, 51, 127–134. [Google Scholar]
- Ndukaife, K.O.; Ndukaife, J.C.; Agwu Nnanna, A.G. Membrane fouling characterization by infrared thermography. Infrared Phys. Technol. 2015, 68, 186–192. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Hou, Y.; Gao, Y.; Li, J.; Guo, W.; Ngo, H.H. In situ investigation of combined organic and colloidal fouling for nanofiltration membrane using ultrasonic time domain reflectometry. Desalination 2015, 362, 43–51. [Google Scholar] [CrossRef]
- Mairal, A.P.; Greenberg, A.R.; Krantz, W.B.; Bond, L.J. Real time measurement of inorganic fouling of RO desalination membranes using ultrasonic time domain reflectometry. J. Membr. Sci. 1999, 159, 185–196. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Greenberg, A.R.; Krantz, W.B.; Chai, G.Y. Study of membrane fouling and cleaning in spiral wound modules using ultrasonic time-domain reflectometry. In New Insights into Membrane Science and Technology Polymeric, Inorganic and Biofunctional Membranes; Butterfield, A.A., Bhattacharyya, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 65–88. [Google Scholar]
- Taheri, A.H.; Sim, S.T.V.; Sim, L.N.; Chong, T.H.; Krantz, W.B.; Fane, A.G. Development of a new technique to predict reverse osmosis fouling. J. Membr. Sci. 2013, 448, 12–22. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Li, H.; Cai, Y.; Cao, Y.; He, B.; Zhang, Y. Non-invasive monitoring of fouling in hollow fiber membrane via UTDR. J. Membr. Sci. 2009, 326, 103–110. [Google Scholar] [CrossRef]
- Tung, K.-L.; Teoh, H.-C.; Lee, C.-W.; Chen, C.-H.; Li, Y.-L.; Lin, Y.-F.; Chen, C.-L.; Huang, M.-S. Characterization of membrane fouling distribution in a spiral wound module using high-frequency ultrasound image analysis. J. Membr. Sci. 2015, 495, 489–501. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: London, UK, 1981. [Google Scholar]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. A review. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef] [PubMed]
- Erickson, D.; Li, D. Streaming Potential and Streaming Current Methods for Characterizing Heterogeneous Solid Surfaces. J. Colloid Interface Sci. 2001, 237, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kodama, S.; Goto, T. X-ray Diffraction Studies on the Deformation and Fracture of Solids; Elsevier: Amsterdam, The Netherlands, 1993; Volume 10. [Google Scholar]
- Kim, J.; Kwon, H.; Lee, S.; Lee, S.; Hong, S. Membrane distillation (MD) integrated with crystallization (MDC) for shale gas produced water (SGPW) treatment. Desalination 2017, 403, 172–178. [Google Scholar] [CrossRef]
- Melián-Martel, N.; Sadhwani, J.J.; Malamis, S.; Ochsenkühn-Petropoulou, M. Structural and chemical characterization of long-term reverse osmosis membrane fouling in a full scale desalination plant. Desalination 2012, 305, 44–53. [Google Scholar] [CrossRef]
- Beckhoff, B.; Kanngießer, B.; Langhoff, N.; Wedell, R.; Wolff, H. Handbook of Practical X-ray Fluorescence Analysis; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Thygesen, O.; Hedegaard, M.A.B.; Zarebska, A.; Beleites, C.; Krafft, C. Membrane fouling from ammonia recovery analyzed by ATR-FTIR imaging. Vib. Spectrosc. 2014, 72, 119–123. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Białończyk, L. Influence of proteins content in the feed on the course of membrane distillation. Desalin. Water Treat. 2013, 51, 2362–2367. [Google Scholar] [CrossRef]
- Nguyen, Q.-M.; Jeong, S.; Lee, S. Characteristics of membrane foulants at different degrees of SWRO brine concentration by membrane distillation. Desalination 2017, 409, 7–20. [Google Scholar] [CrossRef]
- Matthews, B.J.H.; Jones, A.C.; Theodorou, N.K.; Tudhope, A.W. Excitation-emission-matrix fluorescence spectroscopy applied to humic acid bands in coral reefs. Mar. Chem. 1996, 55, 317–332. [Google Scholar] [CrossRef]
- Huang, W.; Chu, H.; Dong, B.; Liu, J. Evaluation of different algogenic organic matters on the fouling of microfiltration membranes. Desalination 2014, 344, 329–338. [Google Scholar] [CrossRef]
- Messaud, F.A.; Sanderson, R.D.; Runyon, J.R.; Otte, T.; Pasch, H.; Williams, S.K.R. An overview on field-flow fractionation techniques and their applications in the separation and characterization of polymers—A review. Polym. Sci. 2009, 34, 351–368. [Google Scholar]
- Giddings, J.C. Field-flow fractionation: Analysis of macromolecular, colloidal, and particulate materials. Science 1993, 260, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Shon, H.K.; Cho, J. Biofouling characteristics using flow field-flow fractionation: Effect of bacteria and membrane properties. Bioresour. Technol. 2010, 101, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Karakulski, K.; Gryta, M.; Sasim, M. Production of process water using integrated membrane processes. Chem. Pap. 2006, 60, 416–421. [Google Scholar] [CrossRef]
- Schäfer, A.; Fane, A.; Waite, T. Nanofiltration: Principles and Applications; Elsevier: Oxford, UK, 2005. [Google Scholar]
- Karakulski, K.; Gryta, M.; Morawski, A. Membrane processes used for potable water quality improvement. Desalination 2002, 145, 315–319. [Google Scholar] [CrossRef]
- Bailey, A.F.G.; Barbe, A.M.; Hogan, P.A.; Johnson, R.A.; Sheng, J. The effect of ultrafiltration on the subsequent concentration of grape juice by osmotic distillation. J. Membr. Sci. 2000, 164, 195–204. [Google Scholar] [CrossRef]
- Song, L.; Ma, Z.; Liao, X.; Kosaraju, P.B.; Irish, J.R.; Sirkar, K.K. Pilot plant studies of novel membrane sand devices for direct contact membrane distillation-based desalination. J. Membr. Sci. 2008, 323, 257–270. [Google Scholar] [CrossRef]
- Jansen, A.E.; Assink, J.W.; Hanemaaijer, J.H.; Medevoort, J.v.; Sonsbeek, E.v. Development and pilot testing of full-scale membrane distillation modules for deployment of waste heat. Desalination 2013, 323, 55–65. [Google Scholar] [CrossRef]
- Gryta, M.; Karakulski, K.; Tomaszewska, M.; Morawski, A. Treatment of effluents from the regeneration of ion exchangers using the MD process. Desalination 2005, 180, 173–180. [Google Scholar] [CrossRef]
- Gryta, M. Desalination of thermally softened water by membrane distillation process. Desalination 2010, 257, 30–35. [Google Scholar] [CrossRef]
- Wang, J.; Qua, D.; Tie, M.; Ren, H.; Peng, X.; Luan, Z. Effect of coagulation pretreatment on membrane distillation process for desalination of recirculating cooling water. Sep. Purif. Technol. 2008, 64, 108–115. [Google Scholar] [CrossRef]
- Kucera, J. Reverse Osmosis: Design, Processes, and Applications for Engineers; Wiley: New York, NY, USA, 2010. [Google Scholar]
- Prihasto, N.; Liu, Q.; Kim, S. Pre-treatment strategies for seawater desalination by reverse osmosis system. Desalination 2009, 249, 308–316. [Google Scholar] [CrossRef]
- Ketrane, R.; Saidant, R.; Gil, O.; Leleyter, L.; Baraud, F. Efficiency of five scale inhibitors on calciumcarbonate precipitation fromhardwater: Effect of temperature and concentration. Desalination 2009, 249, 1397–1404. [Google Scholar] [CrossRef]
- He, F.; Sirkar, K.K.; Gilron, J. Effects of antiscalants to mitigate membrane scaling by direct contact membrane distillation. J. Membr. Sci. 2009, 345, 53–58. [Google Scholar] [CrossRef]
- Franken, A.C.M.; Nolten, J.A.M.; Mulder, M.H.V.; Bargeman, D.; Smolders, C.A. Wetting criteria for the applicability of membrane distillation. J. Membr. Sci. 1987, 33, 315–328. [Google Scholar] [CrossRef]
- Al-Shammiri, M.; Safar, M.; Al-Dawas, M. Evaluation of Two Different Antisclant in Real Operation at the Doha. Res. Plant 2000, 128, 1–16. [Google Scholar]
- Razmjou, A.; Arifin, E.; Dong, G.; Mansouri, J.; Chen, V. Superhydrophobic modification of TiO2 nanocomposite PVDF membranes for applications in membrane distillation. J. Membr. Sci. 2012, 415–416, 850–863. [Google Scholar] [CrossRef]
- Xu, J.B.; Lange, S.; Bartley, J.P.; Johnson, R.A. Alginate-coated microporous PTFE membranes for use in the osmotic distillation of oily feeds. J. Membr. Sci. 2004, 240, 81–89. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, R. Novel membrane surface modification to enhance anti-oil fouling property for membrane distillation application. J. Membr. Sci. 2013, 447, 26–35. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Z.; Li, B.; Wang, Q.; Wang, S. Fabrication and characterization of superhydrophobic poly (vinylidene fluoride) membrane for direct contact membrane distillation. Desalination 2013, 324, 1–9. [Google Scholar] [CrossRef]
- Al-Amoudi, A.; Lovitt, R.W. Fouling strategies and the cleaning system of NF membranes and factors affecting cleaning efficiency. J. Membr. Sci. 2007, 303, 4–28. [Google Scholar] [CrossRef]
- Curcio, E.; Ji, X.; Di Profio, G.; Sulaiman, A.O.; Fontananova, E.; Drioli, E. Membrane distillation operated at high seawater concentration factors: Role of the membrane on CaCO3 scaling in presence of humic acid. J. Membr. Sci. 2010, 346, 263–269. [Google Scholar] [CrossRef]
- Krivorot, M.; Kushmaro, A.; Oren, Y.; Gilron, J. Factors affecting biofilm formation and biofouling in membrane distillation of seawater. J. Membr. Sci. 2011, 376, 15–24. [Google Scholar] [CrossRef]






| Membrane Material and Type | dp (µm) | ε (%) | δ (µm) | Foulant(s) | Tf/Tp (°C) | v (m·s−1) | Jp (kg·m−2·h−1) | FDn (%) | Observations | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| PTFE Flat-sheet | 0.1 | - | 30 | Organic fouling: sludge and brown spots | 40–70/10 | 0.005–0.014 | 1.41–9.22 | 70% at 12g/L 50% at 6 g/L | The feed was from a thermophilic anaerobic membrane bioreactor. After cleaning using deionized (DI) water 15 mg/L of NaOH, the membrane could recover 96% of initial Jp. | [36] |
| 0.5 | - | 20 | Skim milk, Whey proteins | 54/5 | 0.047 | 3 | Skim milk: 85% Whey: 20% | Membrane wetting was not observed even after 20 h operation. | [37] | |
| 0.2 | 80 | 60 | Traditional Chinese medicine (mostly inorganic salt, such as CaCO3) | 60/25 | 0.07–0.13 | 32.78 | 30% at 1.5 g/L | Fouling layer can be effectively limited by increasing either the feed temperature or feed flow velocity. | [38] | |
| 0.2 | 70–80 | 179 | Humid Acid (HA) | 70/25 | 1.1 | 35.7 | 60% | Seawater organic fouling was irreversible in DCMD. CaSO4 reduced the disaggregation of humic substances due to the binding effect. | [39] | |
| 0.5 | - | 20 | Skim milk Whey proteins | 54/5 | 0.047 | 22 | Skim milk: 79% Whey: 11% | Whey proteins had weaker attractive interaction with the membrane and adhesion depended more on the presence of phosphorus near the membrane surface. | [12] | |
| 0.2 | 70–80 | 179 | HA, alginate acid (AA) and bovine serum albumin (BSA) | 50, 70/24 | 1.1 | 35 | HA: 56.2% AA: 44.1% BSA 64.5% | Feed concentration: 10 mg·C·L−1. | [40] | |
| 0.2 | 65 | 41 | NaCl, MgSiO3, MgCO3 CaCO3 | 30–50/24 | 0.32 | ~28, at ΔT = 15 °C | 28% | The PTFE membrane surfaces showed some salt scaling and a larger population of crystals. | [41] | |
| PVDF Flat-sheet | 0.2 | 80 | 197 | ~5, at ΔT = 15 °C | 32% | PVDF membranes showed a smaller population of salt crystals on their surface. | ||||
| 0.45 | 60 | 127 | ~32, at ΔT = 15 °C | 20% | The salt crystals formed were larger than the pore size of the membranes. | |||||
| 0.22 | 0.75 | 125 | HA | 50, 70/20 | 0.23 | 30.6 to 35.1 | 5% | The addition of divalent cations (Ca2+) affected to permeate flux by forming complexes with HA. | [42] | |
| PVDF Hollow fiber | 0.16 | 90.8 | 200 | CaSO4 (RO brine) | 55–77/35 | 0.011 | 2.5–5.8 | 30% | Membrane wetting was more significant at high feed temperatures. Salts promoted membrane pore wetting. | [43] |
| 0.088 | 83.7 | 126 | Rubber wastewater | 55.5/20.0 | - | 7.19 | 79% | Permeate flux decline was due to the presence of complex components (e.g., latex and protein in the rubber effluent). | [44] | |
| PP Flat-sheet | 0.1 | 65–70 | 100 | CaCO3, CaSO4, silica | 60/20 | φ: 0.6/0.5 L·min−1 | 30 | Feed A: 93% Feed B: 27% | Membrane scaling caused a drop of permeate flux and a decrease in salt rejection. Feed A: NaHCO3 + Na2SO4, + CaCl2; Feed B: Na2SiO3·9H2O. | [45] |
| PP Hollow fiber | 0.22 | 73 | 400 | CaCO3 | 85, 90/20 | F: 0.15–0.63 P: 0.12 | 25–38.7 | 40% without antiscalant 20% with antiscalant 0% Rising with HCl | The application of antiscalant minimized the penetration of salts into the pores. A high permeate flux was maintained over 260 h of operation using periodical rinsing with HCl solution | [46] |
| 0.1 0.2 0.6 | 50 60–80 | 150 50 52.5 | CaCO3 and CaCO3–CaSO4 | 70–75–80/20 | φ: 0.084–0.688–1.438 L·min−1 | Uncoated: 14.3–4.8 Coated: 5.5 | 11% | Fluorosilicone coating was proven to be helpful to eliminate membrane scaling. The scaling problem was successfully solved by HCl acidification prior to MD | [35] | |
| PP Capillary | 0.22 | 73 | 400 | CaCO3 | 60–80/50 | F: 0.42–0.96 P: 0.29 | 27.9–22.9 | 10% | Reduction of the number and dimensions of the pores on the membranes surfaces. Large pores were wetted because of CaCO3 depostion inside the pores. Flow rate of distillate was constant. | [47] |
| 0.22 | 73 | 400 | Protein and NaCl (50 g/L) | 85/20 | φ: 0.84 L·min−1 | 12.5 | 25% NaCl up to saturation 0% Boiling feed | The feed solution was NaCl solution containing natural organic matter. Pre-treatment method of the feed does not result in the complete removal of the foulants. | [48] | |
| 0.22 | 73 | 400 | Mainly CaCO3 | 80, 90/20 | F: 0.3–1.4 P: 0.29 | 30.8 (Tf = 80 °C) | 41% at 0.31 m/s 12% at 1.4 m/s | The application of tap water as a feed caused a rapid decline of permeate flux due to the deposition of CaCO3. | [49] | |
| 0.22 | 72 | 200 | NaCl | 70-85/20 | φ: 0.42 L·min−1 | 27.5 | 19% at 1 year 28% at 9 years | Chemical reaction of salt with the hydroxyl and carbonyl groups found on the PP surface. Degradation time dependence. | [50] |
| MD Config. | Membrane Material and Type | dp (µm) | ε (%) | δ (µm) | Foulant | Tf/Tp (°C) pv (kPa) | v (m·s−1) | Jp (kg·m−2·h−1) | FDn (%) | Observations | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AGMD | PTFE Flat-sheet | 1 | 0.85 | 150 | Salts deposition | 77/12 | φ: F: 58 g/s C: 75 g/s | 119 | - | Feed: tap water. | [51] |
| 0.2 | - | 175 | NaCl, MgCl2, Na2CO3, Na2SO4 | 50/10 | φ: F: 1.5 L·min−1 | NaCl: 1.02 Na2SO4: 0.38 Na2CO3: 0.12 | 0% after 5 h | The permeate flux declined as the concentration of salt increased, and increased as the pore size increased. | [1] | ||
| 0.45 | - | 175 | NaCl, MgCl2, Na2CO3, Na2SO4 | 50/10 | φ: F: 1.5 L·min−1 | NaCl: 1.45 Na2SO4: 0.56 Na2CO3: 0.19 | 0% after 5 h | ||||
| VMD | 0.22 | 40 | 175 | CaCO3, CaSO4 organic matter | 25–75 pv: 0.1–10 | 0.4-2.0 | 9.3–8.3 | 24% | Synthetic RO brine feed, Salt concentration: 300 g·L−1. | [53] | |
| 0.2 | - | 50 | Ginseng aqueous solution (polysaccharide, amino acid & biomacromolecule) | 60 pv: 83–89.5 | 0.74 to 0.46 | 24.7 to 21.6 | From 0% to 27% | The results showed the existence of critical fouling operating conditions in VMD process. | [54] | ||
| PVDF Hollow fiber | 0.25 | 79 | 150 | CaCO3 | 52–68 pv: 96 | 0.14 | 8.96–21 (13.43–25 with microwave) | - | Microwave irradiation increased the deposition of calcium carbonate. | [55] | |
| 0.16 | 82–85 | - | Mainly hardness and organic matter | 70 pv: 8.5 | 1 | - | - | The permeate flux was 30% higher when using pretreatment. | [56] | ||
| PP Shell and tube | 0.2 | - | 1500 | Dye and Nacl | 40–70 pv: 0.667 | 0.84 to 3.42 | 8.2 | 95% | The flux was dependent strongly on the feed temperature but was independent of salt concentration. | [57] | |
| PP Flat-sheet | 0.2 | - | - | Inorganic elements: O, S, Fe, Na, Mg, K. Microorganisms and proteins, | 40 °C | φ: F: 4 L·min−1 Strip flow rate: 3 L·min−1 | 42 | 95% | The fouling layer thickness was estimated to 10–15 µm and it becomes severe as the membrane surface changes from hydrophobic to hydrophilic. | [58] | |
| PP Capillary | 0.2 | 45 | 510 | Dye | 40, 50, 60 pv: 1.0 | 0.78–1.67 | 27.5–35–57.0, respectively | 27% | The flux decrease probably due to an interaction with the polymeric membrane. | [59] |
| OD/OMD | Membrane Material and Type | dp (µm) | Ε (%) | δ (µm) | Foulant (s) | Tf/Tp (°C) | v (m·s−1) | Jp (kg·m−2·h−1) | FDn (%) | Observations | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OD | PTFE Flat-sheet | 0.2 0.45 1 | 80 | 178 | Phenolic compounds from crude olive mill wastewater | 30 | 500 rpm | 2.64–2.23 3.01–2.64 3.86–2.85 | 15% 13% 26% | The decrease of the permeate flux with time is more obvious for the membrane which pore size of 1 µm. | [81] |
| 0.2 | 78 | 8.5 | Tomato puree | 20–24 | φ: 0.5 L·min−1 | 1.25 for 6 wt % 0.7 for 21.5 wt % | - | Tomatoes are composed of 95% water, 3–4% carbohydrate, 0.51% protein and 0.1–0.3% fat. | [82] | ||
| 0.2 0.45 1.2 | - | - | Phenolic content of red grape juice | 35 | - | 8–4 | 84% | Initial juice concentration 5 °Brix. Initial concentration of stripping solution 50 wt % CaCl2. | [83] | ||
| PVDF Hollow fiber | 0.2 | 75 | 125 | Glucose | 25, 35, 45 | 0.4; 0.6; 0.8 | 1.67 to 4.73 Concentration factor: From 30 to 40 °Brix | - | Feed concentration and brine velocity have significant effect on OD permeate flux (while the brine concentration was remained constant) Their effects depend on the range of the feed concentration. | [4] | |
| 0.2 | 64 | 170 | Glucose | 25, 35, 45 | 0.2; 0.4; 0.5 | 1.00 to 2.87 Concentration factor: From 45 to 60 °Brix | - | ||||
| PP | 0.2 | - | 125 | Apple juice | 23–33 | φ: 10 L·min−1 | From 2.25 to 0.9 | 71% | OD can be almost free from fouling due to the hydrophobic OD membrane. Few substances (e.g., fat and wax) may stick to the membrane surface. | [84] | |
| OMD | PTFE Flat-sheet | 0.2 | 80 | 178 | Phenolic compounds from crude olive mill wastewater | 40/20 | 500 rpm | From 3.9 to 1.3 | 13.5% | Membrane fouling is of less importance when using OMD by PTFE membranes. | [81] |
| PP Hollow fiber | 0.04 | 40 | 40 | Crystals formed from Na2CO3 | 40/20 | φ: 1.4, 0.6, 0.45 L·min−1 | From 0.12 to 0.078 | 30% | Membrane scaling was observed is due of the accumulation of crystals on the membrane surface. | [86] |
| Pretreatment | MD/OD Set Up | Feed Solution | Species Addressed | Observation | Ref. |
|---|---|---|---|---|---|
| UF, NF, RO | DCMD | Surface water (lake) | Organic compounds, suspended solids, colloids |
| [139] |
| MF | DCMD | Hot brine City water contaning salt (3.5, 6 or 10%) or Seawater | Organic compounds, Colloids and bacteria | Hydrophobicity of the outside membrane surface was reduced. Very limited flux reduction at salts concentration up to 19.5% from seawater. | [141] |
| UF | OD | Grape juice | Fermentable sugars and proteins | Increased flux during subsequent concentration of the permeate by OD and wetting reduction because of increase in juice surface tension. | [140] |
| MF followed by degassing | LGMD | Polluted seawater | Salts, oil, silt, sludge and unknown organic compounds | Distillate with high quality and salt separation factors well above 10.000 have been obtained. | [142] |
| pH contol using HCl at pH = 4.1 | DCMD | Tap water, CaCO3 and mixed CaCO3/CaSO4 solutions | Hardness CaCO3/CaSO4 | Quite stable vapor flux during the experiment time (7 h). | [35] |
| Lime precipitation by Ca(OH)2, sedimentation and filtration | DCMD | Wastewater | CaSO4 and silicon compound | The fouling was significantly diminished. | [143] |
| Coagulation/flocculation and MF | DCMD | OMW | Solids and organic compounds (Phenolic compounds, Sugar and Proteins) | MF pre-treatment improved significantly the DCMD permeate flux compared to coagulation/flocculation pre-treatment. | [85] |
| Thermal softening followed by filtration | DCMD | Tap/ground/lake water | Hardness (bicarbonate) | HCO3 ions was lowered 2–3 times by keeping water at the boiling point for 15 min. | [144] |
| Sedimentation and UF Boiling and MF | DCMD | Bilge water/saline wastewater | Hardness, organic compounds and proteins | Sedimentation and UF showed a significant flux decline. But boiling and MF pretreatment avoided the rapid flux decline. | [33] |
| Coagulation, filtration, acidification and degasification | DCMD | Recirculating Cooling Water | organic matter (NOM), total phosphorus (TP) and suspended substance (SS) | About 23% of improvement of flux was obtained by using coagulation pretreatment after 30 days of operation. | [145] |
| UF with coagulation | VMD | RO-concentrated wastewater from steel plant | Mainly hardness and Organic matter | The flux was 30% higher when the pretreatment was used and the CODcr removal reached 40%. | [56] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laqbaqbi, M.; Sanmartino, J.A.; Khayet, M.; García-Payo, C.; Chaouch, M. Fouling in Membrane Distillation, Osmotic Distillation and Osmotic Membrane Distillation. Appl. Sci. 2017, 7, 334. https://doi.org/10.3390/app7040334
Laqbaqbi M, Sanmartino JA, Khayet M, García-Payo C, Chaouch M. Fouling in Membrane Distillation, Osmotic Distillation and Osmotic Membrane Distillation. Applied Sciences. 2017; 7(4):334. https://doi.org/10.3390/app7040334
Chicago/Turabian StyleLaqbaqbi, Mourad, Julio Antonio Sanmartino, Mohamed Khayet, Carmen García-Payo, and Mehdi Chaouch. 2017. "Fouling in Membrane Distillation, Osmotic Distillation and Osmotic Membrane Distillation" Applied Sciences 7, no. 4: 334. https://doi.org/10.3390/app7040334






