Effects of Three Different Additives and Two Different Bulk Densities on Maize Silage Characteristics, Temperature Profiles, CO2 and O2–Dynamics in Small Scale Silos during Aerobic Exposure
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Ensiling of Material
2.2. Preparation of Buckets
2.3. Experimental Phase
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.E. Factors influencing Silage Quality and their implications for management. J. Dairy Sci. 1988, 71, 2992–3002. [Google Scholar] [CrossRef]
- Ranjit, N.K.; Kung, L. The Effect of Lactobacillus buchneri, Lactobacillus plantarum, or a Chemical Preservative on the Fermentation and Aerobic Stability of Corn Silage. J. Dairy Sci. 2000, 83, 526–535. [Google Scholar] [CrossRef]
- Rotz, C.A. How to maintain forage quality during harvest and storage. Adv. Dairy Technol. 2003, 15, 227–239. [Google Scholar]
- Wilkinson, J.M.; Davies, D.R. The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 2012, 68, 1–19. [Google Scholar] [CrossRef]
- Tabacco, E.; Righi, F.; Quarantelli, A.; Borreani, G. Dry matter and nutrition losses during aerobic deterioration of maize and sorghum silages as influenced by different lactic acid bacteria inocula. J. Dairy Sci. 2011, 94, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Stokes, M.; Lin, C. Silage Additives. In Agronomy Monograph No. 42: Silage Science and Technology; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2003; Chapter 7. [Google Scholar]
- Merry, R.J.; Davies, D.R. Propionibacteria and their role in the biological control of aerobic spoilage in silage. Lait 1999, 79, 149–164. [Google Scholar] [CrossRef]
- Kung, L. Aerobic Stability of Silage. In Proceedings of the California Alfalfa & Forage Symposium and Corn/Cereal Silage Conference, Visalia, CA, USA, 1–2 December 2010. [Google Scholar]
- Muck, R.E. Effects of Corn Silage Inoculants on Aerobic Stability. Trans. ASAE 2004, 47, 1011–1016. [Google Scholar] [CrossRef]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic Acid Increases Stability of Silage under Aerobic Conditions. Appl. Environ. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, Q.; Meng, F.; Buescher, W.; Maack, C.; Ross, F.; Lin, J. Image-based comparison between a c-ray scanner and a dual-sensor penetrometer technique for visual assessment of bale density distribution. Comput. Electron. Agric. 2012, 82, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xu, Q.F.; Gao, W.J.; Wang, Y.X. Harvesting corn silage at different maturity stages. In Proceedings of the XVII International Silage Conference, Piracicaba, Brazil, 1–3 July 2015. [Google Scholar]
- Hussin, G.; Nazli, M.H.; Samsudin, A.A.; Halim, R.A. Plastic container a practical silo for small silage producer in Malaysia. In Proceedings of the XVII International Silage Conference, Piracicaba, Brazil, 1–3 July 2015. [Google Scholar]
- Jungbluth, K.; Maack, C.; Büscher, W.; Sun, Y.; Cheng, Q.; Menghua, L.; Hong, C. A New Ex-Situ Method to Investigate Aerobic Stability of Maize Silage Faces. J. Agric. Sci. Food Technol. 2016, 2, 49–54. [Google Scholar]
- Wulf, S.; Maeting, M.; Clemens, J. Effect of application technique on the emission of trace gases (NH3, N2O, CH4) after speading cofermented slurry on arable and grassland. Part II. Greenhouse gas emissions. J. Environ. Qual. 2002, 31, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.M.; Harrison, J.H.; Davidson, D.; Mahanna, W.C.; Shinners, K.; Linder, D. Corn Silage Management: Effects of Maturity, Inoculation, and Mechanical Processing on Pack Density and Aerobic Stability. J. Dairy Sci. 2002, 85, 434–444. [Google Scholar] [CrossRef]
- Muck, R.E.; Moser, L.E.; Pitt, R.E. Postharvest factors affecting ensiling. In Agronomy Monograph No. 42: Silage Science and Technology; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2003. [Google Scholar]
- Maack, C.; Wagner, A.; Büscher, W. Einfluss der Partikelstruktur auf die Verdichtbarkeit verschiedener Siliergüter (Influence of particle composition on compressibility of different materials adapted for silage). In Proceedings of the 8th Conference on Construction, Engineering and Environment in Livestock Farming, Bonn, Germany, 9–10 October 2007; Association for Technology and Structures in Agriculture (KTBL): Frankfurt am Main, Germany, 2007; pp. 439–444. (In German). [Google Scholar]
- Köhler, B.; Diepolder, M.; Ostertag, J.; Thurner, S.; Spiekers, H. Dry matter losses of grass, lucerne and maize silages in bunker silos. Agric. Food Sci. 2013, 22, 145–150. [Google Scholar]
- Kristensen, N.B.; Sloth, K.H.; Højberg, O.; Spliid, N.H.; Jensen, C.; Thørgersen, R. Effects of microbial inoculants on corn silage fermentation, microbial contents, aerobic stability, and milk production under field conditions. J. Dairy Sci. 2010, 93, 3764–3774. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, F.; Oude Elferink, S.J.; Van Wikselaar, P.G. Fermentation Characteristics and aerobic stability of grass silage inoculated with Lactobacillus buchneri, with or without homofermentative lactic acid bacteria. Grass Forage Sci. 2001, 56, 330–343. [Google Scholar] [CrossRef]
- Ashbell, G.; Weinberg, Z.G. Top silage losses in horizontal silos. Can. Agric. Eng. 1992, 34, 171–175. [Google Scholar]
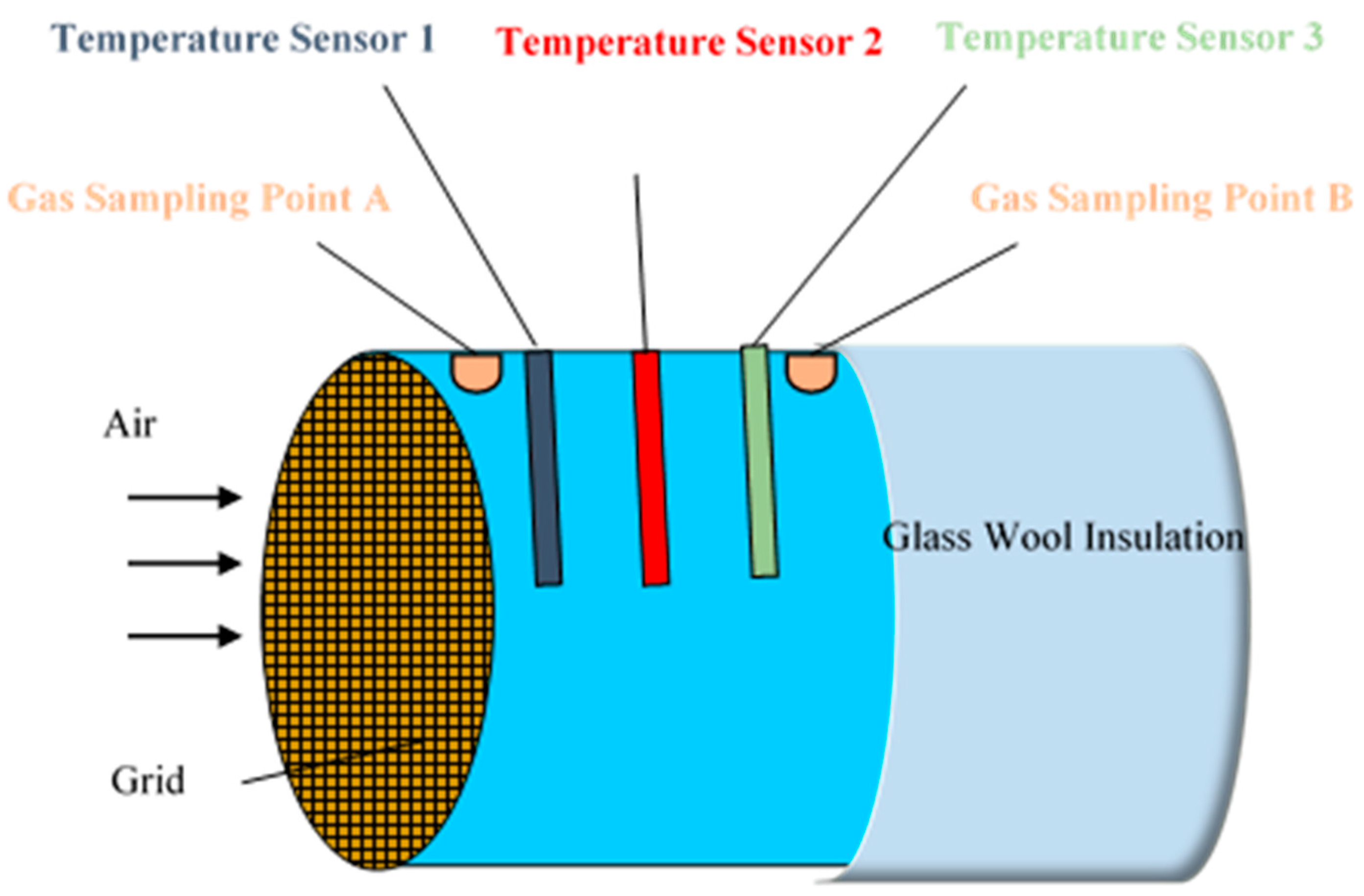
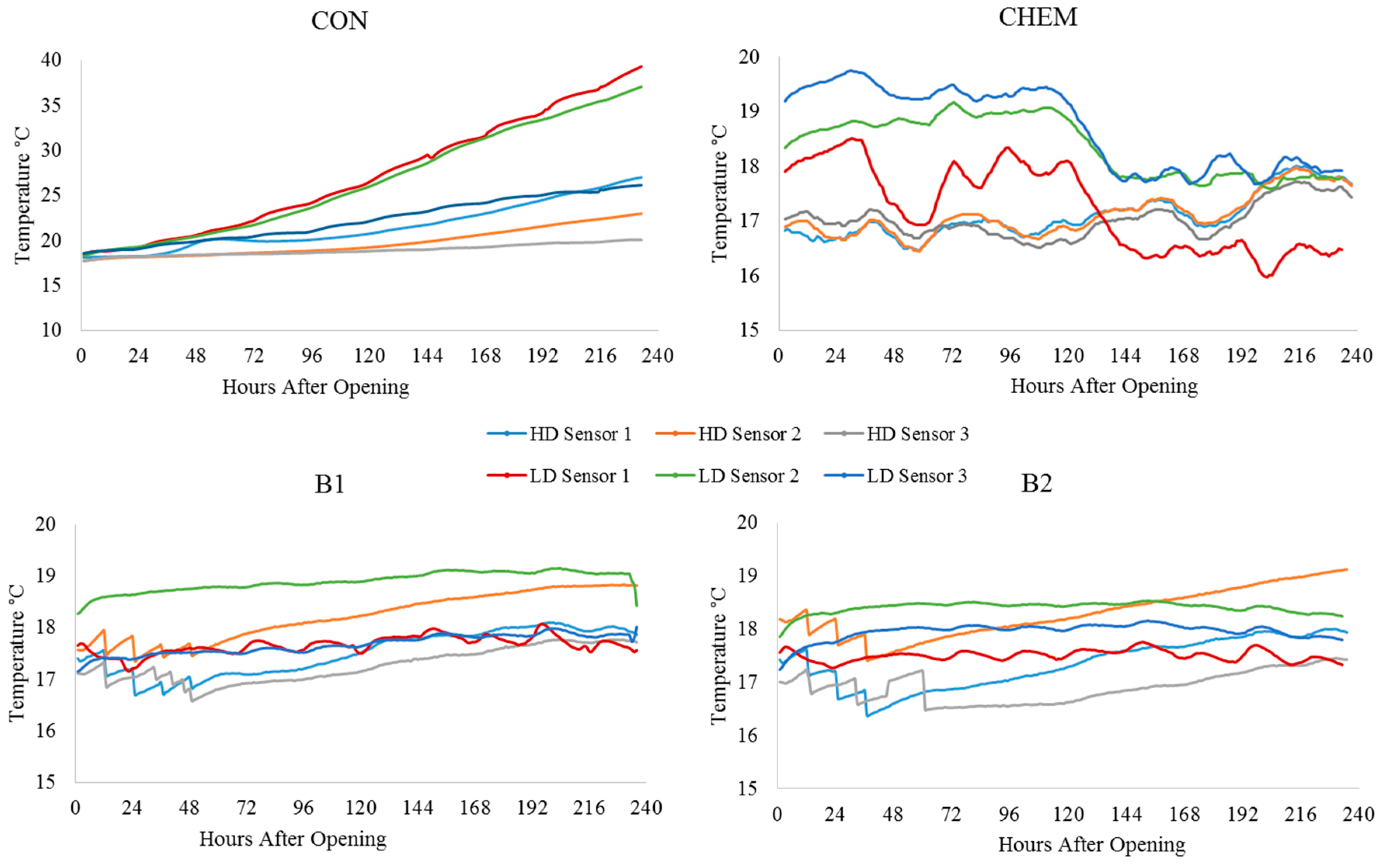
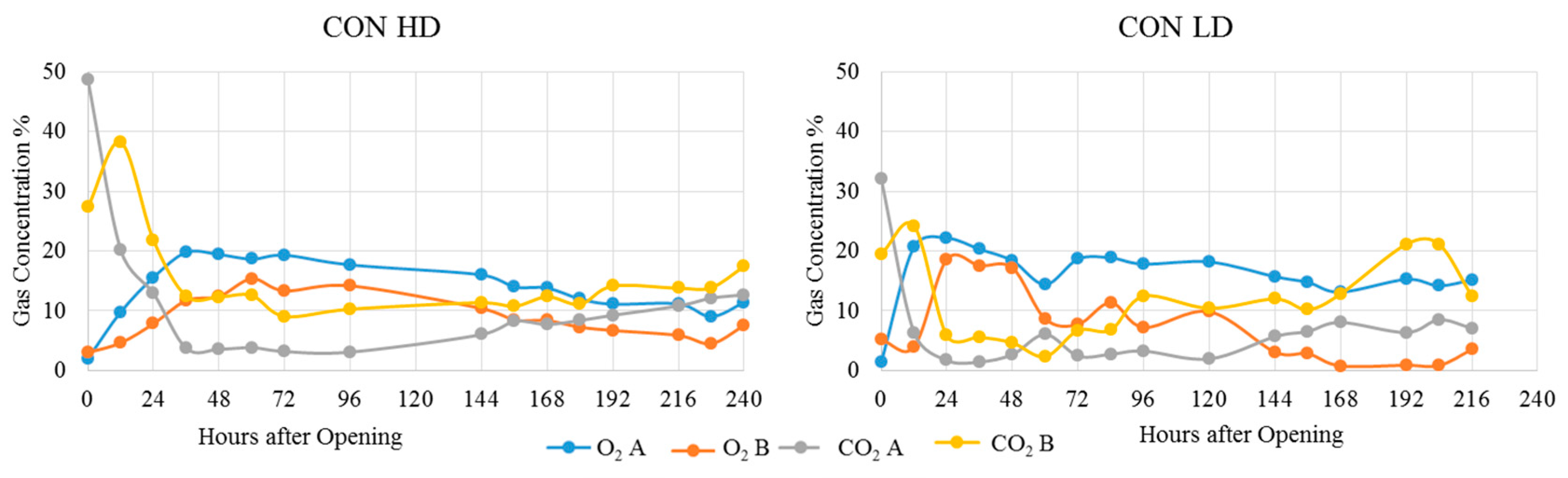
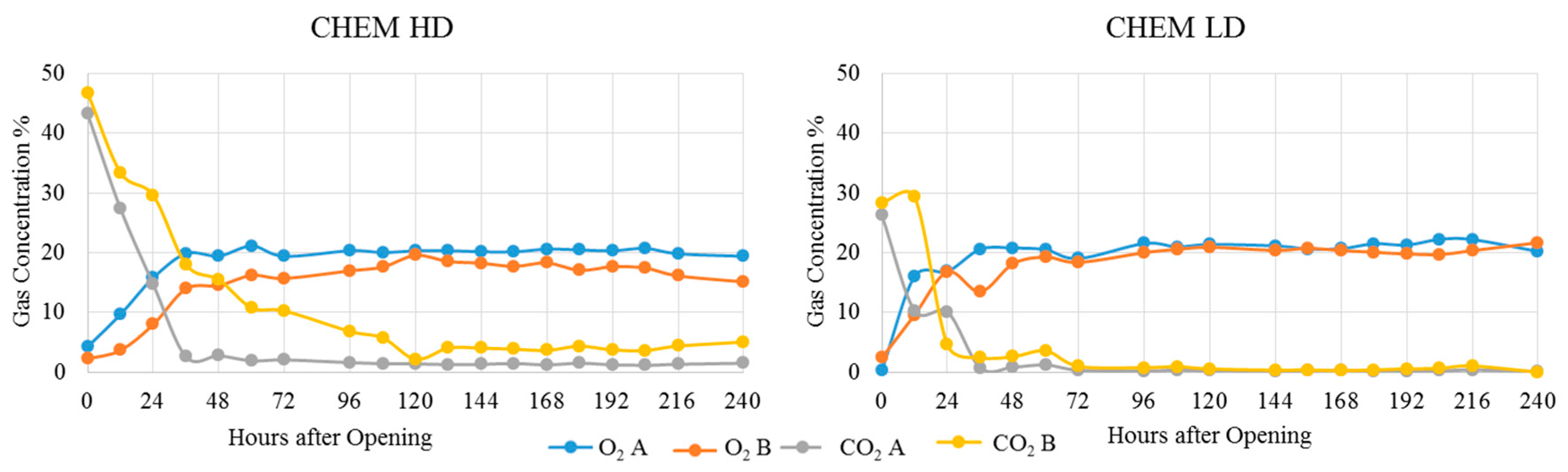
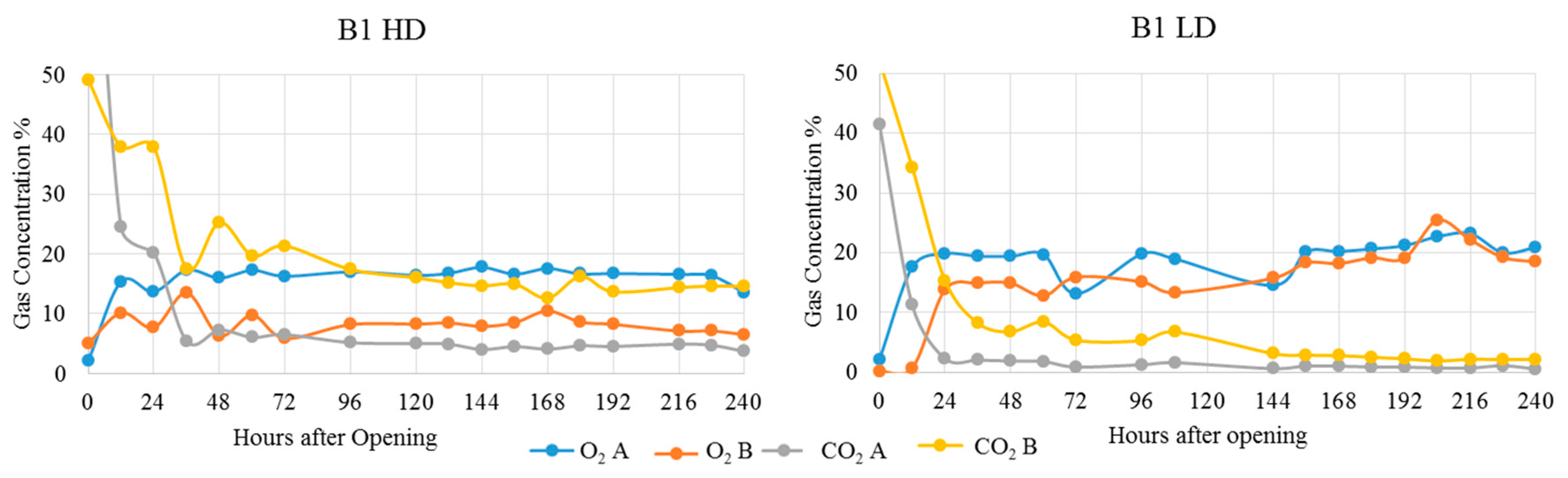
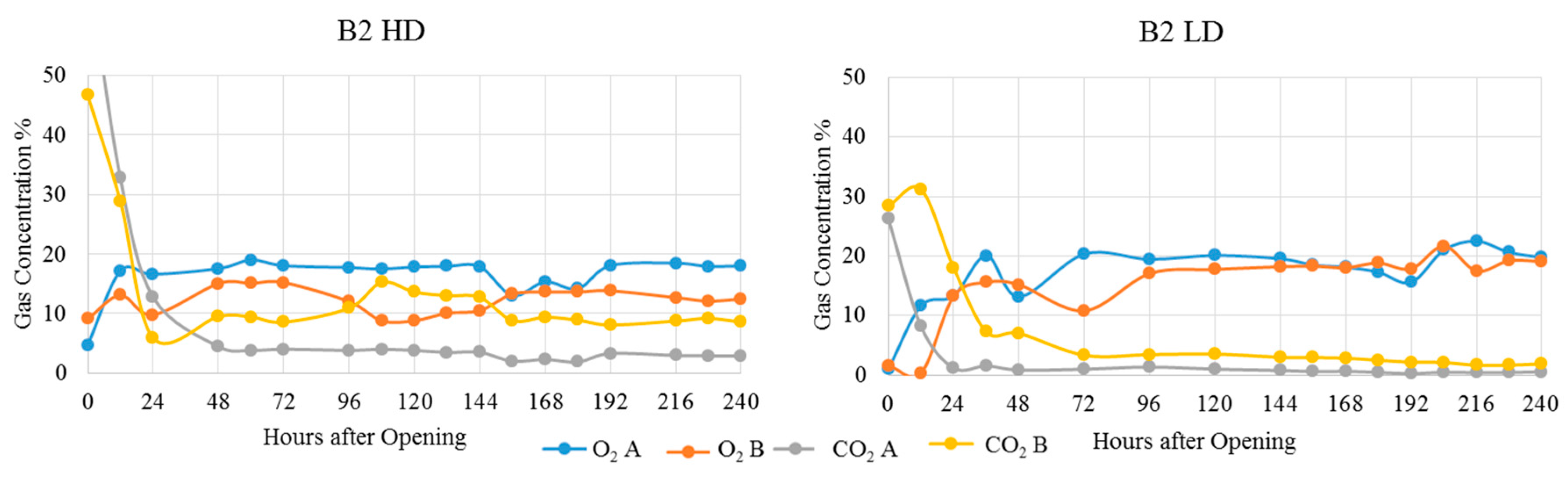
| Treatment | Position | Dry Matter g/kg | Crude Ash g/kg DM | Crude Protein g/kg DM | Crude Fibre g/kg DM | Crude Fat g/kg DM | Starch g/kg DM | pH | aNDFom g/kg DM | ME MJ/kg DM | NEL MJ/kg DM | Lactic Acid % of DM | Acetic Acid % of DM | Propionic Acid % of DM | Ethanol % of DM | 1,2-propandiol % of DM | 1-propanol % of DM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON HD | 0 | 379.6 a,b | 34.4 a | 82.3 a | 171.0 a | 30.6 a | 375.8 a | 4.1 a | 374.4 a,b | 11.4 a,b | 6.9 a,b | 3.4 a,b | 1.1 a | 0.0 a | 0.8 a | 0.0 a | 0.0 a |
| 1 | 377.1 a,b | 32.5 a | 75.6 a | 158.2 a | 32.3 a | 402.6 a | 4.2 a | 370.1 a,b | 11.5 a,b | 7.0 a,b | 2.6 a,b | 0.3 a | 0.0 a | 0.4 a | 0.0 a | 0.0 a | |
| 2 | 371.9 a,b | 31.4 a | 72.2 a | 168.7 a | 31.8 a | 384.4 a | 4.0 a | 371.2 a,b | 11.4 a,b | 7.0 a,b | 3.9 a,b | 0.8 a | 0.0 a | 0.6 a | 0.0 a | 0.0 a | |
| 3 | 372.2 a,b | 31.4 a | 74.9 a | 163.6 a | 31.8 a | 387.1 a | 4.0 a | 367.4 a,b | 11.6 a,b | 7.1 a,b | 4.1 a,b | 0.8 a | 0.1 a | 0.8 a | 0.0 a | 0.0 a | |
| CON LD | 0 | 368.7 a,b | 37.2 a | 84.2 a | 172.7 a | 32.4 a | 368.1 a | 4.1 a | 388.9 a,b | 11.4 a,b | 7.0 a,b | 3.5 a | 1.4 a | 0.2 a | 0.8 a,b | 0.0 a | 0.0 a |
| 1 | 406.6 a,b | 34.5 a | 78.3 a | 175.2 a | 31.0 a | 393.1 a | 4.6 a | 388.4 a,b | 11.3 a,b | 6.9 a,b | 1.9 a | 0.4 a | 0.0 a | 0.3 a,b | 0.0 a | 0.0 a | |
| 2 | 369.3 a,b | 31.3 a | 76.8 a | 161.9 a | 30.3 a | 399.7 a | 4.2 a | 357.0 a,b | 11.5 a,b | 7.1 a,b | 3.0 a | 0.6 a | 0.0 a | 0.6 a,b | 0.0 a | 0.0 a | |
| 3 | 356.6 a,b | 30.0 a | 72.4 a | 162.3 a | 29.9 a | 398.7 a | 4.0 a | 349.6 a,b | 11.5 a,b | 7.1 a,b | 4.3 a | 1.5 a | 0.0 a | 0.7 a,b | 0.0 a | 0.0 a | |
| B1 HD | 0 | 371.4 a,b | 37.3 a,b | 81.7 a | 175.7 a | 34.7 b | 392.7 a | 4.3 a | 416.3 b,c | 11.3 a | 6.8 a | 0.9 c | 2.8 b | 0.0 a | 0.7 a,b,c | 1.4b,c | 0.2 a,b,c |
| 1 | 389.3 a,b | 34.1 a,b | 80.3 a | 151.8 a | 35.2 b | 430.0 a | 4.3 a | 381.3 b,c | 11.5 a | 7.0 a | 1.1 c | 2.5 b | 0.0 a | 0.5 a,b,c | 0.9b,c | 0.2 a,b,c | |
| 2 | 371.2 a,b | 34.0 a,b | 81.9 a | 158.9 a | 31.4 b | 423.4 a | 4.1 a | 390.2 b,c | 11.4 a | 7.0 a | 0.9 c | 3.0 b | 0.0 a | 0.5 a,b,c | 0.5b,c | 0.4 a,b,c | |
| 3 | 355.2 a,b | 36.3 a,b | 81.5 a | 177.5 a | 33.4 b | 379.2 a | 4.1 a | 425.2 b,c | 11.2 a | 6.8 a | 1.5 c | 3.3 b | 0.0 a | 0.6 a,b,c | 0.0b,c | 1.2 a,b,c | |
| B1 LD | 0 | 362.9 a,c | 40.2 a,b | 84.0 a | 180.4 a | 35.3 b | 380.7 a | 4.3 a | 432.4 b,c | 11.2 a | 6.8 a | 0.3 c | 4.6 c | 0.6 b | 0.7 a | 0.4 a,b | 0.5 b,c |
| 1 | 364.1 a,c | 34.6 a,b | 74.5 a | 158.4 a | 35.0 b | 447.3 a | 4.5 a | 376.1 b,c | 11.5 a | 7.1 a | 0.9 c | 4.8 c | 0.5 b | 0.6 a | 0.4 a,b | 0.7 b,c | |
| 2 | 349.4 a,c | 34.1 a,b | 78.8 a | 155.3 a | 36.2 b | 443.1 a | 4.4 a | 373.1 b,c | 11.6 a | 7.1 a | 1.1 c | 4.7 c | 0.5 b | 0.8 a | 0.1 a,b | 1.4 b,c | |
| 3 | 356.6 a,c | 34.6 a,b | 76.6 a | 163.8 a | 36.0 b | 429.9 a | 4.4 a | 383.5 b,c | 11.4 a | 7.0 a | 0.9 c | 5.0 c | 0.6 b | 0.7 a | 0.3 a,b | 1.3 b,c | |
| B2 HD | 0 | 364.2 a,b | 36.8 b | 80.3 a | 169.4 a | 37.1 b | 410.7 a | 4.3 a | 402.3 c | 11.4 a | 7.0 a | 0.9 c | 3.0 b | 0.0 a | 0.7 a | 1.5 d | 0.4 a,b |
| 1 | 374.8 a,b | 36.5 b | 78.7 a | 166.7 a | 33.3 b | 424.1 a | 4.3 a | 400.8 c | 11.3 a | 6.9 a | 0.6 c | 3.2 b | 0.0 a | 0.6 a | 1.7 d | 0.4 a,b | |
| 2 | 366.1 a,b | 34.8 b | 81.1 a | 166.6 a | 35.1 b | 411.5 a | 4.3 a | 407.7 c | 11.4 a | 6.9 a | 1.2 c | 3.2 b | 0.2 a | 0.6 a | 0.9 d | 0.4 a,b | |
| 3 | 374.1 a,b | 37.5 b | 79.1 a | 172.1 a | 34.7 b | 403.6 a | 4.2 a | 406.1 c | 11.3 a | 6.9 a | 1.6 c | 3.0 b | 0.0 a | 0.8 a | 1.4 d | 0.5 a,b | |
| B2 LD | 0 | 351.0 c | 40.9 b | 81.9 a | 183.7 a | 33.2 b | 382.5 a | 4.4 a | 431.5 c | 11.1 a | 6.7 a | 0.6 c | 6.0 d | 1.0 c | 1.1 d | 1.1 c,d | 0.9 c |
| 1 | 346.1 c | 37.1 b | 76.6 a | 172.8 a | 37.1 b | 410.5a | 4.5 a | 415.9 c | 11.3 a | 6.9 a | 0.5 c | 6.0 d | 0.7 c | 0.8 d | 1.1c,d | 0.9 c | |
| 2 | 337.2 c | 36.0 b | 77.0 a | 166.0 a | 34.4 b | 422.1 a | 4.5 a | 394.4 c | 11.3 a | 6.9 a | 0.8 c | 5.6 d | 0.7 c | 1.2 d | 0.7c,d | 1.3 c | |
| 3 | 334.6 c | 33.7 b | 74.2 a | 153.0 a | 37.5 b | 454.5 a | 4.4 a | 361.4 c | 11.7 a | 7.2 a | 0.7 c | 5.5 d | 0.7 c | 1.1 d | 1.0c,d | 1.1 c | |
| CHEM HD | 0 | 392.3 b | 35.7 a | 81.0 a | 168.2 a | 33.1 a | 380.0 a | 3.9 b | 379.8 a | 11.5 b | 7.0 b | 3.4 a,b | 1.3 a | 0.1 a | 0.3 c | 0.0 a | 0.1 a |
| 1 | 402.6 b | 31.7 a | 83.8 a | 141.2 a | 32.2 a | 433.0 a | 4.0 b | 323.9 a | 11.9 b | 7.3 b | 3.2 a,b | 0.9 a | 0.0 a | 0.2 c | 0.0 a | 0.0 a | |
| 2 | 385.4 b | 31.1 a | 77.5 a | 159.5 a | 32.3 a | 405.6 a | 3.8 b | 353.8 a | 11.6 b | 7.1 b | 4.1 a,b | 1.1 a | 0.0 a | 0.4 c | 0.0 a | 0.1 a | |
| 3 | 391.2 b | 32.0 a | 80.9 a | 148.2 a | 31.6 a | 428.7 a | 3.8 b | 336.5 a | 11.8 b | 7.2 b | 3.9 a,b | 1.0 a | 0.0 a | 0.1 c | 0.0 a | 0.0 a | |
| CHEM LD | 0 | 392.1 b | 36.3 a | 80.3 a | 165.3 a | 31.4 a | 397.9 a | 4.1 b | 374.5 a | 11.4 b | 7.0 b | 3.8 b | 1.4 a | 0.2 a | 0.2 b,c | 0.1 a | 0.1 a |
| 1 | 397.1 b | 32.9 a | 77.8 a | 159.4 a | 32.3 a | 413.8 a | 4.0 b | 358.6 a | 11.5 b | 7.1 b | 4.5 b | 1.3 a | 0.0 a | 0.2 b,c | 0.0 a | 0.1 a | |
| 2 | 388.4 b | 31.4 a | 78.0 a | 146.4 a | 31.7 a | 438.9 a | 4.0 b | 342.2 a | 11.7 b | 7.2 b | 4.6 b | 1.3 a | 0.0 a | 0.3 b,c | 0.0 a | 0.1 a | |
| 3 | 379.0 b | 31.2 a | 77.1 a | 154.5 a | 33.0 a | 425.3 a | 3.9 b | 336.6 a | 11.7 b | 7.2 b | 5.0 b | 1.6 a | 0.0 a | 0.4 b,c | 0.0 a | 0.1 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jungbluth, K.H.; Trimborn, M.; Maack, G.-C.; Büscher, W.; Li, M.; Cheng, H.; Cheng, Q.; Sun, Y. Effects of Three Different Additives and Two Different Bulk Densities on Maize Silage Characteristics, Temperature Profiles, CO2 and O2–Dynamics in Small Scale Silos during Aerobic Exposure. Appl. Sci. 2017, 7, 545. https://doi.org/10.3390/app7060545
Jungbluth KH, Trimborn M, Maack G-C, Büscher W, Li M, Cheng H, Cheng Q, Sun Y. Effects of Three Different Additives and Two Different Bulk Densities on Maize Silage Characteristics, Temperature Profiles, CO2 and O2–Dynamics in Small Scale Silos during Aerobic Exposure. Applied Sciences. 2017; 7(6):545. https://doi.org/10.3390/app7060545
Chicago/Turabian StyleJungbluth, Kerstin Helena, Manfred Trimborn, Gerd-Christian Maack, Wolfgang Büscher, Menghua Li, Hong Cheng, Qiang Cheng, and Yurui Sun. 2017. "Effects of Three Different Additives and Two Different Bulk Densities on Maize Silage Characteristics, Temperature Profiles, CO2 and O2–Dynamics in Small Scale Silos during Aerobic Exposure" Applied Sciences 7, no. 6: 545. https://doi.org/10.3390/app7060545







