1. Introduction
The discharge of dyes and pigments from various industries has generated large quantities of colored wastewater, which pose a potential threat to the environment [
1,
2,
3,
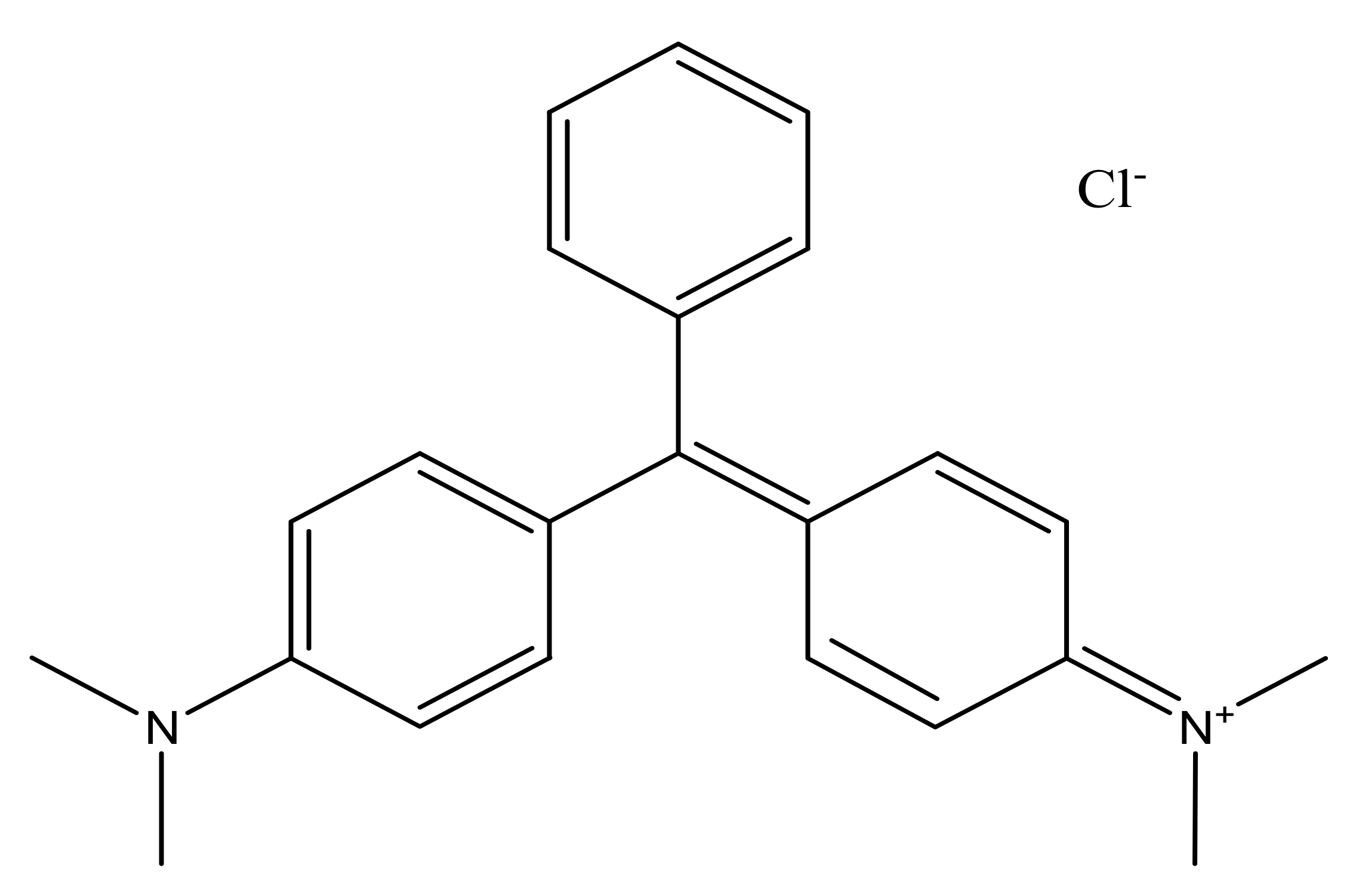
4]. Malachite green (MG) (shown in
Figure 1) is a cationic dye belonging to the triphenylmethane dye category, which is widely used for dyeing of textile, leather and paper, printing, and treating parasites, fungal and bacterial infections in fish and fish eggs [
5]. However, this noxious dye can cause carcinogenic, mutagenic and teratogenic effects on humans and animals [
6,
7,
8,
9]. Thereby, the removal of MG from wastewater before being discharged into surface water bodies is indispensible and significant. A variety of decolorization methods have been used for the removal of MG, including physical (adsorption), chemical (coagulating) and biological (decolorizsation–fermentation processes) [
10,
11]. Since the chemical treatment method is unsuited for highly soluble dyes and longer time is required for biological method, adsorption is considered the efficient method due to its comparatively low cost and high removal efficiency [
12,
13].
Nanomaterials have been used as promising materials for environmental remediation due to a series of unique properties, e.g., small size effect, surface effect and quantum size effect [
14,
15]. Nano-sized zinc oxide is a wide-gap semiconductor with a high photo-catalysis activity, which has been widely used in the photodegradation of organic pollutants in wastewater [
16,
17]. A wide range of studies have discussed the application of nanoscale zerovalent iron (nZVI) in the removal of heavy metals and organic pollutants [
18,
19]. Due to its high reactivity, zerovalent zinc exhibited a faster removal of organic compounds than zerovalent iron [
20]. To our knowledge, hitherto there have been no reports available in the literature regarding MG removal by nanoscale zerovalent zinc (nZVZ).
Modeling and optimization are a vital stage in the contaminant removal process, which are beneficial for determining the optimum experimental condition and obtaining maximum removal efficiency [
21]. Response surface methodology (RSM) as an experimental design tool was used to build models, evaluate the effects of factors and search for the optimum experimental conditions [
22]. Artificial intelligence (AI) has recently emerged as a promising technology with tremendous development potential for actual applications, including intelligent search, autonomous driving, robots, big data, fingerprint identification and human-computer games [
23,
24]. Artificial neural networks (ANNs) are a key AI tool commonly used as a black box that works better than RSM in terms of modeling and prediction, but they cannot obtain the global optimal solutions [
25,
26,
27]. Particle swarm optimization (PSO) is inspired by the behavior of bird flocks, which has a potential to find the optimal solutions via “particles” movement [
28]. The genetic algorithms (GA) based on the natural mechanics and natural selection is utilized for finding optimum solutions [
29]. PSO or GA coupled with ANNs is used to achieve the optimum removal efficiency because it does not easily get trapped in a local optimum [
30].
The aim of the present study was to investigate the feasibility that the the commercially available nZVZ could be utilized as a potential adsorbent for the removal of MG from aqueous solutions with the aid of the RSM, ANN-PSO and ANN-GA models. First of all, the nZVZ was characterized via X-ray diffraction and X-ray photoelectron spectroscopy. Then, the experiments for the adsorption of MG onto nZVZ were performed based on RSM design using the selected parameters, viz. initial pH, temperature, contact time and initial concentration. The RSM, ANN-PSO and ANN-GA models were applied to find the optimum process variables and maximum removal efficiency of MG. Next, the performance of these models was evaluated and the relative importance of the variables were obtained by sensitivity analysis. Besides, the equilibrium data for uptake of MG by nZVZ was fitted to the Langmuir, Freundlich, Temkin isotherms. The pseudo-first-order kinetics and the pseudo-second-order kinetics were employed to examine the effect between the removal efficiency and contact time. Finally, thermodynamic parameters ΔG0, ΔH0 and ΔS0 were calculated from the Van’t Hoff plot of lnKc vs. 1/T to discuss the removal mechanism of MG.
2. Materials and Methods
2.1. Materials
In the present study, all chemicals and reagents (HCl, NaOH) used are of analytical grade and used without further purification. The commercially available nZVZ (the specific surface area: 33.01 m2/g, average particles diameter: 50.00 nm) was purchased from Shanghai Naiou Nanotechnology Co., Ltd. (Shanghai, China), and the target chemical MG was obtained from Tianjin Kemio Chemical Co., Tianjin, China. The MG stock solution (1000.00 mg/L) was prepared by dissolving in deionized water, which was further diluted for a desired concentration (600.00, 700.00 and 800.00 mg/L). The pH values of the solutions were measured with a digital pH meter (PHB-5, Hangzhou Qiwei Co., Ltd., Hangzhou, Zhejiang, China) using a standard buffer solution to calibrate the solid electrode, and pH values of the test solutions were adjusted by addition of 0.1 M HCl or 0.1 M NaOH. Absorbance of the MG solutions was determined by using UVmini-1240 spectrophotometer (SHIMADZU, Kyoto, Japan).
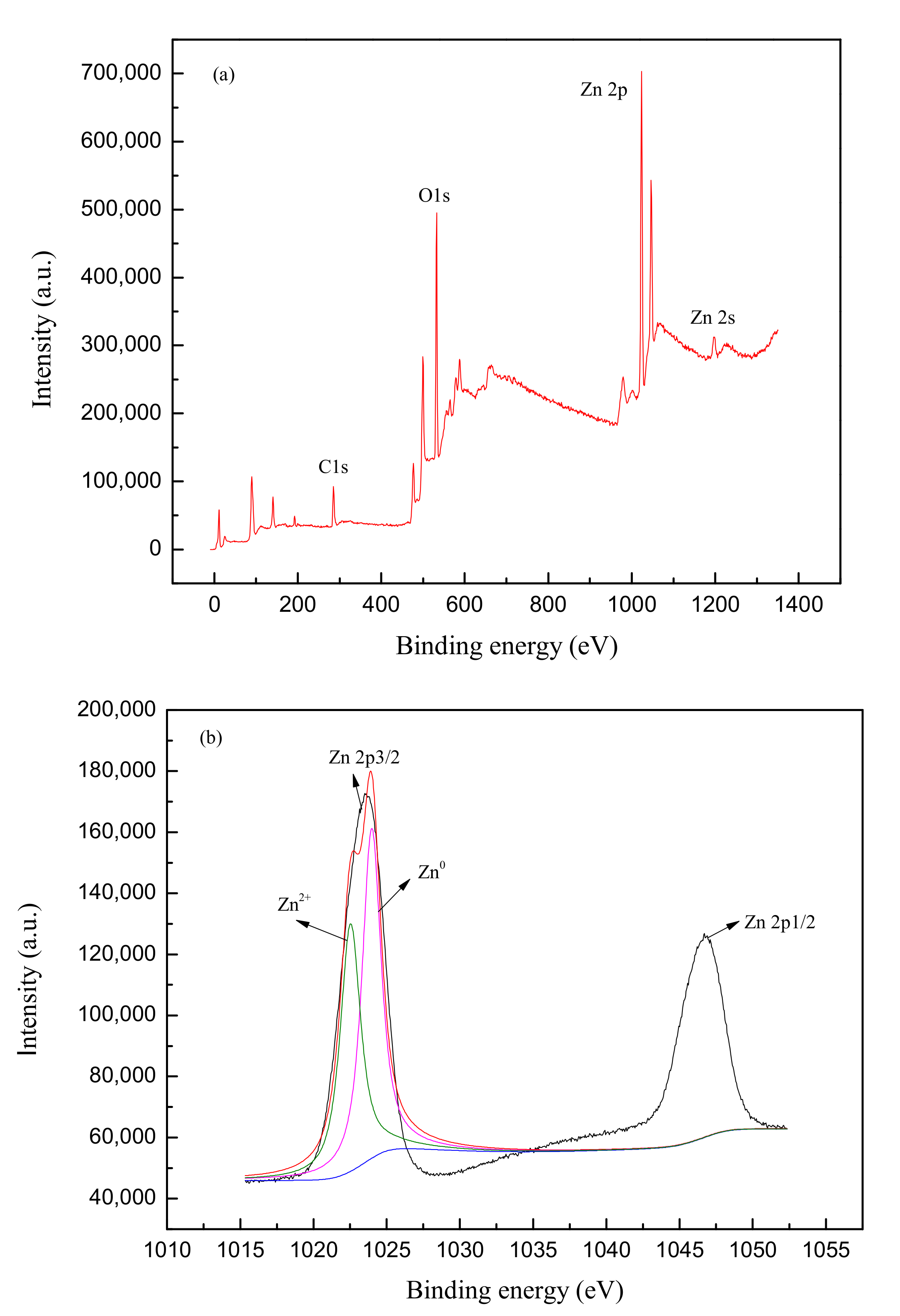
2.2. Characterization of the Commercially Available nZVZ
The crystalline structure of nZVZ was measured by X-ray diffraction (XRD) on a Philips Analytical X-ray (Lelyweg 1 7602, EA, Almelo, The Netherlands) with a Cu-Kα X-ray source (generator tension 40 kV, current 40 mA). The commercial nZVZ was placed in a glass holder and scanned from 5° to 90°. The X-ray photoelectron spectroscopy (XPS) data of nZVZ were collected using an ESCALAB 250Xi spectrometer (Thermo Electron Corporation, Waltham, MA, USA) with monochromatized Al Kα radiation (1486.6 eV) and the peak energies were corrected with the C1s peak as a reference.
2.3. Batch Adsorption Experiments
The adsorption experiments were performed using nZVZ as the adsorbent and MG as the adsorbate. The experiment was carried out using 100 mL conical flask with 50 mL solution of known initial pH and initial MG concentration by adding 30 mg of the adsorbent. Then, the conical flask was mounted on a temperature controlled water bath shaker and agitated at 200 rpm for the desired temperature (20, 25 or 30 °C) and contact time (60, 90 or 120 min). After the experiment, the suspension was centrifuged at 4500 rpm for 5 min, and the concentration of the supernatant was measured at the maximum absorbance wavelength (616 nm). The removal efficiency of MG and the amount of adsorption at equilibrium (
qe, mg/g) was calculated by using the following equation:
where
Y is the removal efficiency of MG,
c0 is the initial concentration of MG (mg/L), and
ct is the MG concentration at any time
t (mg/L).
where
qe is the amount of adsorption at equilibrium (mg/g),
v and
m represent the solution volume (L) and the mass of adsorbent used (g), respectively.
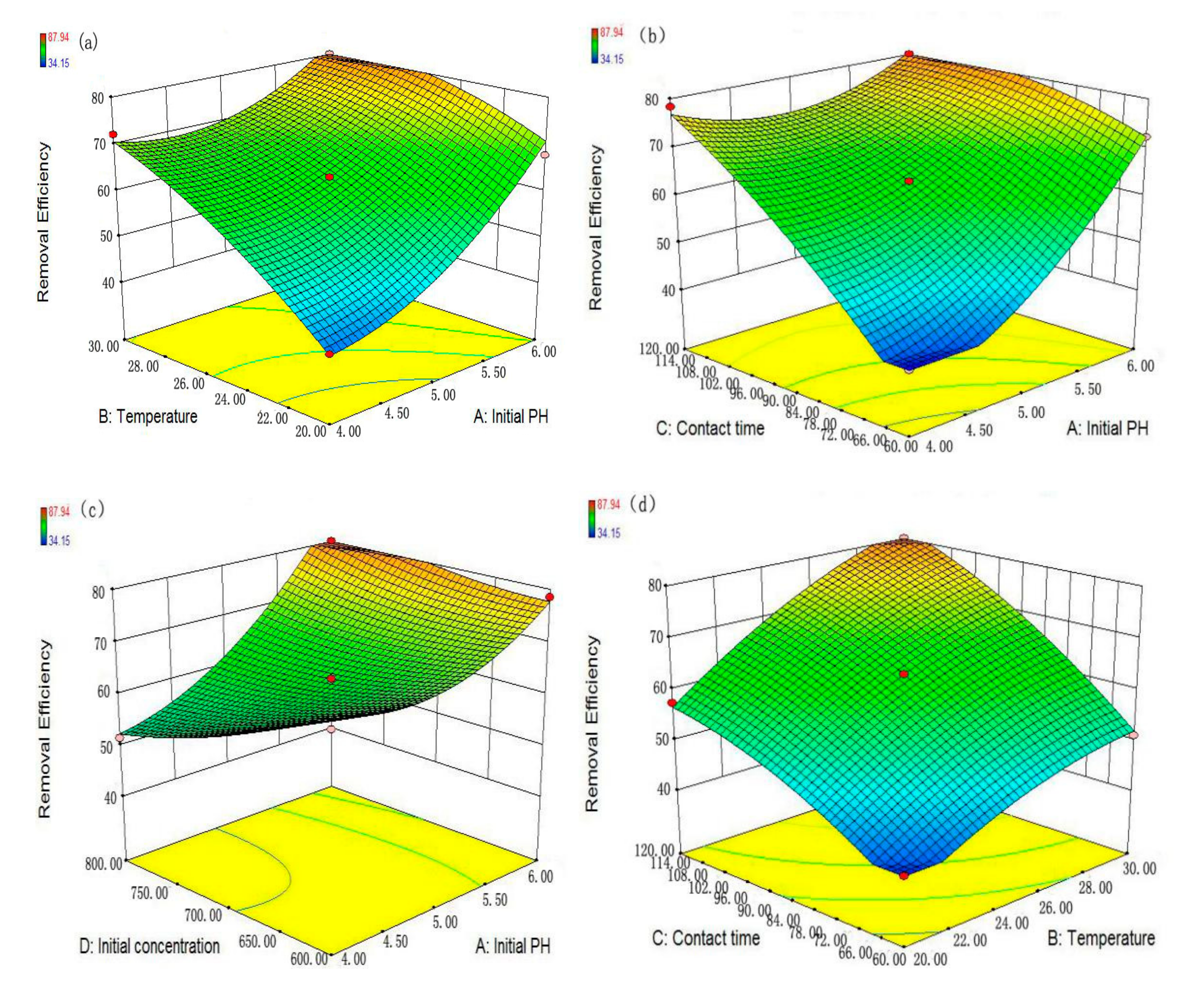
Responses surface methodology (RSM) is a collection of mathematical and statistical methods, which can help better understand the interactive effect of the process parameters and provide a smaller number of experimental runs [
20]. In this study, the optimal conditions for maximum removal efficiency of MG by nZVZ were identified by the 3-level 4-factor Box–Behnken design (BBD) under RSM. The four important independent variables, viz. initial pH (A), temperature (B), contact time (C) and initial concentration (D), were selected to design experiments, whereas the removal efficiency (
Y) was considered as the dependent variable. This design included 29 experiments with 5 central points. The levels of factors were coded as −1 (low), 0 (middle) and 1 (high) (
Table 1) [
31]. The relationship between the response and the independent variables can be represented by the following quadratic equation:
where
Y is the predicted removal efficiency of MG, β
0 is the intercept of quadratic model, β
1–β
14 are the estimated coefficients, and A, B, C and D are the independent variables.
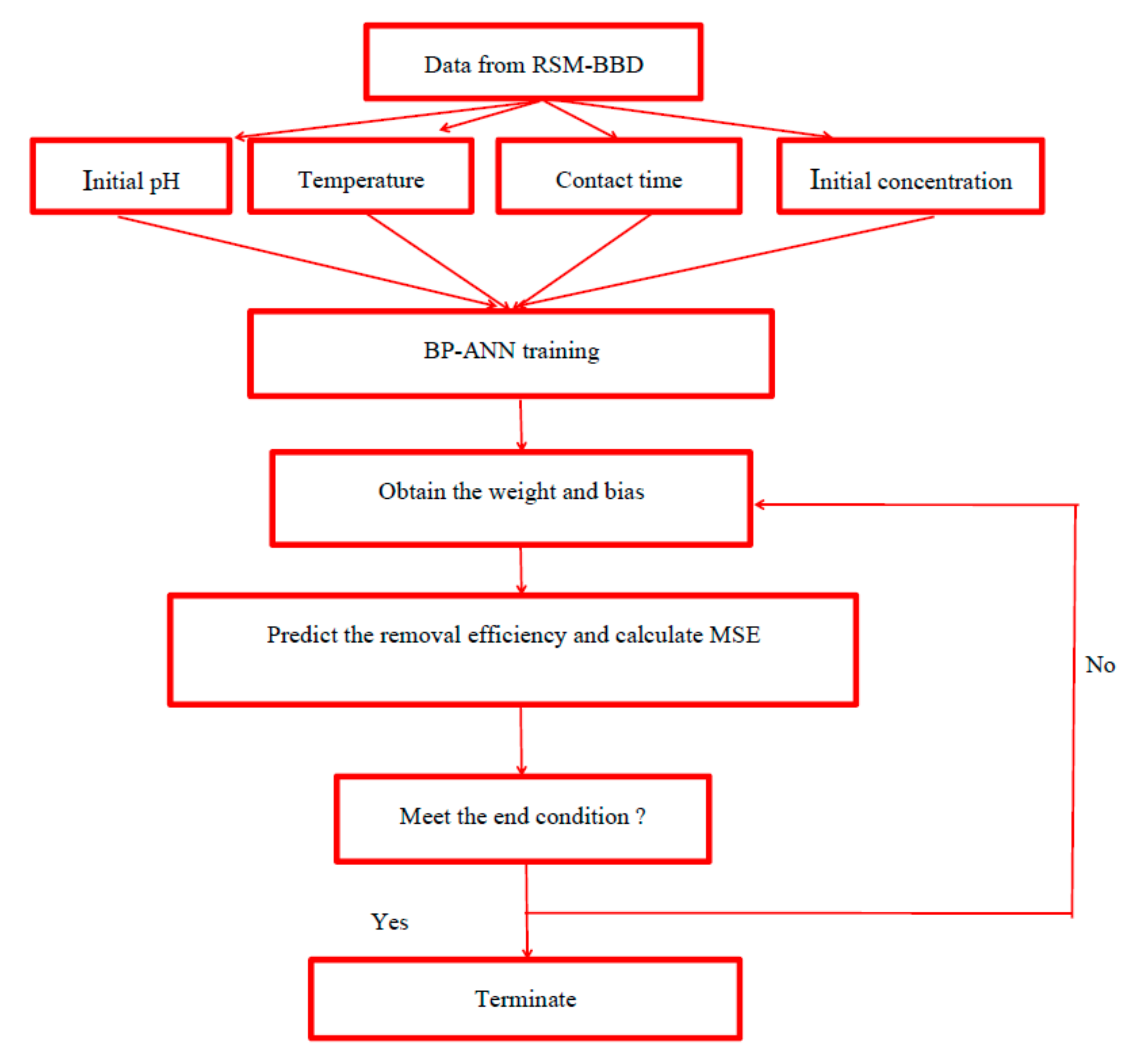
2.4. Back Propagation Artificial Neural Network (BP-ANN)
ANNs have captured the focus of much attention due to their wide range of applicability, which can deal with complicated problems [
32]. The Neural Network Toolbox of MATLAB software (2010a) (the MathWorks, Inc., Natick, MA, USA) was chosen in this work to develop the ANN models using neural network toolbox. Commonly used ANNs include multilayer perception (MLP), self-organization mapping (SOM), radial basis function (RBF) and fuzzy network [
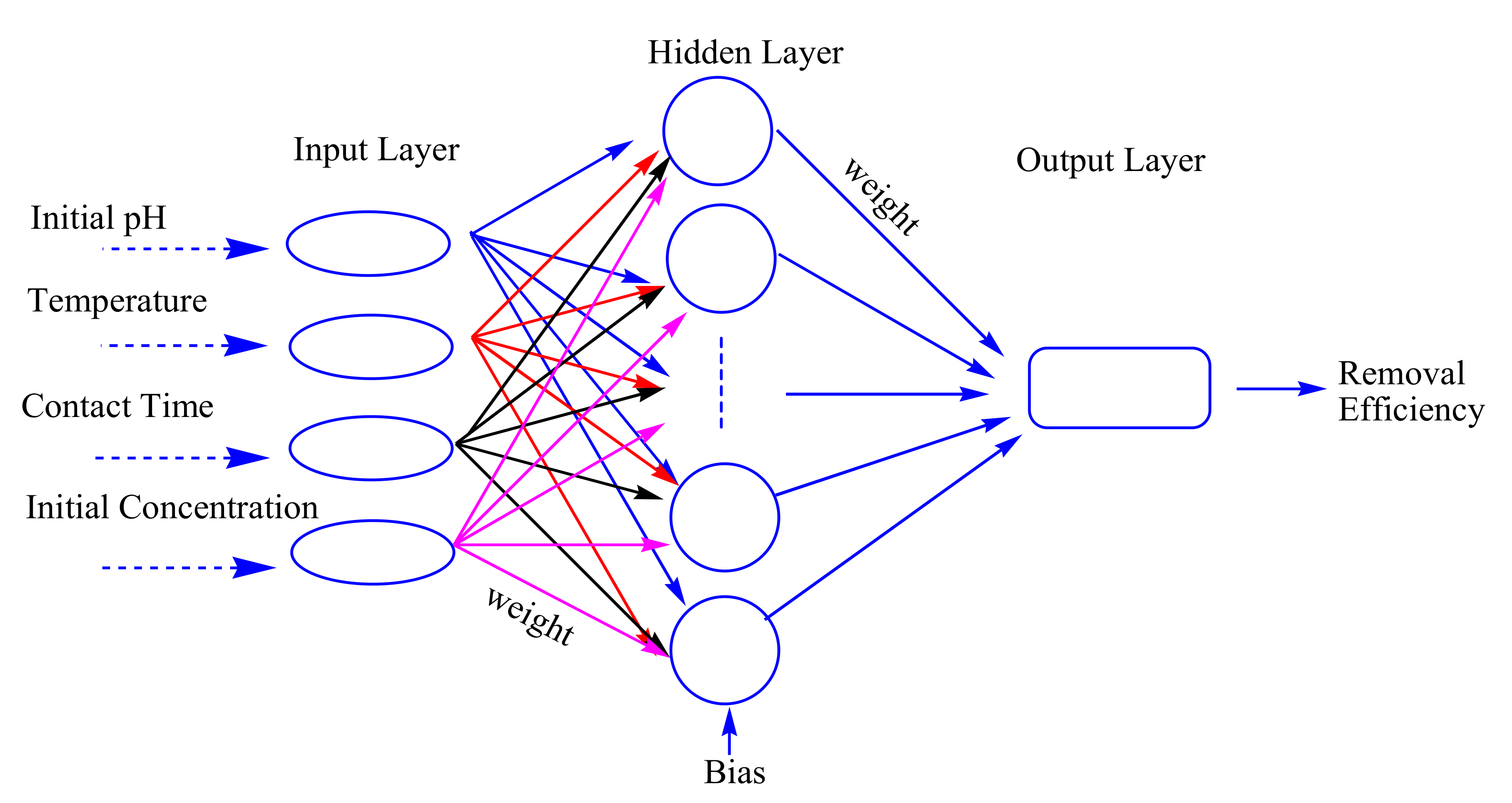
33]. Back propagation (BP) with the multilayer perception architecture is the most popular and widely used ANN with a good generalization ability and high reliability, which is based on the supervised learning procedure. In the present study, a three layer (input layer, hidden layer and output layer) feed-forward artificial neural network was selected to develop the predictive model (
Figure 2). The output in each layer of BP-ANN was produced by summing all the input weight through the activation function [
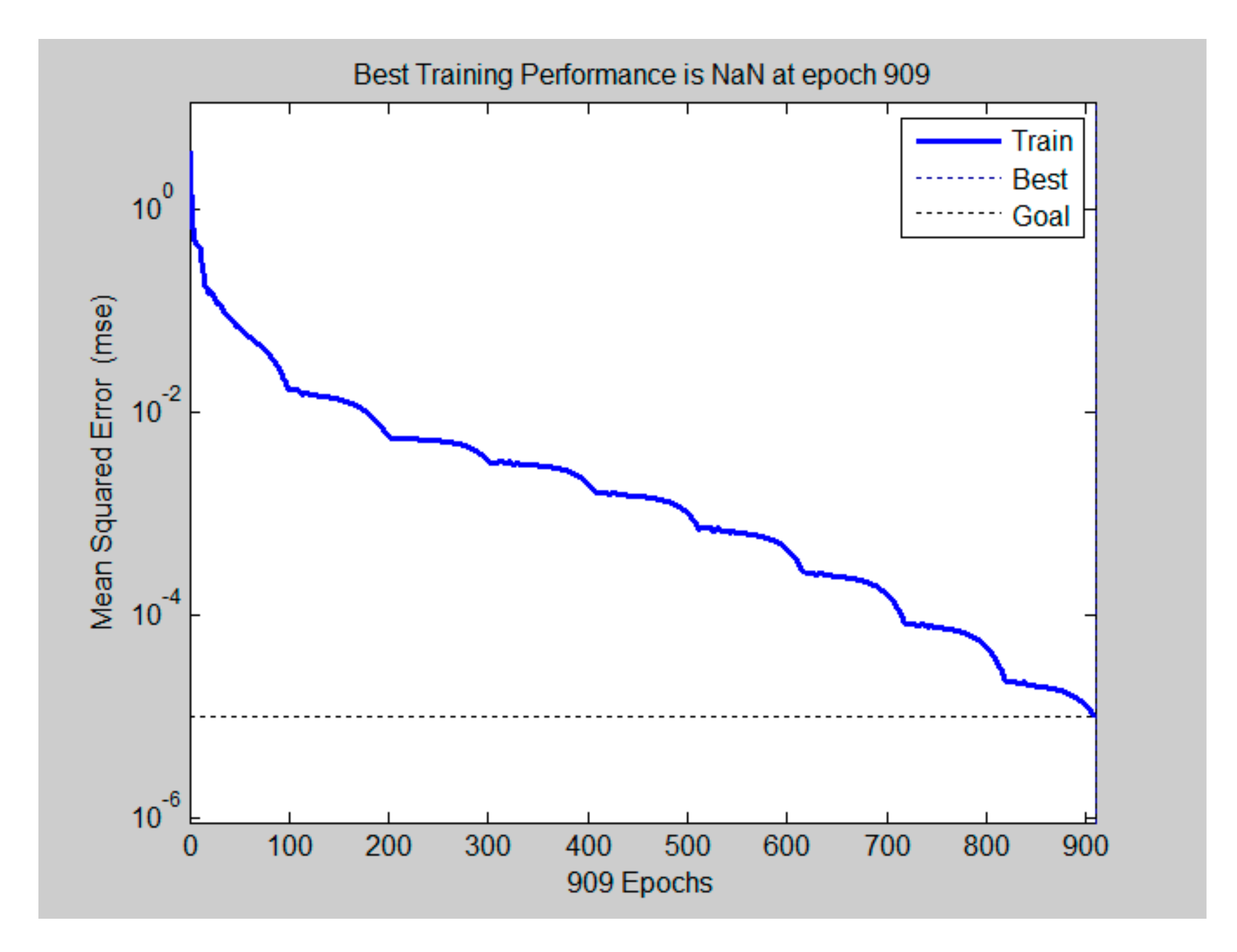
34]. A tangent sigmoid transfer function (tansig) at input-hidden layer was adopted (Equation (7)), while a linear transfer function (purelin) was used in hidden-output layer (Equation (8)). The network was trained by traingdx algorithm and the number of training epochs was set as 2000 with the learning rate at 10
−5 (
Figure 3).
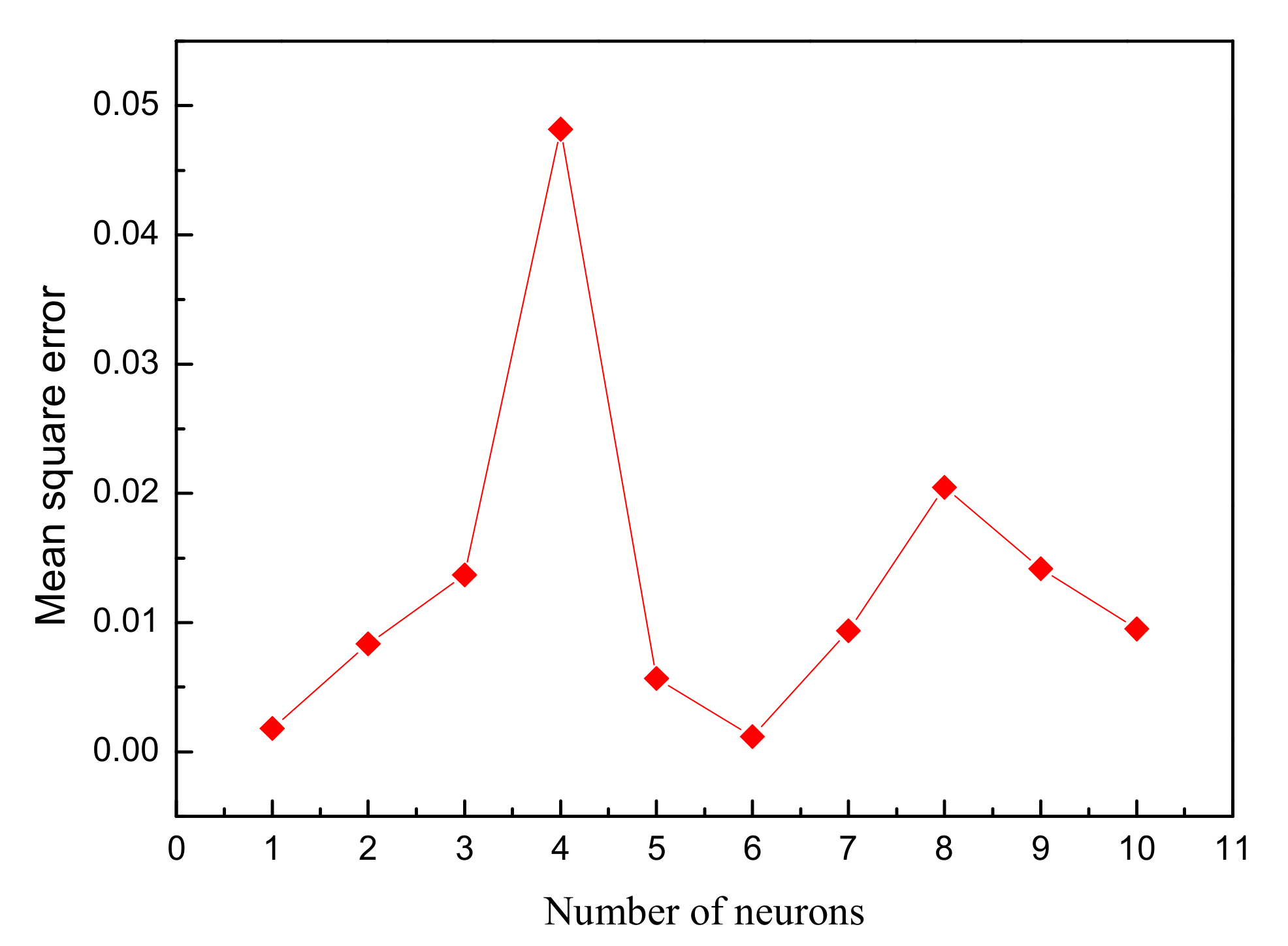
It is an important step to determine the number of neurons in hidden layer for training the network. In the present research, the computations were performed in which the number of neurons was varied from 1 to 10. The inputs (independent variables) and output (dependent variable) was normalized in the range of −1–+1 to avoid numerical overflow due to excessively large or small weights. The normalization equation is described as follows:
where
yi is the normalized value of
x, and the
xmin and
xmax are the minimum and maximum values of
x, respectively. The weight of a neuron in hidden layer is calculated as follows:
where
xi is value of neuron
i in input layer,
wij is the corresponding connection weight between neuron (
i) in input layer and neuron (
j) in hidden layer, and the weight of a neuron in output layer is calculated as follows:
where
xj is value of neuron j in hidden layer,
wjl is the corresponding connection weight between neuron (
j) in hidden layer and neuron (
l) in output layer. The tangent sigmoid (tansig) was used as transfer function between input and hidden layers which is given by:
The following is linear transfer function (purelin) used between hidden and output layers:
The output was produced by weight and bias of neuron through the activation function using Equation (9):
where
y,
f,
w and
b represent the output, activation function, weight and bias in hidden layer or output layer, respectively.
The relative influence of the individual variable was calculated by the following Garson equation [
35,
36]:
where
Iab is the relative importance of the
jth input variable on the output variable,
wx is the connection weight,
a,
g and
b are the number of neurons in the input layer, hidden layer and output layer, respectively.
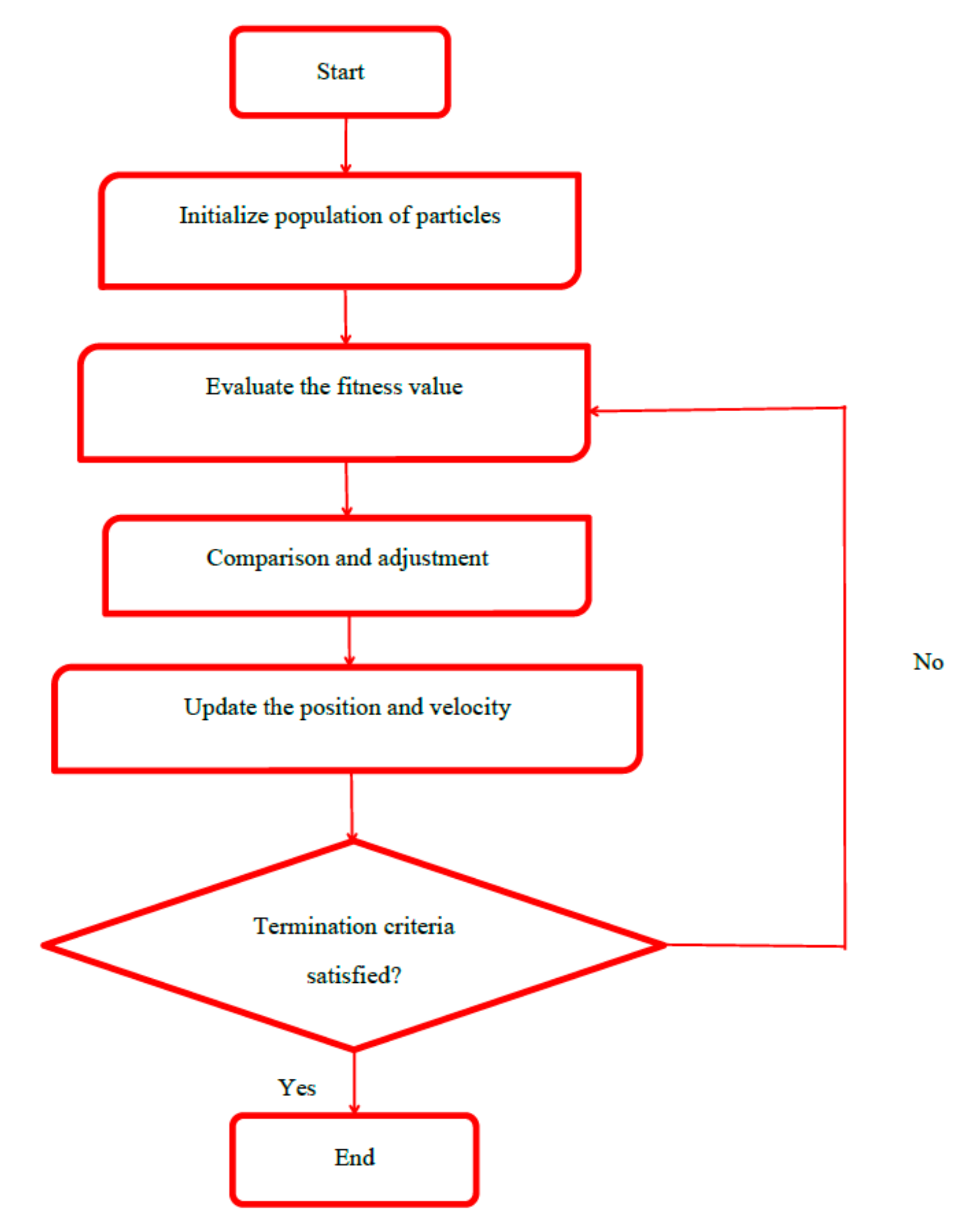
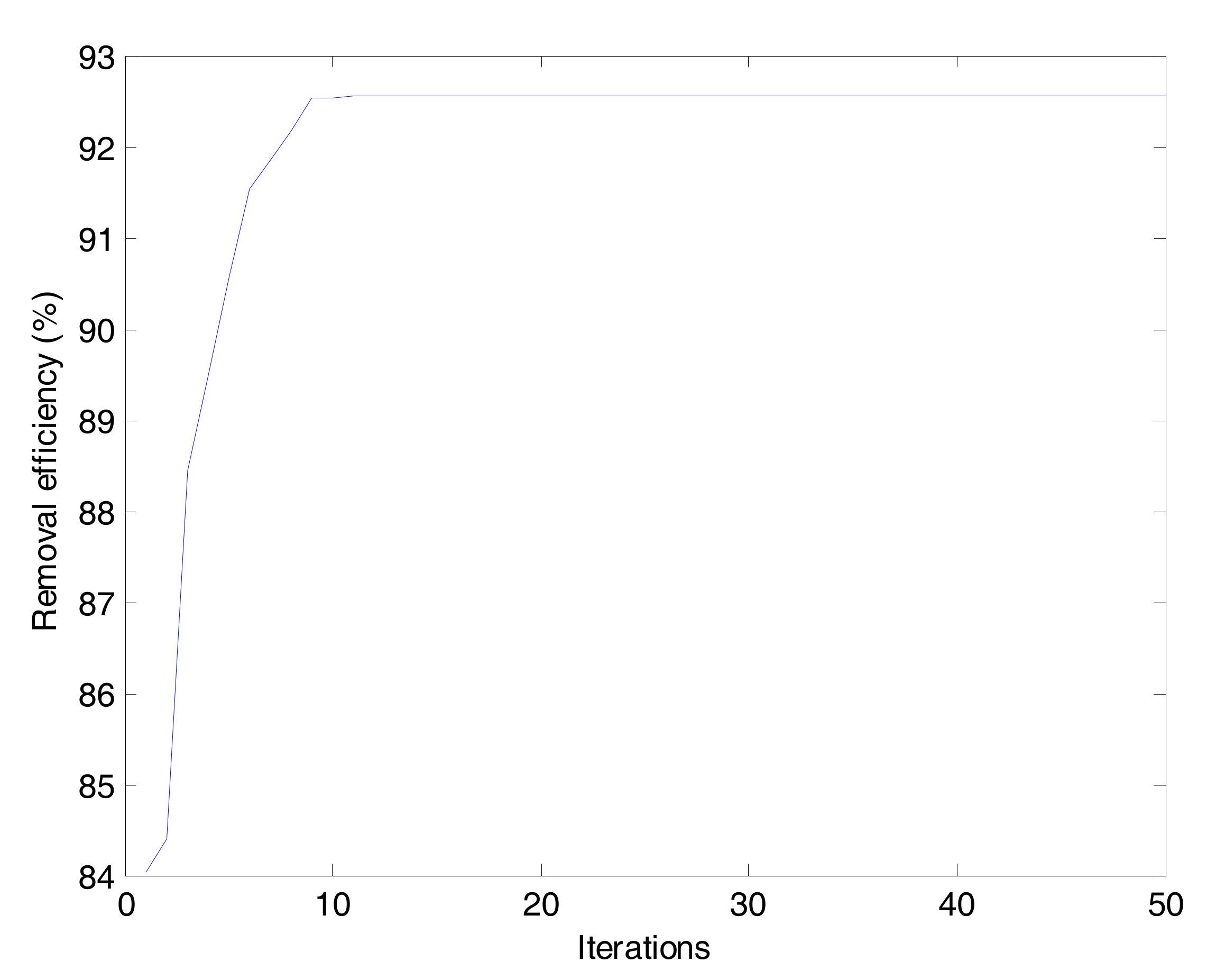
2.5. Particle Swarm Optimization (PSO)
Particle swarm optimization (PSO) was first introduced by Kennedy and Eberhart, which is an efficient optimization technique simulating the social behavior, e.g., bird flocking and fish training [
37]. An initial population randomly generated was called particles (solutions). Each particle had a position and a velocity, which was scattered throughout the multi-dimensional search space. The fitness function was used for evaluating fitness values of the particles. In every iteration, the particles could update their position and velocity according to their own history (personal best) and the knowledge of the swarm (global best) in comparison with the particle’s fitness values [
38,
39]. The particles continued to move in the search space until the maximum iteration number or the desired value of the objective function was reached (
Figure 4). The operation parameters were as follows: 20 for the swarm size, 50 for maximum iteration, 2 for personal learning coefficient (c1), 2 for global learning coefficient (c2), 0.3 for minimum inertia weight and 0.9 for maximum inertia weight.
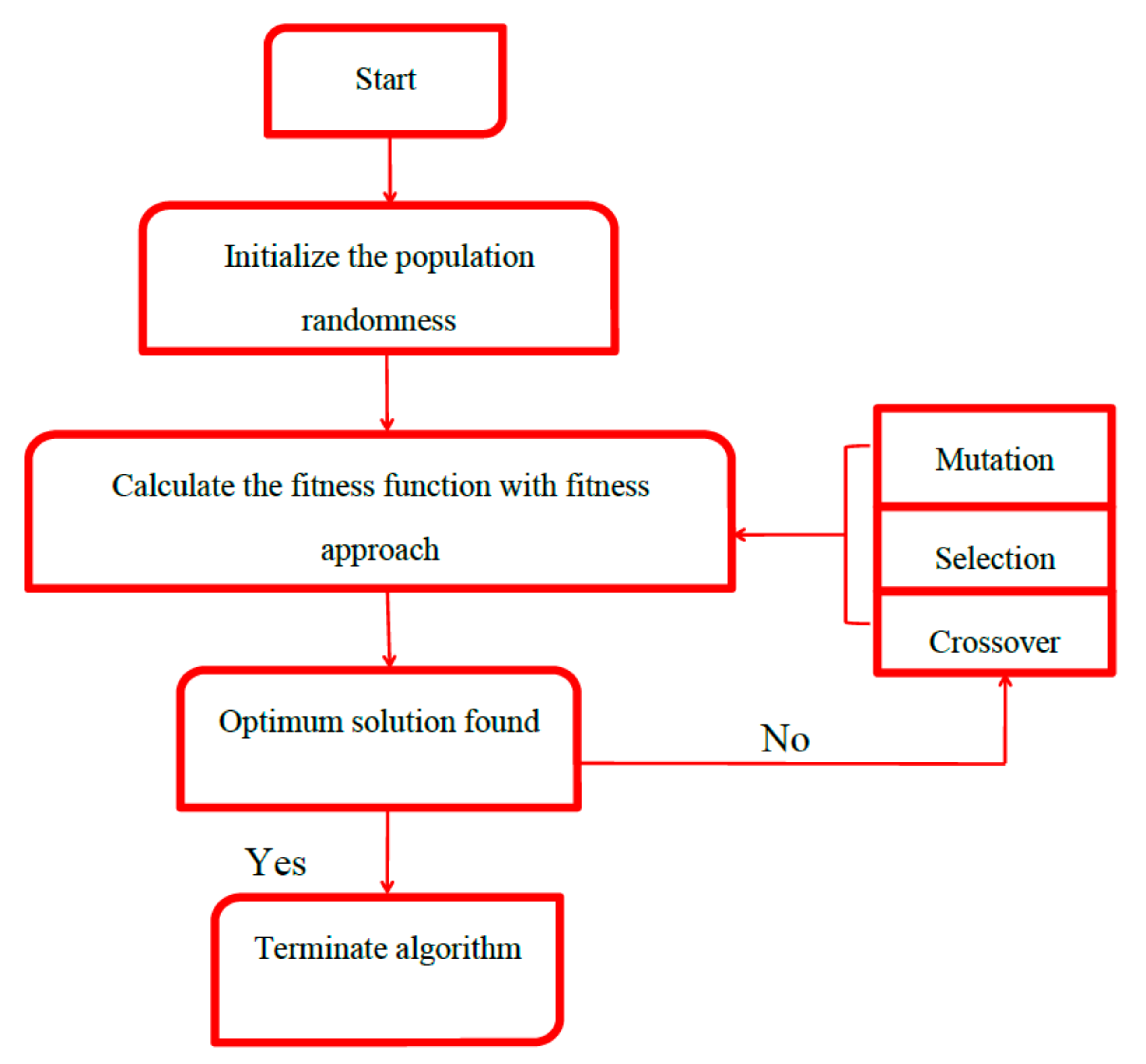
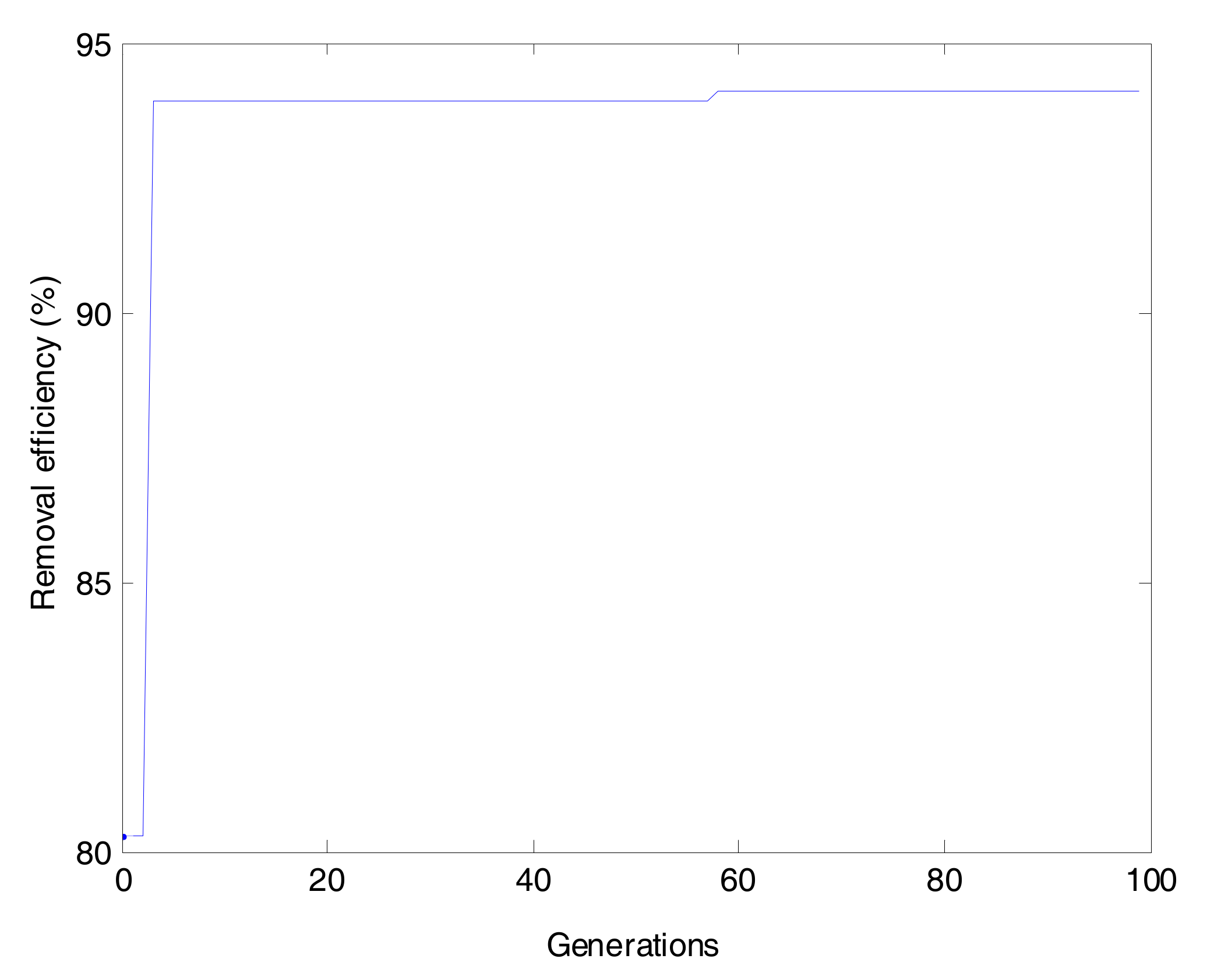
2.6. Genetic Algorithm (GA)
The genetic algorithm operation was started by the blind random population (a possible solution) that was represented by a set of chromosomes coded as a binary string [
40]. Each of chromosomes included four different genes, i.e., initial pH, temperature, contact time and initial concentration. First of all, the initialization of populations was identified. The fitness function was applied for evaluating the merit of each chromosome. Then, selection operation was to choose some individuals from initial generation (parents) to breed a new generation (offspring) by utilizing the roulette wheel selection method [
41]. Next, crossover operator was aimed to swap the information of parents that led to generating new individuals for the next generation. With the aid of crossover, the offspring had both the strength and weakness of its parents. The last operator was the mutation that was able to increase the randomness of individuals and prevent the GA from getting stuck in a local optimum (
Figure 5) [
42]. The optimum parameters used for GA optimization were size of population (20), number of generation (100), probability of crossover (0.8) and probability of mutation (0.01).
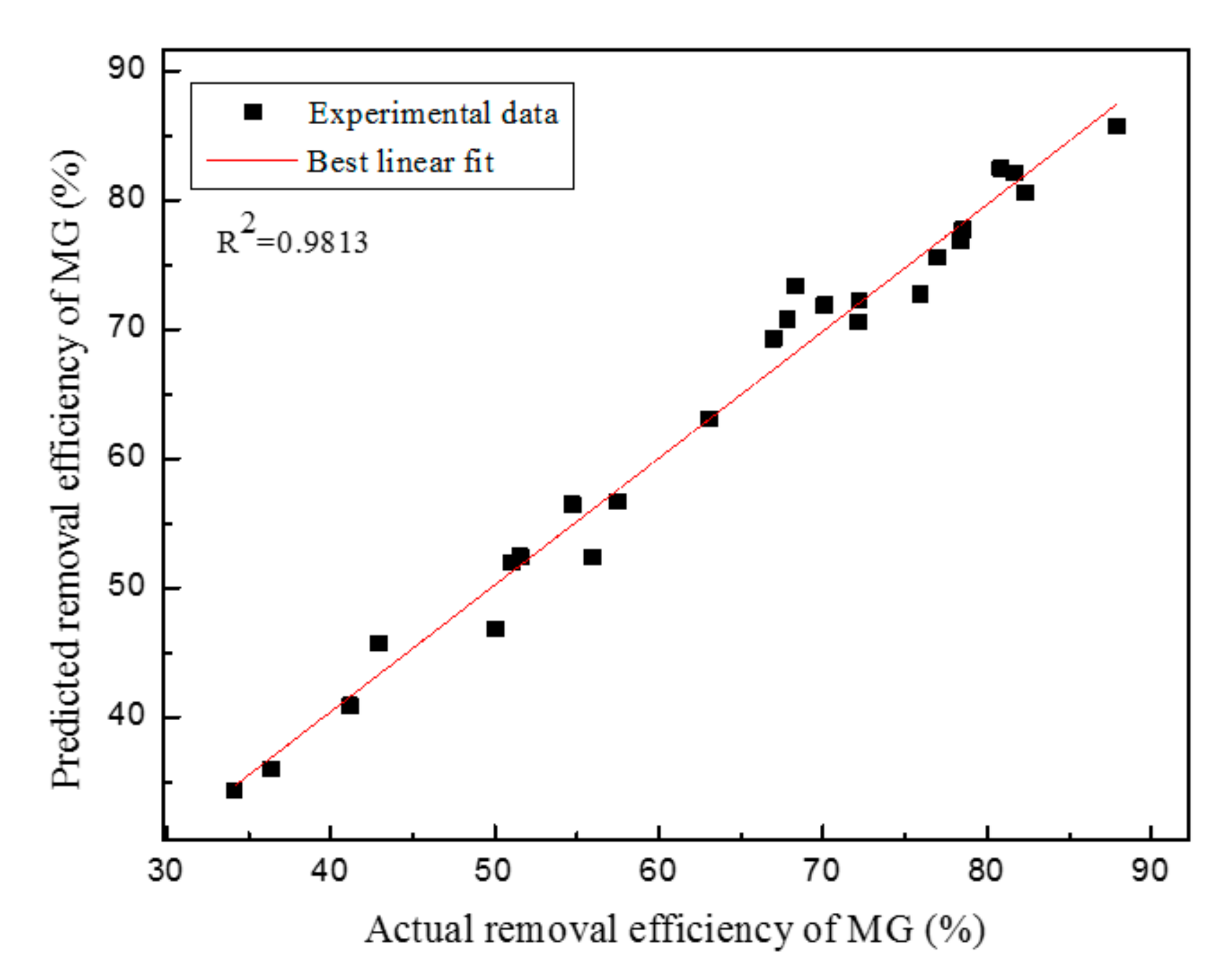
4. Conclusions
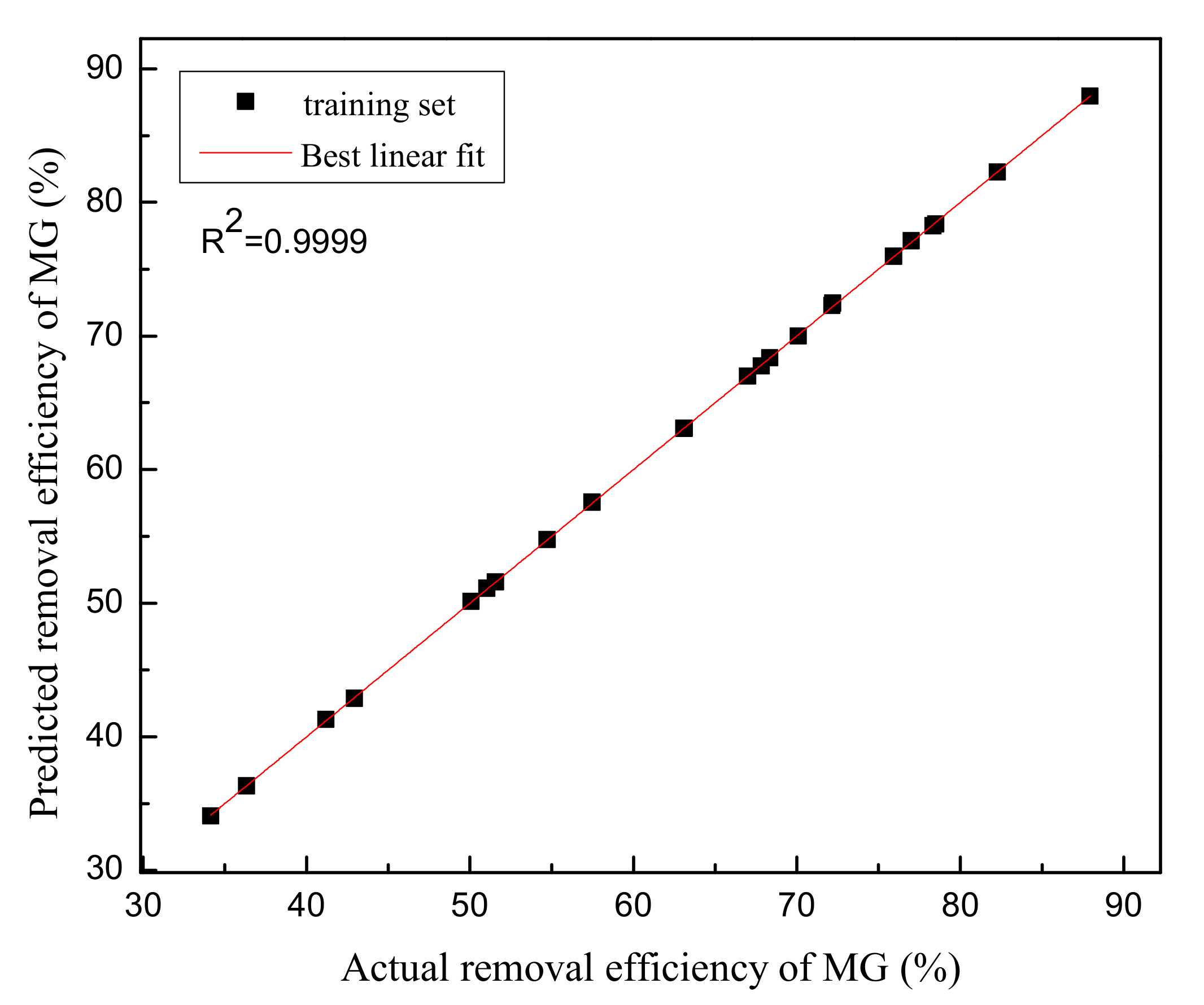
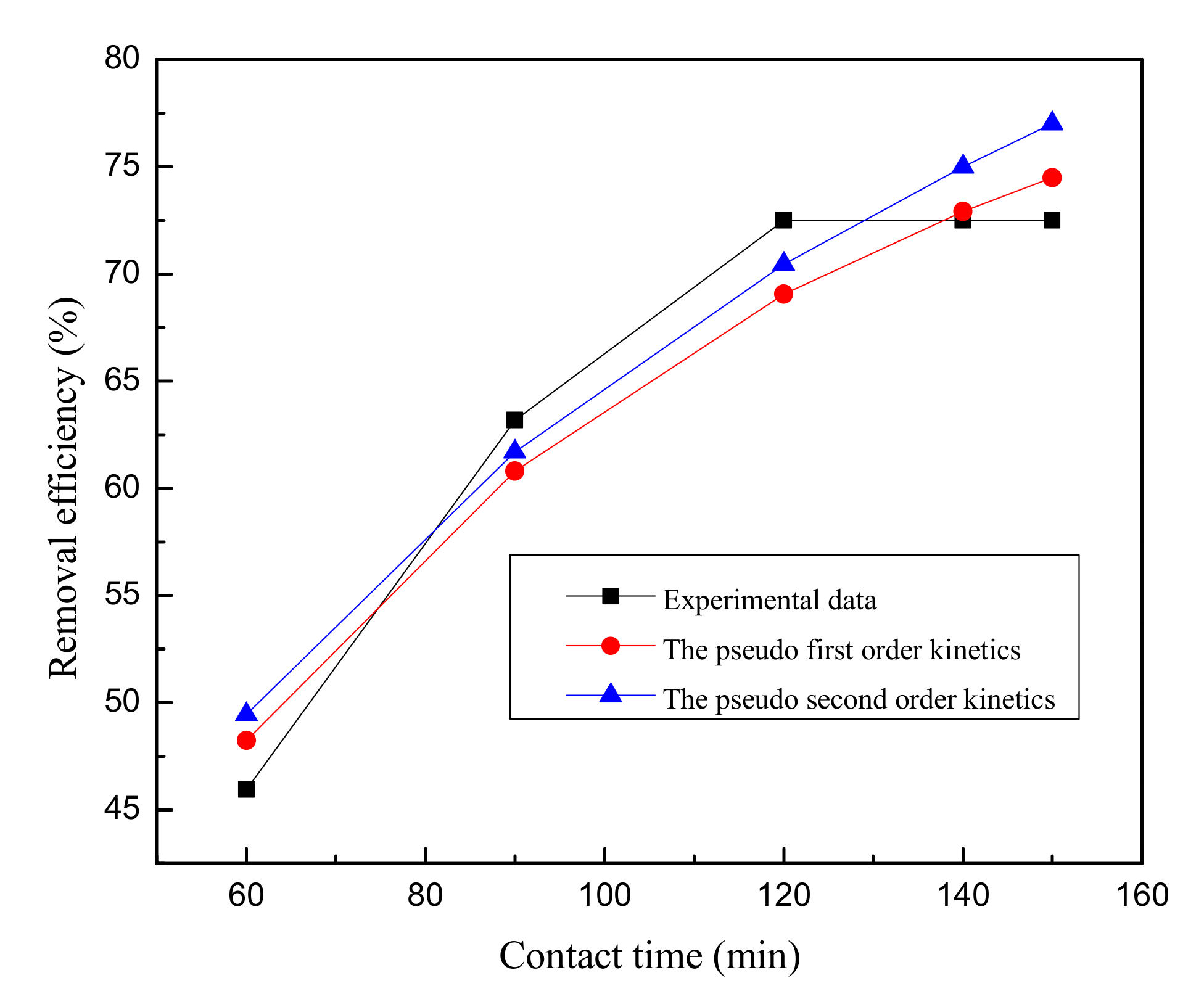
In this study, the commercially available nZVZ was characterized by means of XRD and XPS. Comparison between the experimental and prediction values obtained by the RSM and the BP-ANN models showed that the BP-ANN model was more accurate based on the value of R2 (0.9999). The maximum removal efficiency of MG under the optimum conditions was obtained with the aid of the RSM, ANN-PSO and ANN-GA models. The mean absolute errors between confirmatory and predicted results for the RSM, ANN-PSO, and ANN-GA models are 12.13%, 7.68% and 3.40%, respectively, which demonstrate that the ANN-GA model manifest a better agreement in predicting the removal efficiency of MG than the other two models. Additionally, both the ANOVA and sensitivity analysis demonstrate that the important process parameter is contact time. The equilibrium data of MG uptake by nZVZ were fitted to Langmuir, Freundlich and Temkin isotherms. The Langmuir model was found to fit the adsorption data well with maximum adsorption capacity of 1000.00 mg/g. The experimental data were well described by the pseudo-second order model (R2 = 0.9463) with higher correlation coefficients than the pseudo-second order model (R2 = 0.9302). From the thermodynamic study, it is concluded that the adsorption of spontaneous nature occurred in the range of temperature studied (293 K–308 K) due to the negative value of ΔG0. The positive value of ΔH0 indicated the endothermic nature of the adsorption interaction. This is in agreement with the results where the MG dye adsorption increases with an increase in temperature. The positive value of ΔS0 reflects the affinity of the adsorbate for the adsorbent and the increased randomness at the solid-solution interface during the adsorption process. In conclusion, nZVZ can be a promising adsorbent for the removal of MG from aqueous solutions. Further studies should investigate the regeneration efficiency of the nZVZ for the removal of MG or other dyes (e.g., Methyl orange, Methylene Blue) as well as the removal efficiency of dyes via the permeable reactive barrier in the laboratory.