Healable, Flexible Supercapacitors Based on Shape Memory Polymers
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Fabrication and Morphologies of the Composite
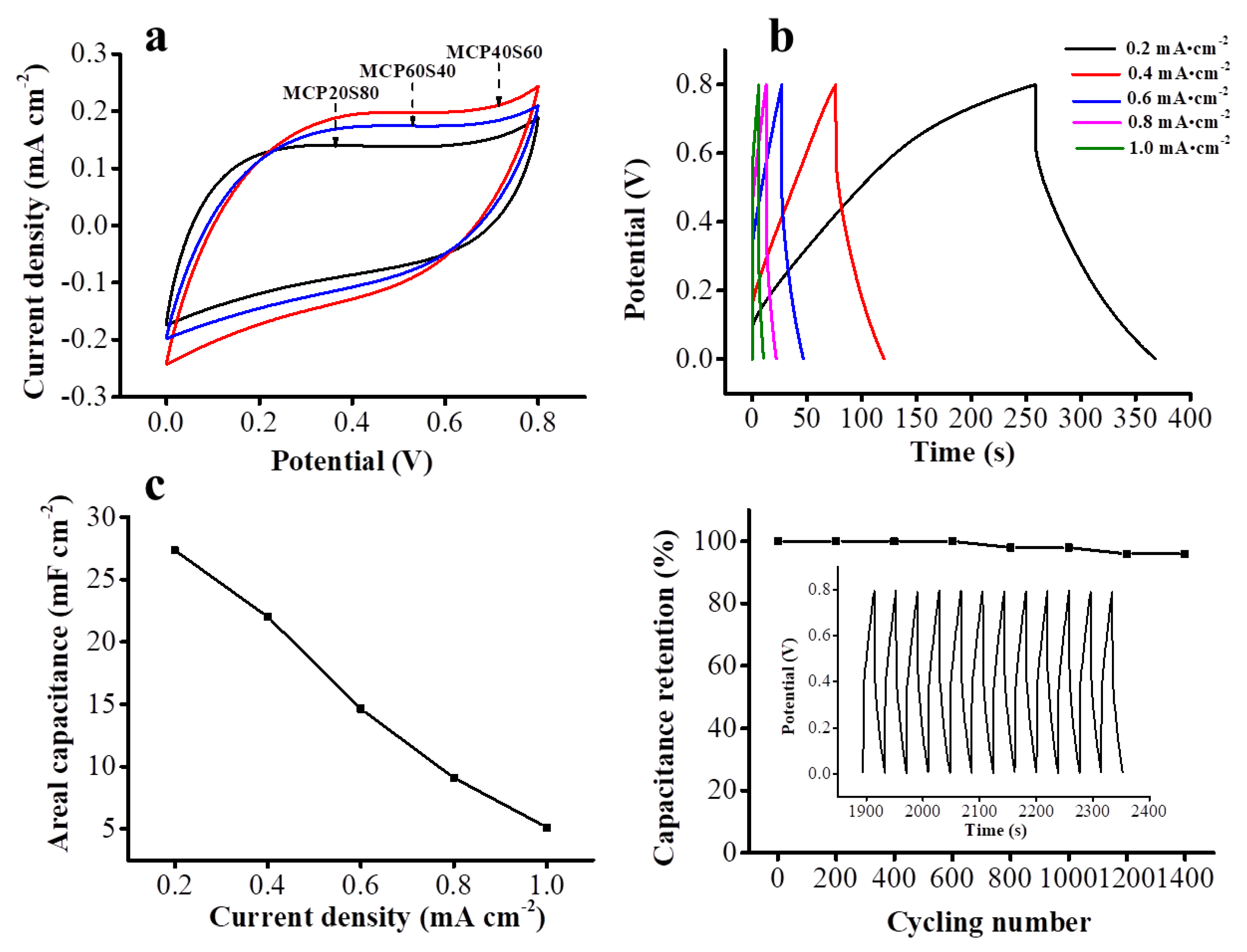
3.2. Capacitance Measurements of the Supercapacitors
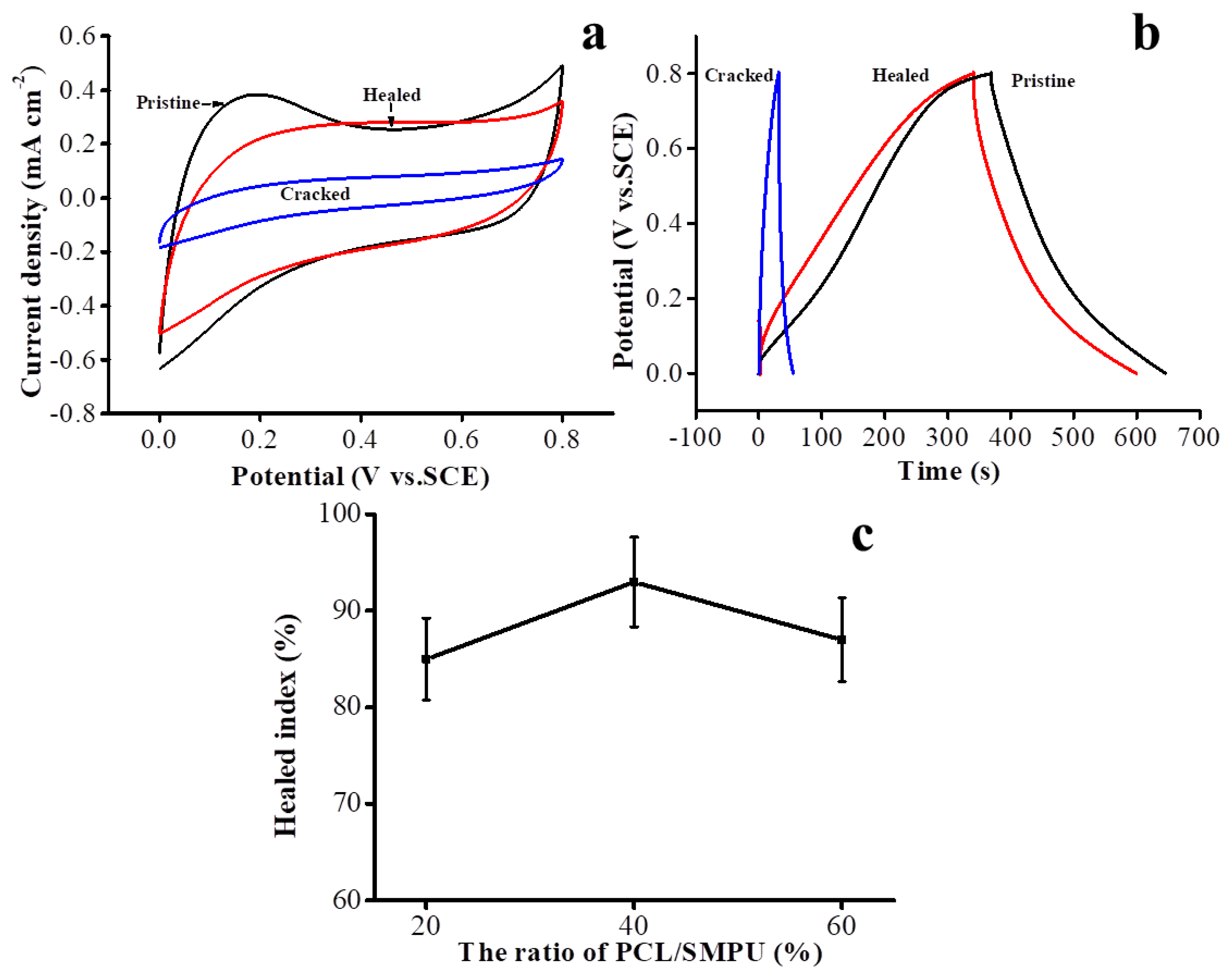
3.3. Investigations on the Heal-Ability of the Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mather, P.T.; Luo, X.F.; Rousseau, I.A. Shape memory polymer research. Annu. Rev. Mater. Res. 2009, 39, 445–471. [Google Scholar] [CrossRef]
- Rodriguez, E.D.; Luo, X.F.; Mather, P.T. Linear/network poly(epsilon-caprolactone) blends exhibiting shape memory assisted self-healing (smash). ACS Appl. Mater. Interfaces 2011, 3, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.F.; Mather, P.T. Shape memory assisted self-healing coating. ACS Macro Lett. 2013, 2, 152–156. [Google Scholar] [CrossRef]
- Mather, P.T.; Luo, X.F. Self-healing coatings utilizing a shape memory effect. In Proceedings of the 2013 ICSHM 4th International Conference on Self-Healing Materials, Ghent, Belgium, 16–20 June 2013. [Google Scholar]
- Wei, H.Q.; Yao, Y.T.; Liu, Y.J.; Leng, J.S. A dual-functional polymeric system combining shape memory with self-healing properties. Compos. Part B Eng. 2015, 83, 7–13. [Google Scholar] [CrossRef]
- Ge, J.; Cheng, G.H.; Chen, L.W. Transparent and flexible electrodes and supercapacitors using polyaniline/single-walled carbon nanotube composite thin films. Nanoscale 2011, 3, 3084–3088. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.Z.; Yang, L.; Hou, L.R.; Shen, L.F.; Zhang, X.G.; Lou, X.W. Growth of ultrathin mesoporous Co3O4 nanosheet arrays on ni foam for high-performance electrochemical capacitors. Energy Environ. Sci. 2012, 5, 7883–7887. [Google Scholar] [CrossRef]
- Lu, X.H.; Wang, G.M.; Zhai, T.; Yu, M.H.; Xie, S.L.; Ling, Y.C.; Liang, C.L.; Tong, Y.X.; Li, Y. Stabilized tin nanowire arrays for high-performance and flexible supercapacitors. Nano Lett. 2012, 12, 5376–5381. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhong, M.; Huang, Y.; Zhu, M.S.; Pei, Z.X.; Wang, Z.F.; Xue, Q.; Xie, X.M.; Zhi, C.Y. A self-healable and highly stretchable supercapacitor based on a dual crosslinked polyelectrolyte. Nat. Commun. 2015, 6, 10310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, T.J.; Bhattacharjya, D.; Yu, J.S.; Kumar, A. Functionalized agarose self-healing ionogels suitable for supercapacitors. ChemsusChem 2015, 8, 3294–3303. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, B.W.; Jiang, W.C.; Yang, Y.; Leow, W.R.; Wang, H.; Chen, X.D. A mechanically and electrically self-healing supercapacitor. Adv. Mater. 2014, 26, 3638–3643. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, Y.; Zhu, M.S.; Meng, W.J.; Pei, Z.X.; Liu, C.; Hu, H.; Zhi, C.Y. Magnetic-assisted, self-healable, yarn-based supercapacitor. ACS Nano 2015, 9, 6242–6251. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Z.; Zhou, X.; Tang, Q.Q.; Bao, H.; Wang, G.C.; Saha, P. A self-healable and easily recyclable supramolecular hydrogel electrolyte for flexible supercapacitors. J. Mater. Chem. A 2016, 4, 8769–8776. [Google Scholar] [CrossRef]
- Zhou, X.D.; Luo, H.S.; Zhang, Y.H.; Wang, H.Q.; Lin, Y.L.; Zhao, G.R.; Yi, G.B.; Yuan, S.J.; Zhu, Z.Q. Tunable water sensitive polymeric composites with synergistic graphene and carbon nanotubes. Mater. Lett. 2017, 199, 160–163. [Google Scholar] [CrossRef]
- Luo, H.S.; Zhoua, X.D.; Ma, Y.Y.; Yi, G.B.; Cheng, X.L.; Zhu, Y.; Zu, X.H.; Zhang, N.J.; Huang, B.H.; Yu, L.F. Shape memory-based tunable resistivity of polymer composites. Appl. Surf. Sci. 2016, 363, 59–65. [Google Scholar] [CrossRef]
- Luo, H.S.; Li, Z.W.; Yi, G.B.; Zu, X.H.; Wang, H.; Wang, Y.J.; Huang, H.L.; Hu, J.W.; Liang, Z.F.; Zhong, B.B. Electro-responsive silver nanowire-shape memory polymer composites. Mater. Lett. 2014, 134, 172–175. [Google Scholar] [CrossRef]
- Yu, M.H.; Zhang, Y.F.; Zeng, Y.X.; Balogun, M.S.; Mai, K.C.; Zhang, Z.S.; Lu, X.H.; Tong, Y.X. Water surface assisted synthesis of large-scale carbon nanotube film for high-performance and stretchable supercapacitors. Adv. Mater. 2014, 26, 4724–4729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.S.; Zhai, T.; Lu, X.H.; Yu, M.H.; Tong, Y.X.; Mai, K.C. Conductive membranes of eva filled with carbon black and carbon nanotubes for flexible energy-storage devices. J. Mater. Chem. A 2013, 1, 505–509. [Google Scholar] [CrossRef]
- Zhi, J.; Reiser, O.; Huang, F.Q. Hierarchical MnO2 spheres decorated by carbon-coated cobalt nanobeads: Low-cost and high-performance electrode materials for supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 8452–8459. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Yi, G.B.; Wang, J.C.; Wang, H.; Luo, H.S.; Zu, X.H. Shape-controllable and -tailorable multi-walled carbon nanotube/MnO2/shape-memory polyurethane composite film for supercapacitor. Synth. Met. 2017, 223, 67–72. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.L.; Yeung, K.W.; Choi, K.F.; Liu, Y.Q.; Liem, H.M. Effect of cationic group content on shape memory effect in segmented polyurethane cationomer. J. Appl. Polym. Sci. 2007, 103, 545–556. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Sun, X.Z.; Ma, Y.W. Conducting polymer hydrogel materials for high-performance flexible solid-state supercapacitors. Sci. China Mater. 2016, 59, 412–420. [Google Scholar] [CrossRef]
- Banerjee, D.; Das, N.S.; Chattopadhyay, K.K. Enhancement of field emission and hydrophobic properties of silicon nanowires by chemical vapor deposited carbon nanoflakes coating. Appl. Surf. Sci. 2012, 261, 223–230. [Google Scholar] [CrossRef]
- Gambou-Bosca, A.; Belanger, D. Chemical mapping and electrochemical performance of manganese dioxide/activated carbon based composite electrode for asymmetric electrochemical capacitor. J. Electrochem. Soc. 2015, 162, A5115–A5123. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Luo, H.; Zhou, X.; Wang, H.; Yao, Y.; Lin, W.; Yi, G. Healable, Flexible Supercapacitors Based on Shape Memory Polymers. Appl. Sci. 2018, 8, 1732. https://doi.org/10.3390/app8101732
Zhou H, Luo H, Zhou X, Wang H, Yao Y, Lin W, Yi G. Healable, Flexible Supercapacitors Based on Shape Memory Polymers. Applied Sciences. 2018; 8(10):1732. https://doi.org/10.3390/app8101732
Chicago/Turabian StyleZhou, Huankai, Hongsheng Luo, Xingdong Zhou, Huaquan Wang, Yangrong Yao, Wenjing Lin, and Guobin Yi. 2018. "Healable, Flexible Supercapacitors Based on Shape Memory Polymers" Applied Sciences 8, no. 10: 1732. https://doi.org/10.3390/app8101732




