The Enhancing Effects of Amelogenin Exon 5-Encoded Peptide from Enamel Matrix Derivative on Odontoblast-Like KN-3 Cells
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
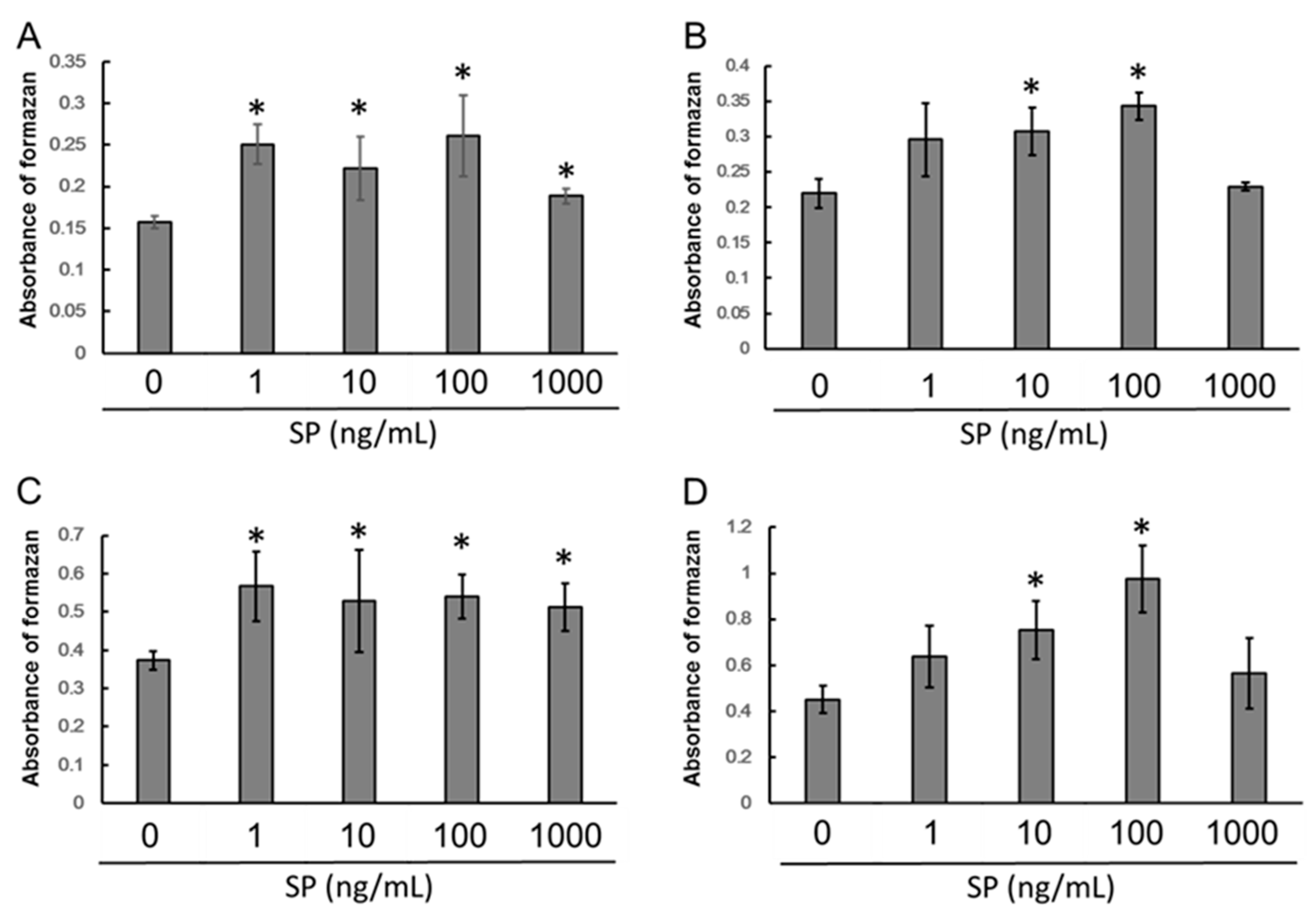
2.2. Cell Proliferation Assay
2.3. Morphological Analysis
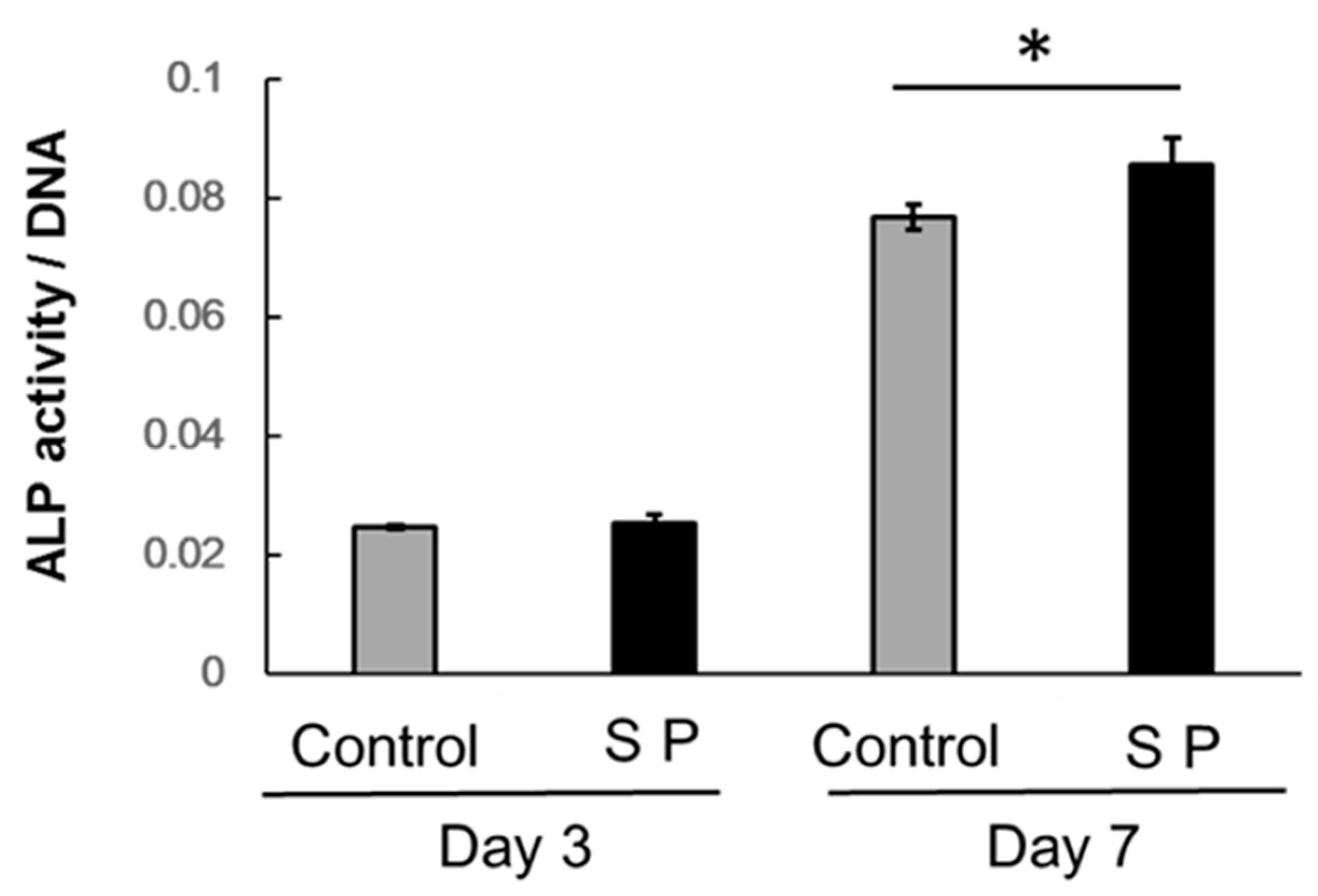
2.4. Alkaline Phosphatase (ALP) Activity Assay
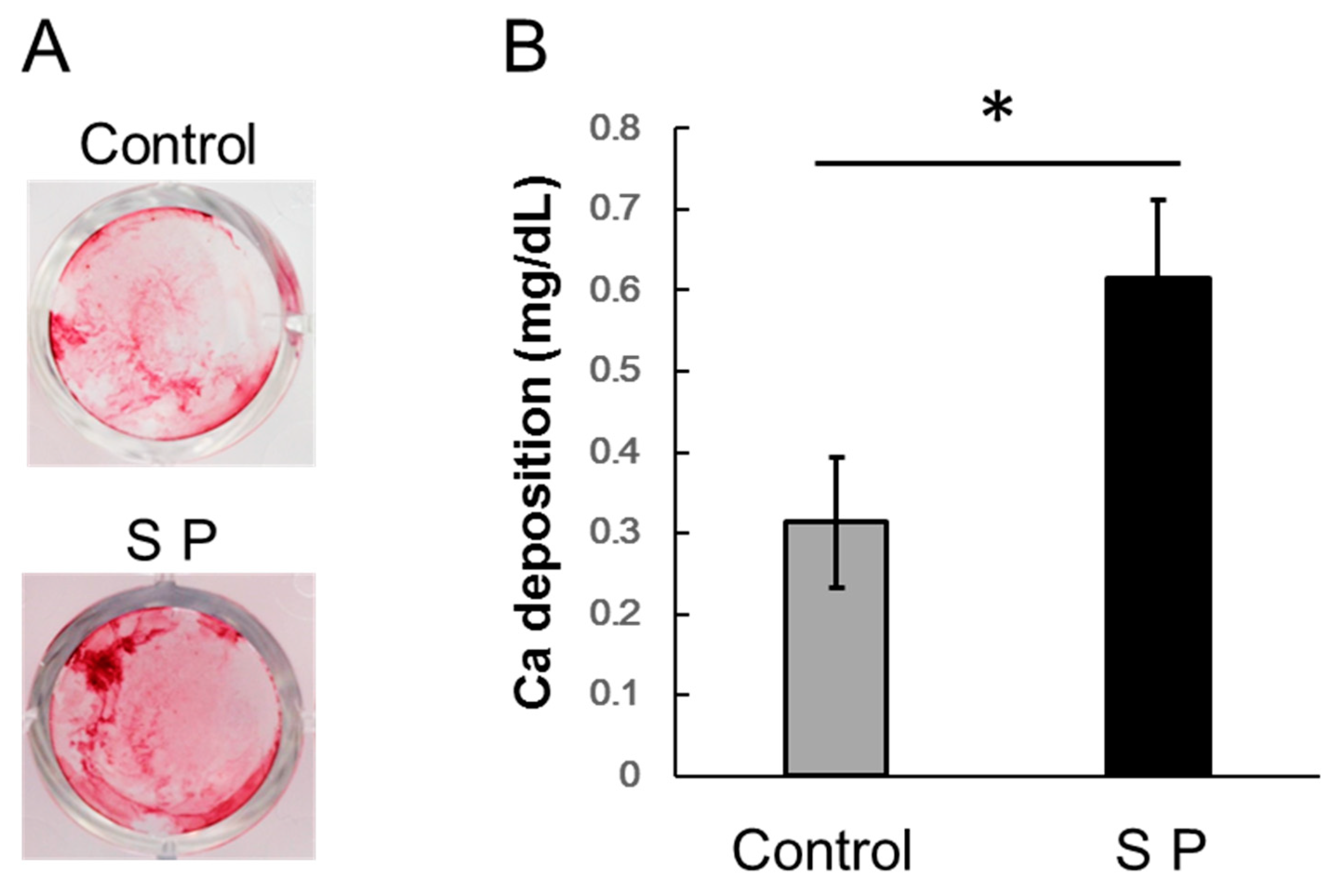
2.5. Extracellular Matrix Mineralization
2.6. Quantitative Real-Time Polymerase Chain Reaction (PCR)
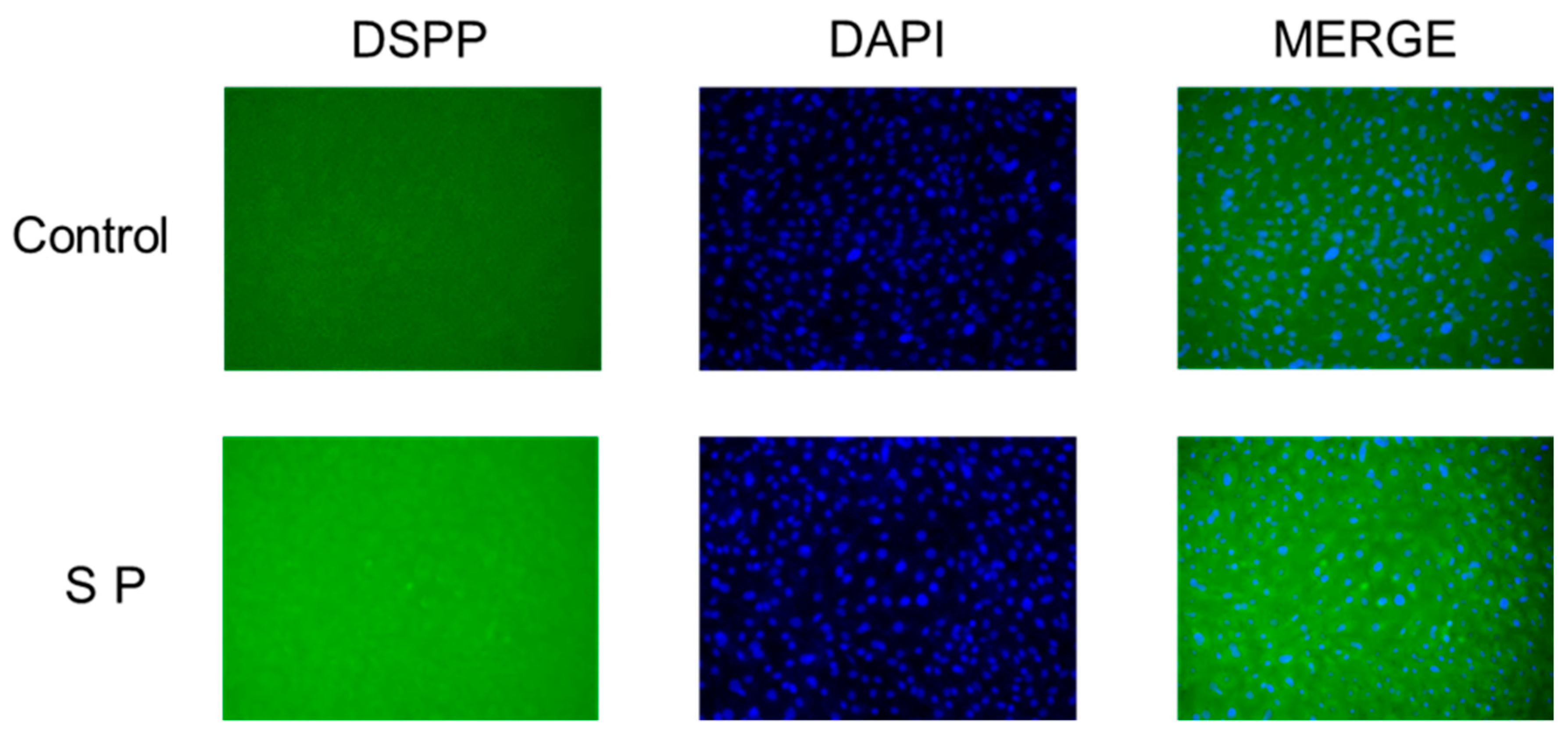
2.7. Immunofluorescence Staining
2.8. Statistical Analysis
3. Results
3.1. Cell Proliferation
3.2. Cell Morphology
3.3. ALP Activity
3.4. Extracellular Matrix Mineralization
3.5. mRNA Expression of Dentin Sialoprotein (DSPP)
3.6. Immunofluorescence Expression of DSPP
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hammarström, L. Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 1997, 24, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Hammarström, L.; Heijl, L.; Gestrelius, S. Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J. Clin. Periodontol. 1997, 24, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Tominaga, K.; Tanaka, A. Analysis of eosinophilic round bodies formed after injection of enamel matrix derivative into the backs of rats. J. Periodontol. 2005, 76, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Hida, T.; Tominaga, K.; Tanaka, A. Tissue Reaction to synthetic oligopeptide derived from enamel matrix derivative in rats. Oral Sci. Int. 2010, 7, 26–33. [Google Scholar] [CrossRef]
- Noguchi, M.; Tominaga, K.; Tanaka, A.; Ueda, M. Hard tissue formation induced by synthetic oligopeptide derived from an enamel matrix derivative. Oral Med. Pathol. 2012, 16, 75–80. [Google Scholar] [CrossRef]
- Miki, H.; Tominga, K.; Takahashi, T.; Tanaka, A.; Umeda, M. Comparison of the influence of a new synthetic peptide and enamel matrix derivative in the early wound healing process of artificial periodontal tissue defects in rats. Jpn. J. Conserv. Dent. 2018, 61, 17–29. [Google Scholar]
- Kawanaka, A.; Tominaga, K.; Tanaka, A. Effect of peptide derived from Emdogain on human periodontal ligament fibroblasts. J. Osaka Dent. Univ. 2009, 43, 111–117. [Google Scholar]
- Katayama, N.; Kato, H.; Taguchi, Y.; Tanaka, A.; Umeda, M. The effects of synthetic oligopeptide derived from enamel matrix derivative on cell proliferation and osteoblastic differentiation of human mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 14026–14043. [Google Scholar] [CrossRef] [PubMed]
- Yasui, N.; Taguchi, Y.; Tanaka, A.; Ueda, M.; Umeda, M. Biological effects of emdogain-derived oligopeptides on rat bone marrow cells in vitro. J. Oral Tissue Eng. 2012, 9, 126–135. [Google Scholar]
- Kato, H.; Katayama, N.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. A synthetic oligopeptide derived from enamel matrix derivative promotes the differentiation of human periodontal ligament stem cells into osteoblast-like cells with increased mineralization. J. Periodontol. 2013, 84, 1476–1483. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Hsu, C.W.; Tsai, W.H. The induction and possible subsequent effect of human antibodies against porcine enamel matrix derivative. J. Periodontol. 2006, 77, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Shinnick, T.M.; Sutcliffe, J.G.; Green, N.; Lerner, R.A. Synthetic peptide immunogens as vaccines. Ann. Rev. Microbiol. 1983, 37, 425–446. [Google Scholar] [CrossRef] [PubMed]
- Lerner, R.A. Tapping the immunological repertoire to produce antibodies of predetermined specificity. Nature 1982, 299, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Bergenholtz, G.; Mjör, I.A.; Cotton, W.R.; Hanks, C.T.; Kim, S.; Torneck, C.D.; Trowbridge, H.O. The biology of dentin and pulp. Consensus report. J. Dent. Res. 1985, 64, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Couve, E. Ultrastructural changes during the life cycle of human odontoblasts. Arch. Oral Biol. 1986, 31, 643–651. [Google Scholar] [CrossRef]
- Bergenholtz, G. Advances since the paper by Zander and Glass (1949) on the pursuit of healing methods for pulpal exposures: Historical perspectives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2005, 100, S102–S108. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H. Dynamics of the pulpo-dentin complex. Crit. Rev. Oral Biol. Med. 1996, 7, 104–133. [Google Scholar] [CrossRef] [PubMed]
- Zander, H.A.; Glass, R.L. The healing of phenolized pulp exposures. Oral Surg. Oral Med. Oral Pathol. 1949, 2, 803–810. [Google Scholar] [CrossRef]
- Nomiyama, K.; Kitamura, C.; Tsujisawa, T.; Nagayoshi, M.; Morotomi, T.; Terashita, M.; Nishihara, T. Effects of lipopolysaccharide on newly established rat dental pulp-derived cell line with odontoblastic properties. J. Endod. 2007, 33, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Washio, A.; Kitamura, C.; Morotomi, T.; Terashita, M.; Nishihara, T. Possible involvement of Smad signaling pathways in induction of odontoblastic properties in KN-3 cells by bone morphogenetic protein-2: A growth factor to induce dentin regeneration. Int. J. Dent. 2012, 2012, 258469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hase, N.; Ozeki, N.; Hiyama, T.; Yamaguchi, H.; Kawai, R.; Kondo, A.; Nakata, K.; Mogi, M. Products of dentin matrix protein-1 degradation by interleukin-1β-induced matrix metalloproteinase-3 promote proliferation of odontoblastic cells. Biosci. Trends 2015, 9, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, T.; Ozeki, N.; Mogi, M.; Yamaguchi, H.; Kawai, R.; Nakata, K.; Kondo, A.; Nakamura, H. Matrix metalloproteinase-3 in odontoblastic cells derived from ips cells: Unique proliferation response as odontoblastic cells derived from ES cells. PLoS ONE 2013, 8, e83563. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, V.; Rota, C.; Silvestri, M.; Piacentini, C.; Forlino, A.; Gallanti, A.; Rasperini, G.; Cetta, G. Effect of enamel matrix derivative on human periodontal fibroblasts: Proliferation, morphology and root surface colonization. An in vitro study. J. Periodontal Res. 2003, 38, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Guida, L.; Annunziata, M.; Carinci, F.; Di Feo, A.; Passaro, I.; Oliva, A. In vitro biologic response of human bone marrow stromal cells to enamel matrix derivative. J. Periodontol. 2007, 78, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.; Yasui, N.; Takahashi, S.; Tominaga, K.; Kato, H.; Komasa, S.; Shida, M.; Hayashi, H.; Tanaka, A.; Umeda, M. Hard tissue formation by human periodontal ligament fibroblast Cells treated with an emdogain-derived oligopeptide in vitro. J. Hard Tissue Biol. 2012, 21, 375–384. [Google Scholar] [CrossRef]
- Weinreb, M.; Shinar, D.; Rodan, G.A. Different pattern of alkaline phosphatase, osteopontin, and osteocalcin expression in developing rat bone visualized by in situ hybridization. J. Bone Miner. Res. 1990, 5, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Magne, D.; Bluteau, G.; Lopez-Cazaux, S.; Weiss, P.; Pilet, P.; Ritchie, H.H.; Daculsi, G.; Guicheux, J. Development of an odontoblast in vitro model to study dentin mineralization. Connect. Tissue Res. 2004, 45, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zou, B.; Narayanan, K.; George, A. Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone 2004, 34, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Sreenath, T.; Thyagarajan, T.; Hall, B.; Longenecker, G.; D’Souza, R.; Hong, S.; Wright, J.T.; MacDougall, M.; Sauk, J.; Kulkarni, A.B. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J. Biol. Chem. 2003, 278, 24874–24880. [Google Scholar] [CrossRef] [PubMed]
- Yeom, K.H.; Ariyoshi, W.; Okinaga, T.; Washio, A.; Morotomi, T.; Kitamura, C.; Nishihara, T. Platelet-rich plasma enhances the differentiation of dental pulp progenitor cells into odontoblasts. Int. Endod. J. 2016, 49, 271–278. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, H.; Taguchi, Y.; Imai, K.; Ruan, Y.; Tsai, Y.-W.; Chen, Y.-C.; Shida, M.; Taguchi, R.; Tominaga, K.; Umeda, M. The Enhancing Effects of Amelogenin Exon 5-Encoded Peptide from Enamel Matrix Derivative on Odontoblast-Like KN-3 Cells. Appl. Sci. 2018, 8, 1890. https://doi.org/10.3390/app8101890
Kato H, Taguchi Y, Imai K, Ruan Y, Tsai Y-W, Chen Y-C, Shida M, Taguchi R, Tominaga K, Umeda M. The Enhancing Effects of Amelogenin Exon 5-Encoded Peptide from Enamel Matrix Derivative on Odontoblast-Like KN-3 Cells. Applied Sciences. 2018; 8(10):1890. https://doi.org/10.3390/app8101890
Chicago/Turabian StyleKato, Hirohito, Yoichiro Taguchi, Kazutaka Imai, Yaru Ruan, Yu-Wei Tsai, Yi-Chie Chen, Muneyasu Shida, Reiko Taguchi, Kazuya Tominaga, and Makoto Umeda. 2018. "The Enhancing Effects of Amelogenin Exon 5-Encoded Peptide from Enamel Matrix Derivative on Odontoblast-Like KN-3 Cells" Applied Sciences 8, no. 10: 1890. https://doi.org/10.3390/app8101890
APA StyleKato, H., Taguchi, Y., Imai, K., Ruan, Y., Tsai, Y.-W., Chen, Y.-C., Shida, M., Taguchi, R., Tominaga, K., & Umeda, M. (2018). The Enhancing Effects of Amelogenin Exon 5-Encoded Peptide from Enamel Matrix Derivative on Odontoblast-Like KN-3 Cells. Applied Sciences, 8(10), 1890. https://doi.org/10.3390/app8101890




