Efficient Recovery of Silver from Crystalline Silicon Solar Cells by Controlling the Viscosity of Electrolyte Solvent in an Electrochemical Process
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Dissolution of Ag in an Organic Solvent
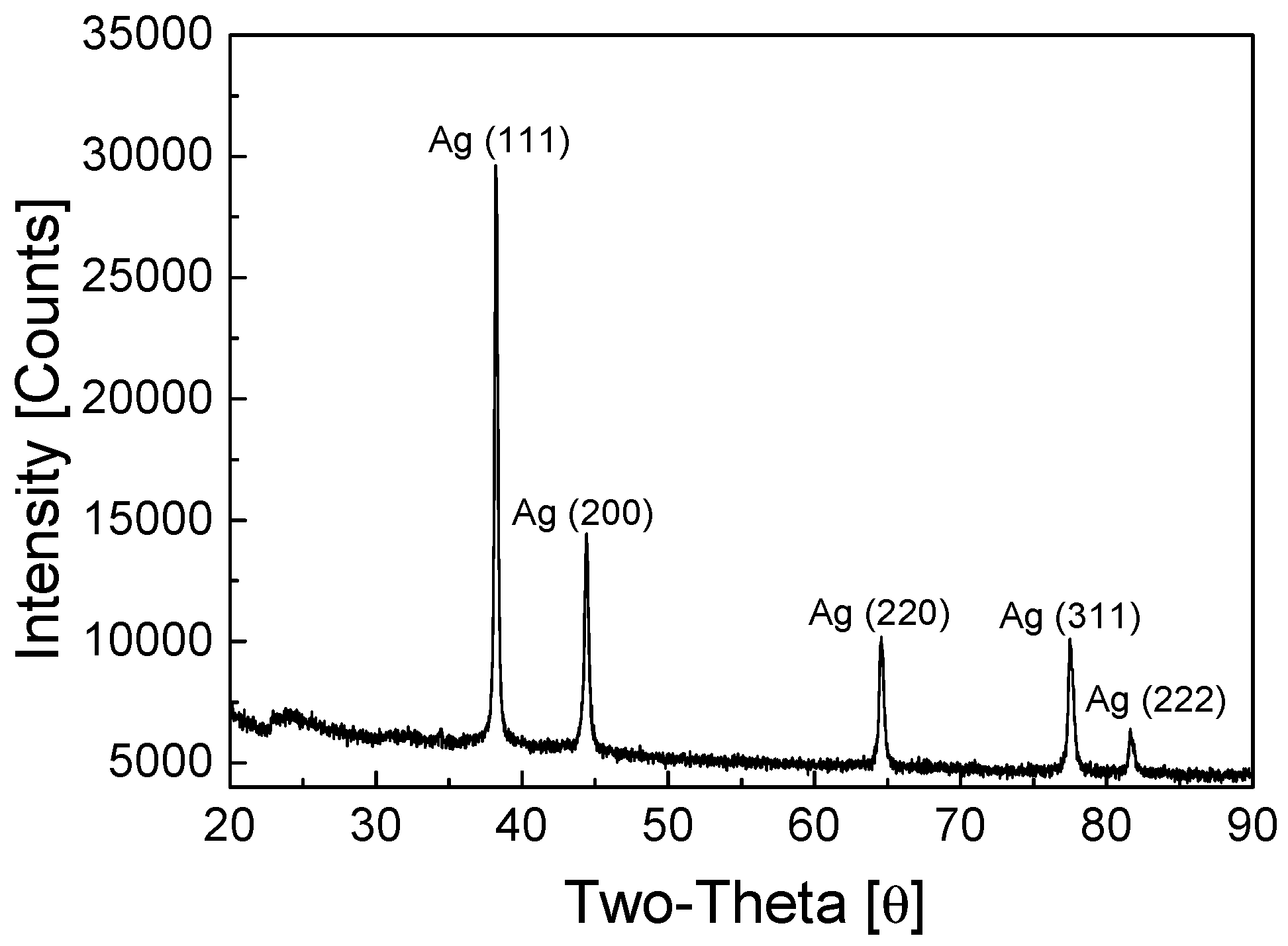
2.2. Recovery of Ag by Electrowinning
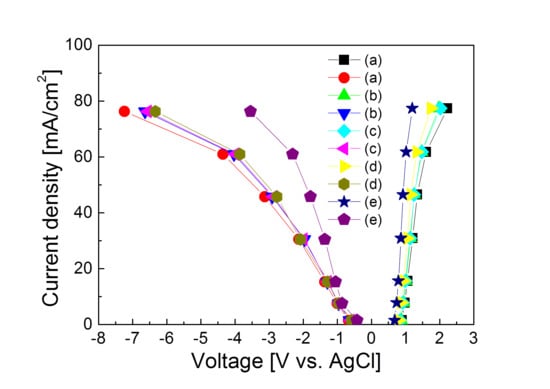
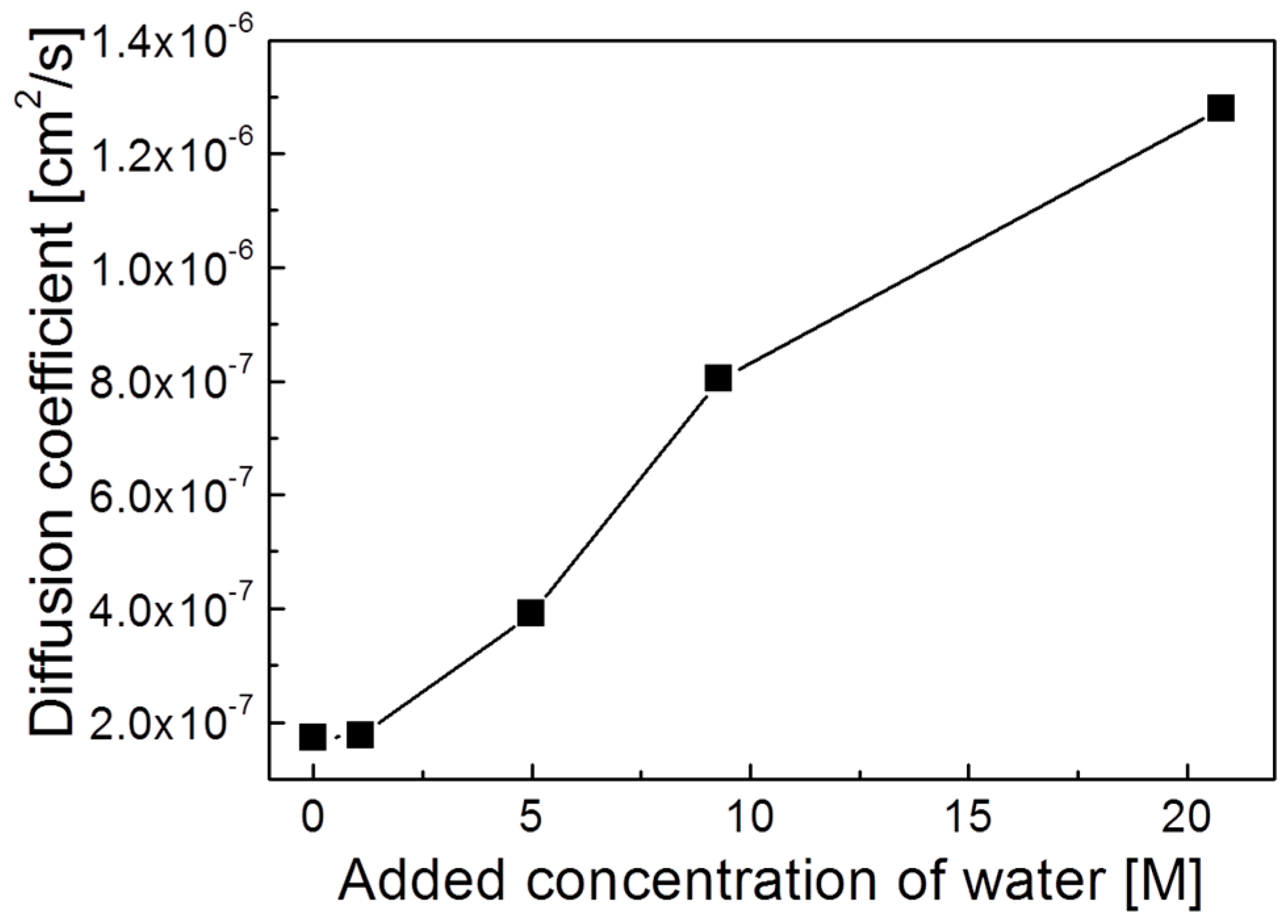
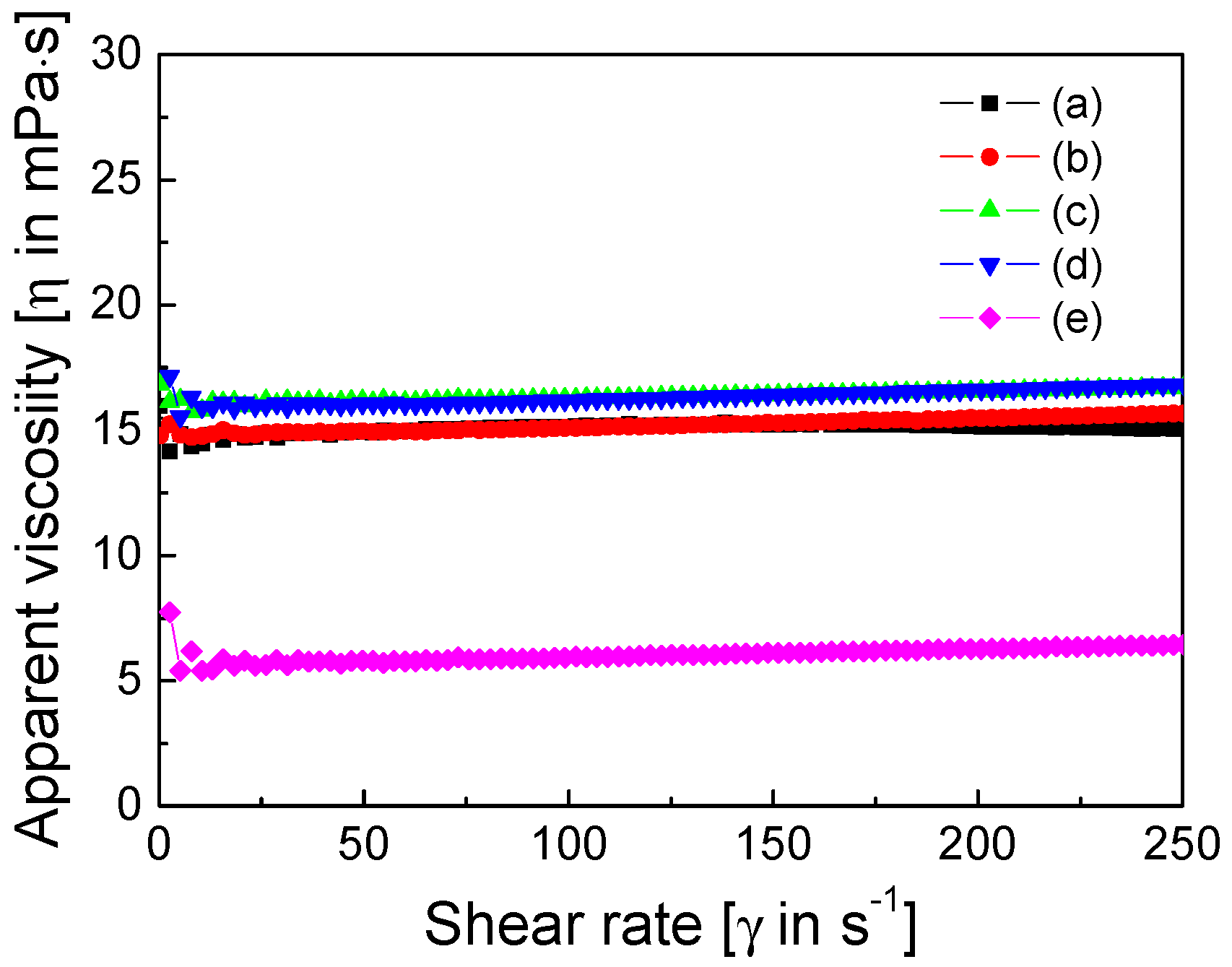
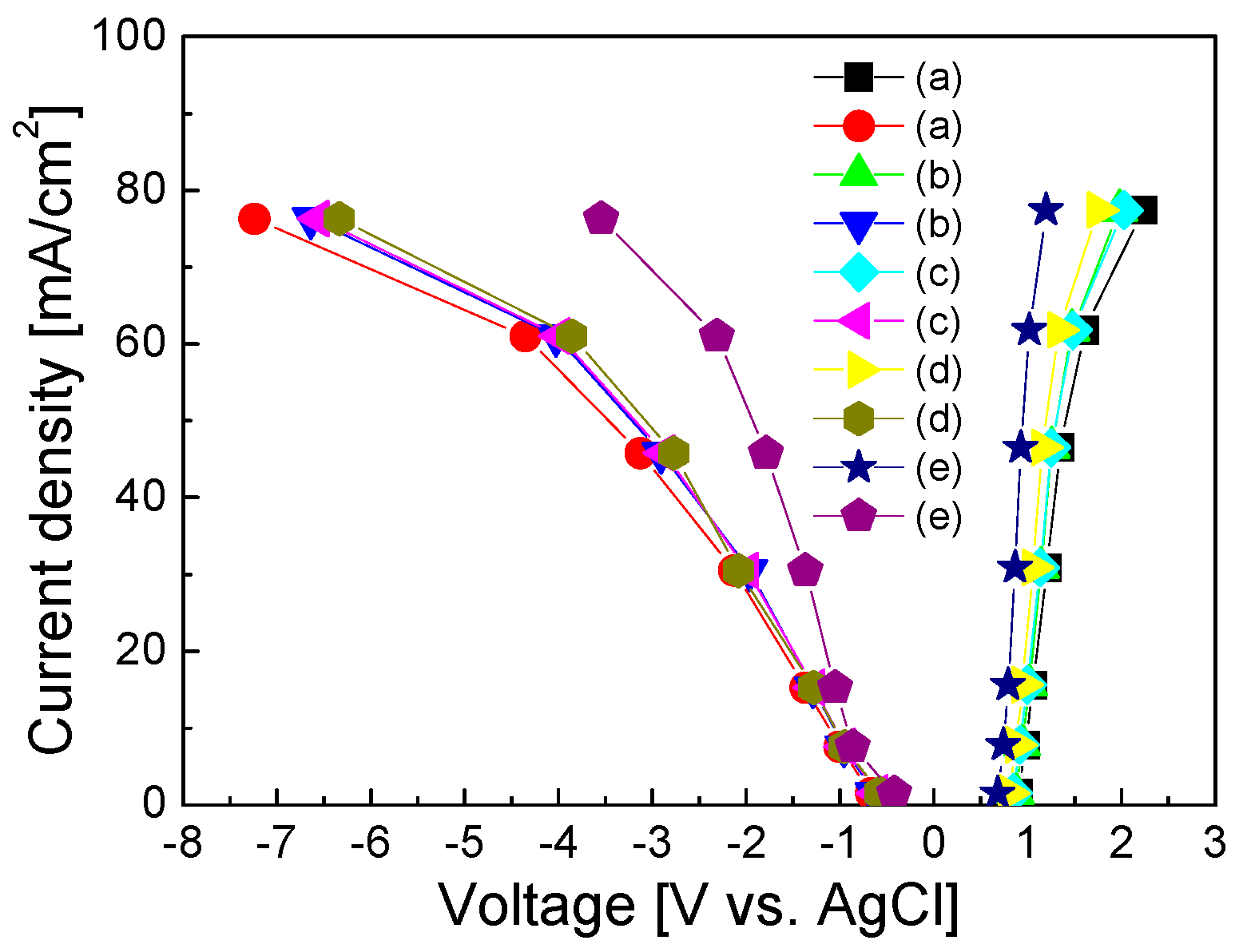
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tao, J.; Yu, S. Review on feasible recycling pathways and technologies of solar photovoltaic modules. Sol. Energy Mater. Sol. Cells 2015, 141, 108–124. [Google Scholar] [CrossRef]
- Doi, T.; Tsuda, I.; Unagida, H.; Murata, A.; Sakuta, K.; Kurokawa, K. Experimental study on PV module recycling with organic solvent method. Sol. Energy Mater. Sol. Cells 2001, 67, 397–403. [Google Scholar] [CrossRef]
- Granata, G.; Pagnanelli, F.; Moscardini, E.; Havlik, T.; Toro, L. Recycling of photovoltaic panels by physical operations. Sol. Energy Mater. Sol. Cells 2014, 123, 239–248. [Google Scholar] [CrossRef]
- Directive, E.C. Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on waste electrical and electronic equipment, WEEE. Off. J. Eur. Union L 2012, 197, 38–71. [Google Scholar]
- Weckend, S.; Wade, A.; Heath, G. End-of-Life Management: Solar Photovoltaic Panels; No. NREL/BK-6A20-66178; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2016.
- Nieland, S.; Neuhaus, U.; Pfaff, T.; Radlein, E. New approaches for component recycling of crystalline solar modules. In Proceedings of the Electronics Goes Green 2012+(EGG), Berlin, Germany, 9–12 September 2012; pp. 1–5. [Google Scholar]
- Palitzsch, W.; Loser, U. A new and intelligent de-metalization step of broken silicon cells and silicon cell production waste in the recycling procedure of crystalline Si modules. In Proceedings of the 37th IEEE Photovoltaic Specialists Conference (PVSC), Seattle, WA, USA, 19–24 June 2011. [Google Scholar]
- Dias, P.; Javimczik, S.; Benevit, M.; Veit, H.; Bernardes, A.M. Recycling WEEE: Extraction and concentration of silver from waste crystalline silicon photovoltaic modules. Waste Manag. 2016, 57, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.K.; Kim, H.S.; Tran, T.; Hong, S.K.; Kim, M.J. Recovering valuable metals from recycled photovoltaic modules. J. Air Waste Manag. Assoc. 2014, 64, 797–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.K.; Lee, J.S.; Ahn, Y.S.; Kang, G.H. Effect of current density on morphology of silver thin film recovered from crystalline silicon solar cell by electrochemical process. Thin Solid Films 2018, 663, 143–147. [Google Scholar] [CrossRef]
- Klugmann-Radziemska, E. Recycling of Photovoltaic Solar Cells and Modules-The State-Of-Art; Lambert Academic Publishing: Saarbrücken, Germany, 2014; pp. 1–53. ISBN 978-3-659-51951-2. [Google Scholar]
- Lee, C.H.; Hung, C.E.; Tsai, S.L.; Popuri, S.R.; Liao, C.H. Resource recovery of scrap silicon solar battery cell. Waste Manag. Res. 2013, 31, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Kuczyńska-Łażewska, A.; Klugmann-Radziemska, E.; Sobczak, Z.; Klimczuk, T. Recovery of silver metallization from damaged silicon cells. Sol. Energy Mater. Sol. Cells 2018, 176, 190–195. [Google Scholar] [CrossRef]
- Hiskey, J.B.; Sanchez, V.M. Mechanistic and kinetic aspects of silver dissolution in cyanide solutions. J. Appl. Electrochem. 1990, 20, 479–487. [Google Scholar] [CrossRef]
- Huang, W.H.; Shin, W.J.; Wang, L.; Sun, W.C.; Tao, M. Strategy and technology to recycle wafer-silicon solar modules. Sol. Energy 2017, 144, 22–31. [Google Scholar] [CrossRef]
- Yang, E.H.; Lee, J.K.; Lee, J.S.; Ahn, Y.S.; Kang, G.H.; Cho, C.H. Environmentally friendly recovery of Ag from end-of-life c-Si solar cell using organic acid and its electrochemical purification. Hydrometallurgy 2017, 167, 129–133. [Google Scholar] [CrossRef]
- Gernon, M.D.; Wu, M.; Buszta, T.; Janney, P. Environmental benefits of methanesulfonic acid. Comparative properties and advantages. Green Chem. 1999, 1, 127–140. [Google Scholar] [CrossRef]
- Suo, L.; Hu, Y.S.; Li, H.; Armand, M.; Chen, L. A new class of solvent-in-salt electrolyte for high-energy rechargeable metallic lithium batteries. Nat. Commun. 2013, 4, 1481. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Hayamizu, K.; Tsuzuki, S.; Takahashi, K.; Ishino, Y.; Kato, M.; Nozaki, E.; Watanabe, H.; Umebayashi, Y. Density, viscosity, ionic conductivity, and self-diffusion coefficient of organic liquid electrolytes: Part I. Propylene carbonate + Li, Na, Mg and Ca cation salts. J. Electrochem. Soc. 2018, 165, A542–A546. [Google Scholar] [CrossRef]
- Bandara, T.M.W.J.; Mellander, B.E. Evaluation of mobility, diffusion coefficient and density of charge carriers in ionic liquids and novel electrolytes based on a new model for dielectric response. In Ionic Liquids: Theory, Properties, New Approaches; InTech: London, UK, 2011; pp. 383–406. ISBN 978-953-307-349-1. [Google Scholar]
- Neghmouche, N.S.; Lanez, T. Calculation of electrochemical parameters starting from the polarization curves of ferrocene at glassy carbon electrode. Int. Lett. Chem. Phys. Astron. 2013, 4, 37–45. [Google Scholar] [CrossRef]
- Edward, J.T. Molecular volumes and the Stokes-Einstein equation. J. Chem. Educ. 1970, 47, 261. [Google Scholar] [CrossRef]
- Hazza, A.; Pletcher, D.; Wills, R. A novel flow battery: A lead acid battery based on an electrolyte with soluble lead (II) Part I. Preliminary studies. Phys. Chem. Chem. Phys. 2014, 6, 1773–1778. [Google Scholar] [CrossRef]
- Stankovic, V. Metal removal from effluents by electrowinning and a new design concept in wastewater purification technology. Chem. Biochem. Eng. Q. 2007, 21, 33–45. [Google Scholar]
- Rahman, H.A.; Moustafa, A.H.E.; Magid, S.A. High rate copper electrodeposition in the presence of inorganic salts. Int. J. Electrochem. Sci. 2012, 7, 6959–6975. [Google Scholar]
- Wang, K.; Pei, P.; Ma, Z.; Chen, H.; Xu, H.; Chen, D.; Wang, X. Dendrite growth in the recharging process of zinc–air batteries. J. Mater. Chem. A 2015, 3, 22648–22655. [Google Scholar] [CrossRef]
- Borikar, D.K.; Umare, S.S.; Viswanath, S.G. Electrowinning of Nickel from ammonical sulphate bath and effect of acetone on morphology of nickel deposit and its correlation with kinetic parameters. Metalurgija 2005, 45, 3–8. [Google Scholar]
- Szmant, H.H. Organic Building Blocks of the Chemical Industry; John Wiley & Sons: New York, NY, USA, 1989; p. 92. ISBN 0-471-85545-6. [Google Scholar]


| Contents | (a) | (b) | (c) | (d) | (e) |
|---|---|---|---|---|---|
| Concentration of added water (M) | 0 | 1.1 | 5.0 | 9.3 | 20.8 |
| MSA/oxidizing agent | 9:1 | ||||
| Concentration of silver (M) | 5.56 × 10−5 | ||||
| Cathode | Tungsten plate | ||||
| Anode | Platinum wire | ||||
| Elements | Concentration (ppmw) | Elements | Concentration (ppmw) |
|---|---|---|---|
| Li | <0.005 | Cr | 1.5 |
| Be | <0.001 | Mn | 0.09 |
| B | <0.005 | Fe | 5.4 |
| Na | 0.45 | Co | 0.006 |
| Mg | 0.06 | Ni | 0.87 |
| Al | 0.98 | Cu | 0.11 |
| Si | 2.9 | Zn | 0.03 |
| P | 5.8 | Ga | <0.1 |
| S | 220 | Ge | <0.1 |
| Cl | ~600 | Ag | Matrix |
| K | 0.16 | Sn | 12 |
| Ca | 0.19 | Pb | <0.05 |
| Ti | 0.07 | Bi | 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-K.; Lee, J.-S.; Ahn, Y.-S.; Kang, G.-H. Efficient Recovery of Silver from Crystalline Silicon Solar Cells by Controlling the Viscosity of Electrolyte Solvent in an Electrochemical Process. Appl. Sci. 2018, 8, 2131. https://doi.org/10.3390/app8112131
Lee J-K, Lee J-S, Ahn Y-S, Kang G-H. Efficient Recovery of Silver from Crystalline Silicon Solar Cells by Controlling the Viscosity of Electrolyte Solvent in an Electrochemical Process. Applied Sciences. 2018; 8(11):2131. https://doi.org/10.3390/app8112131
Chicago/Turabian StyleLee, Jun-Kyu, Jin-Seok Lee, Young-Soo Ahn, and Gi-Hwan Kang. 2018. "Efficient Recovery of Silver from Crystalline Silicon Solar Cells by Controlling the Viscosity of Electrolyte Solvent in an Electrochemical Process" Applied Sciences 8, no. 11: 2131. https://doi.org/10.3390/app8112131