A Modified Interposer Fabrication Process by Copper Nano-Pillars Filled in Anodic Aluminum Oxide Film for 3D Electronic Package
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effect of Parameters on Pore-Filling Percentage
3.2. Growth of CNPs on ITO
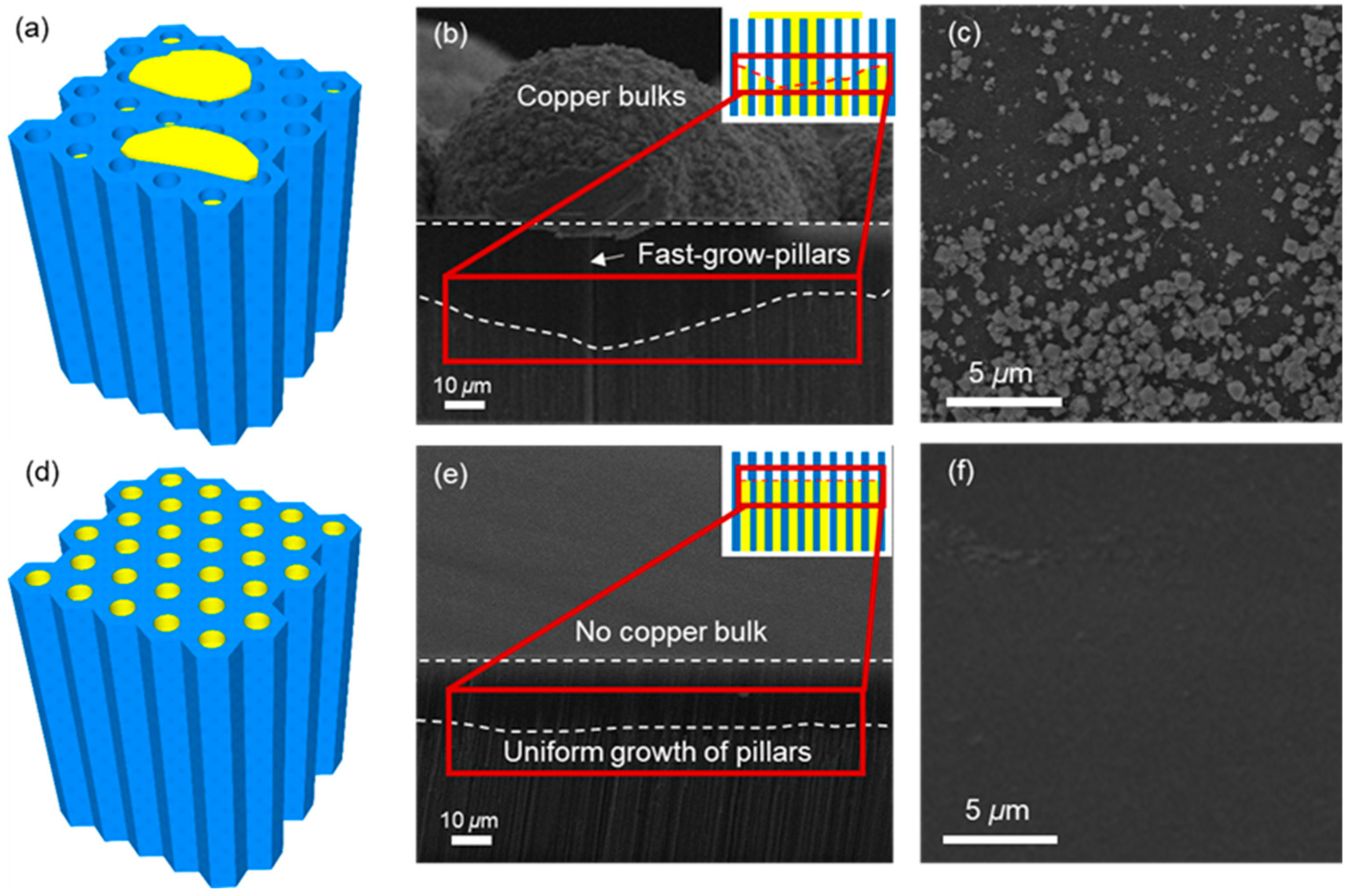
3.3. Performance of Cu-AAO Interposer
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waldrop, M.M. The chips are down for Moore’s law. Nature 2016, 530, 144–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.; Lin, Y.; Wu, S.; Lee, C.; Huang, S.; Chang, H.; Chang, T.; Chen, K. Warpage characteristics and process development of through silicon via-less interconnection technology. J. Nanosci. Nanotechnol. 2018, 18, 5558–5565. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, B.M.D.; Suzuki, Y.; Furuya, R.; Nair, C.; Huang, T.; Smet, V.; Panayappan, K.; Sundaram, V.; Tummala, R. Design and demonstration of a 2.5-D glass interposer BGA package for high bandwidth and low cost. IEEE Trans. Compon. Packag. Manuf. Technol. 2017, 7, 552–562. [Google Scholar] [CrossRef]
- Su, J.; Ma, R.; Chen, X.; Han, X.L.; Yang, R.; Zhang, W. Low-loss shielded through-silicon vias filled with multi-walled carbon nanotube bundle. Microelectron. J. 2016, 58, 83–88. [Google Scholar] [CrossRef]
- Wu, B.; Kumar, A.; Pamarthy, S. High aspect ratio silicon etch: A review. J. Appl. Phys. 2010, 108, 051101. [Google Scholar] [CrossRef]
- Lu, T.T.; Serafy, C.; Yang, Z.Y.; Samal, S.K.; Lim, S.K.; Srivastava, A. TSV-based 3-D ICs: Design methods and tools. IEEE Trans. Comput. Aid D 2017, 36, 1593–1619. [Google Scholar] [CrossRef]
- Lee, K.W.; Bea, J.C.; Fukushima, T.; Ramalingam, S.; Wu, X.; Tanaka, T.; Koyanagi, M. Novel hybrid bonding technology using ultra-high density Cu nano-pillar for exascale 2.5D/3D integration. IEEE Electron. Device Lett. 2015, 37, 81–83. [Google Scholar] [CrossRef]
- Qian, L.; Xia, Y.; Shi, G.; Wang, J.; Ye, Y.; Du, S. Electrical–thermal characterization of through packaging vias in glass interposer. IEEE Trans. Nanotechnol. 2017, 16, 901–908. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hu, G.; Li, Y. Fabrication of ZnO nanostructure within the AAO template by electrochemical deposition. J. Mater. Sci. Mater Electron. 2010, 21, 950–953. [Google Scholar] [CrossRef]
- Hun, C.W.; Chiu, Y.; Luo, Z.; Chen, C.C.; Chen, S.H. A new technique for batch production of tubular anodic aluminum oxide films for filtering applications. Appl. Sci. 2018, 8, 1055. [Google Scholar] [CrossRef]
- Chien, Y.; Weng, H.C. Magnetic Nanofluid Droplet Impact on an AAO Surface with a Magnetic Field. Appl. Sci. 2018, 8, 1059. [Google Scholar] [CrossRef]
- Gao, T.; Meng, G.M.; Zhang, J.; Wang, Y.W.; Liang, C.H.; Fan, J.C.; Zhang, L.D. Template synthesis of single-crystal Cu nanowire arrays by electrodeposition. Appl. Phys. A 2001, 73, 251–254. [Google Scholar] [CrossRef]
- Yang, R.; Sui, C.; Gong, J.; Qu, L. Silver nanowires prepared by modified AAO template method. Mater. Lett. 2007, 61, 900–903. [Google Scholar] [CrossRef]
- Wang, S.; Tian, Y.; Ding, S.; Huang, Y. Rapid synthesis of long silver nanowires by controlling concentration of Cu2+ ions. Mater. Lett. 2016, 172, 175–178. [Google Scholar] [CrossRef]
- Gao, T.; Meng, G.; Wang, Y.; Sun, S.; Zhang, L. Electrochemical synthesis of copper nanowires. J. Phys. Condens. Mater. 2002, 14, 355–363. [Google Scholar] [CrossRef]
- Lv, X.; Hu, G.; Tang, J.; Wang, Y. New thoughts into the fabrication of ZnO nanoparticles: Confined growth in the channels of AAO membrane and its formation mechanisms. J. Mater. Sci. Mater. Electron. 2017, 28, 14163–14169. [Google Scholar] [CrossRef]
- Inguanta, R.; Piazza, S.; Sunseri, C. Influence of the electrical parameters on the fabrication of copper nanowires into anodic alumina templates. Appl. Surf. Sci. 2009, 255, 8816–8823. [Google Scholar] [CrossRef] [Green Version]
- Tian, M.; Wang, J.; Kurtz, J.; Mallouk, T.E.; Chan, M.H.W. Electrochemical growth of single-crystal metal nanowires via a two-dimensional nucleation and growth mechanism. Nano Lett. 2003, 3, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Duan, H.; Zhou, Z.; Liang, J. Fabrication of free-standing Cu nanorod arrays on Cu disc by template-assisted electrodeposition. J. Gnanaraj. Nanotechnol. 2008, 19, 365306. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, G.; Dhanasekaran, R.; Subraman, C. Electrodeposition and optical characterisation of CdS thin films on ITO-coated glass. Thin Solid Films 1997, 302, 71–76. [Google Scholar] [CrossRef]
- Lupan, O.; Guérin, V.M.; Tiginyanu, I.M.; Ursaki, V.V.; Chow, L.; Heinrich, H.; Pauporté, T. Well-Aligned Arrays of Vertically Oriented ZnO Nanowires Electrodeposited on ITO-Coated Glass and Their Integration in Dye Sensitized Solar Cell. J. Photochem. Photobiol. A 2010, 211, 65–73. [Google Scholar] [CrossRef]
- Tan, M.; Chen, X. Growth mechanism of single crystal nanowires of fcc metals (Ag, Cu, Ni) and hcp metal (Co) electrodeposited. J. Electrochem. Soc. 2012, 159, K15–K20. [Google Scholar] [CrossRef]
- Hsiao, H.; Liu, C.; Lin, H.; Liu, T.; Lu, C.; Huang, Y.; Tu, K.N. Unidirectional Growth of Microbumps on (111)-Oriented and Nanotwinned Copper. Science 2012, 336, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, B.S.; Kim, K.M.; Kong, B.H.; Cho, H.H. Tuning the morphology of copper nanowires by controlling the growth processes in electrodeposition. J. Mater. Chem. 2011, 21, 17967–17971. [Google Scholar] [CrossRef]
- Riveros, G.; Gomez, H.; Cortes, A.; Marotti, R.E.; Dalchiele, E.A. Crystallographically-oriented single-crystalline copper nanowire arrays electrochemically grown into nanoporous anodic alumina templates. Appl. Sci. A Mater. Sci. Process. 2005, 81, 17–24. [Google Scholar] [CrossRef]
- Gerein, N.J.; Haber, J.A. Effect of ac electrodeposition conditions on the growth of high aspect ratio copper nanowires in porous aluminum oxide templates. J. Phys. Chem. B 2005, 109, 17372–17385. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Chopra, K.L. Thermal conductivity of copper films. Thin Solid Films 1974, 20, 53–62. [Google Scholar] [CrossRef]
- Ghidelli, M.; Sebastiani, M.; Johanns, K.E.; Pharr, G.M. Effects of indenter angle on micro-scale fracture toughness measurement by pillar splitting. J. Am. Ceram. Soc. 2017, 100, 5731–5738. [Google Scholar] [CrossRef]






| Item | Value |
|---|---|
| ρ (g/cm3) | 4.5 |
| C (J/(kg·K−1)) | 631 |
| αh (m2/s) | 1.123 × 10−6 |
| αv (m2/s) | 32.521 × 10−6 |
| λhe (W·m−1·K−1) | 3.19 |
| λve (W·m−1·K−1) | 92.34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hang, C.; Zhang, H.; Tian, Y.; Wang, C.; Huang, Y.; Zheng, Z.; Wang, C. A Modified Interposer Fabrication Process by Copper Nano-Pillars Filled in Anodic Aluminum Oxide Film for 3D Electronic Package. Appl. Sci. 2018, 8, 2188. https://doi.org/10.3390/app8112188
Hang C, Zhang H, Tian Y, Wang C, Huang Y, Zheng Z, Wang C. A Modified Interposer Fabrication Process by Copper Nano-Pillars Filled in Anodic Aluminum Oxide Film for 3D Electronic Package. Applied Sciences. 2018; 8(11):2188. https://doi.org/10.3390/app8112188
Chicago/Turabian StyleHang, Chunjin, He Zhang, Yanhong Tian, Chenxi Wang, Yuan Huang, Zhen Zheng, and Chunqing Wang. 2018. "A Modified Interposer Fabrication Process by Copper Nano-Pillars Filled in Anodic Aluminum Oxide Film for 3D Electronic Package" Applied Sciences 8, no. 11: 2188. https://doi.org/10.3390/app8112188





