Adsorption of Nonylphenol to Multi-Walled Carbon Nanotubes: Kinetics and Isotherm Study
Abstract
:1. Introduction
2. Material and Method
2.1. Materials
2.2. Water Sample Preparation
2.3. Instrumentation and Techniques
2.4. Batch Adsorption Experiments
3. Result and Discussion
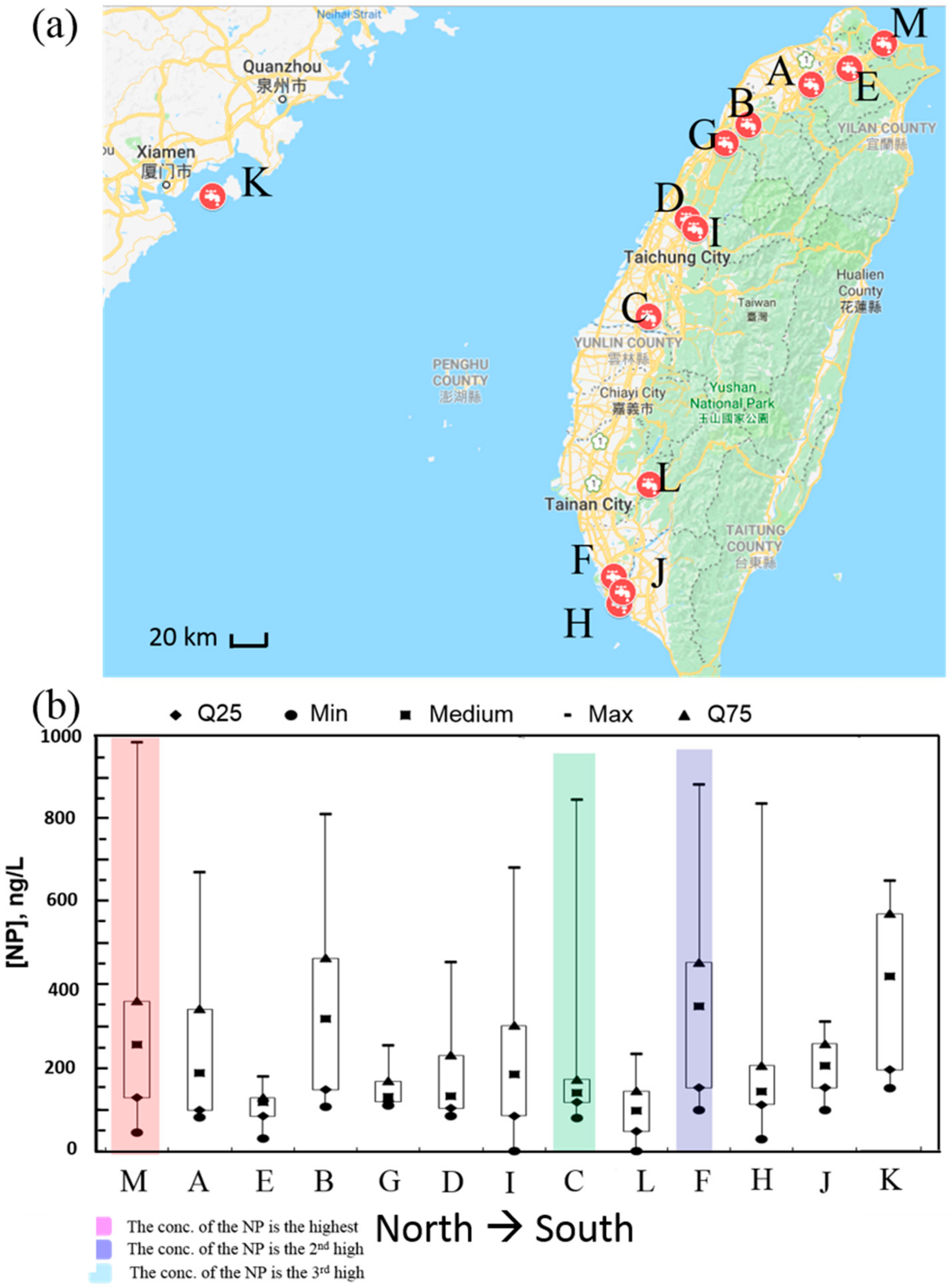
3.1. NP Concentrations of the Source and Treated Water Samples
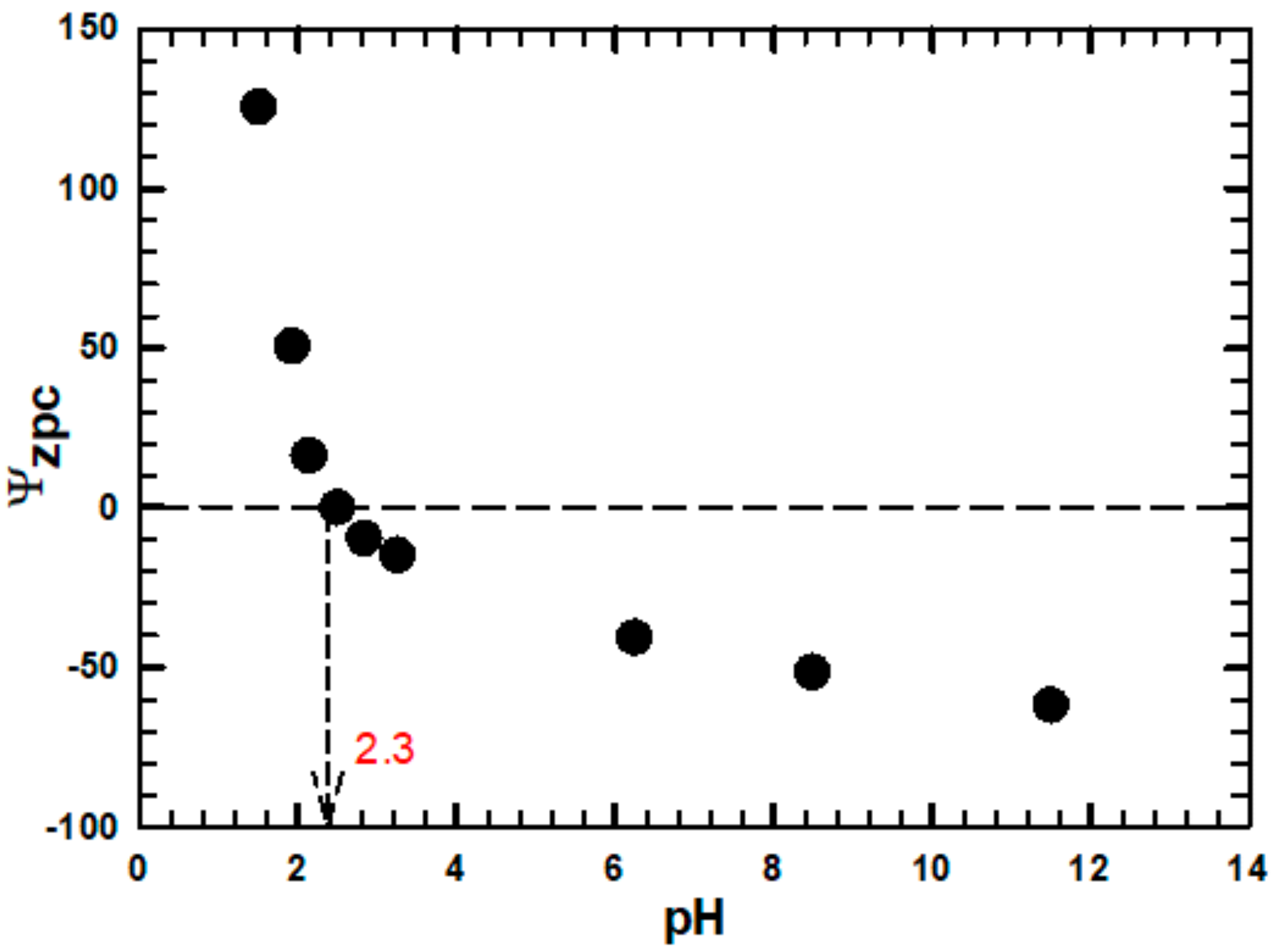
3.2. Characterization of Adsorbent
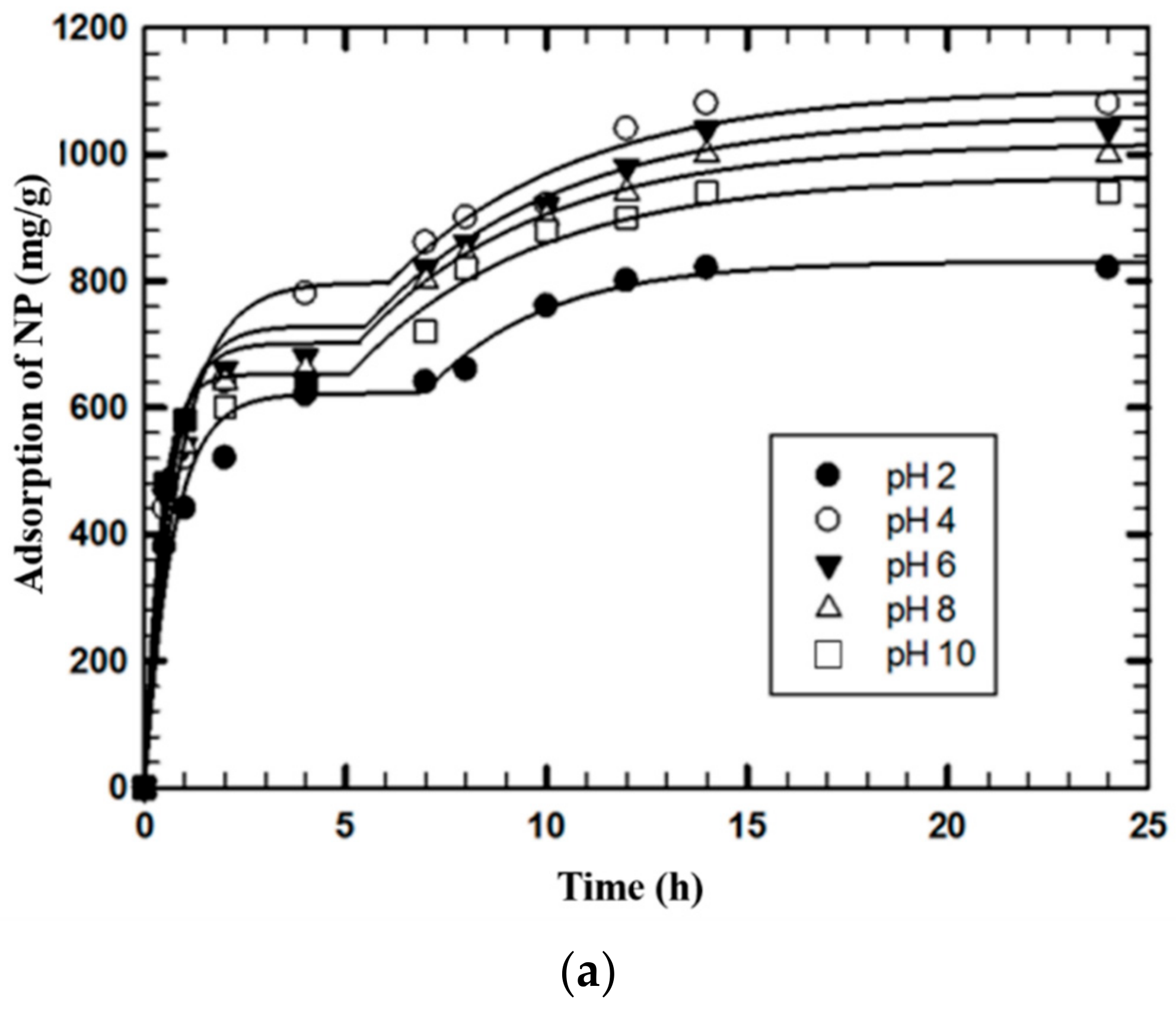
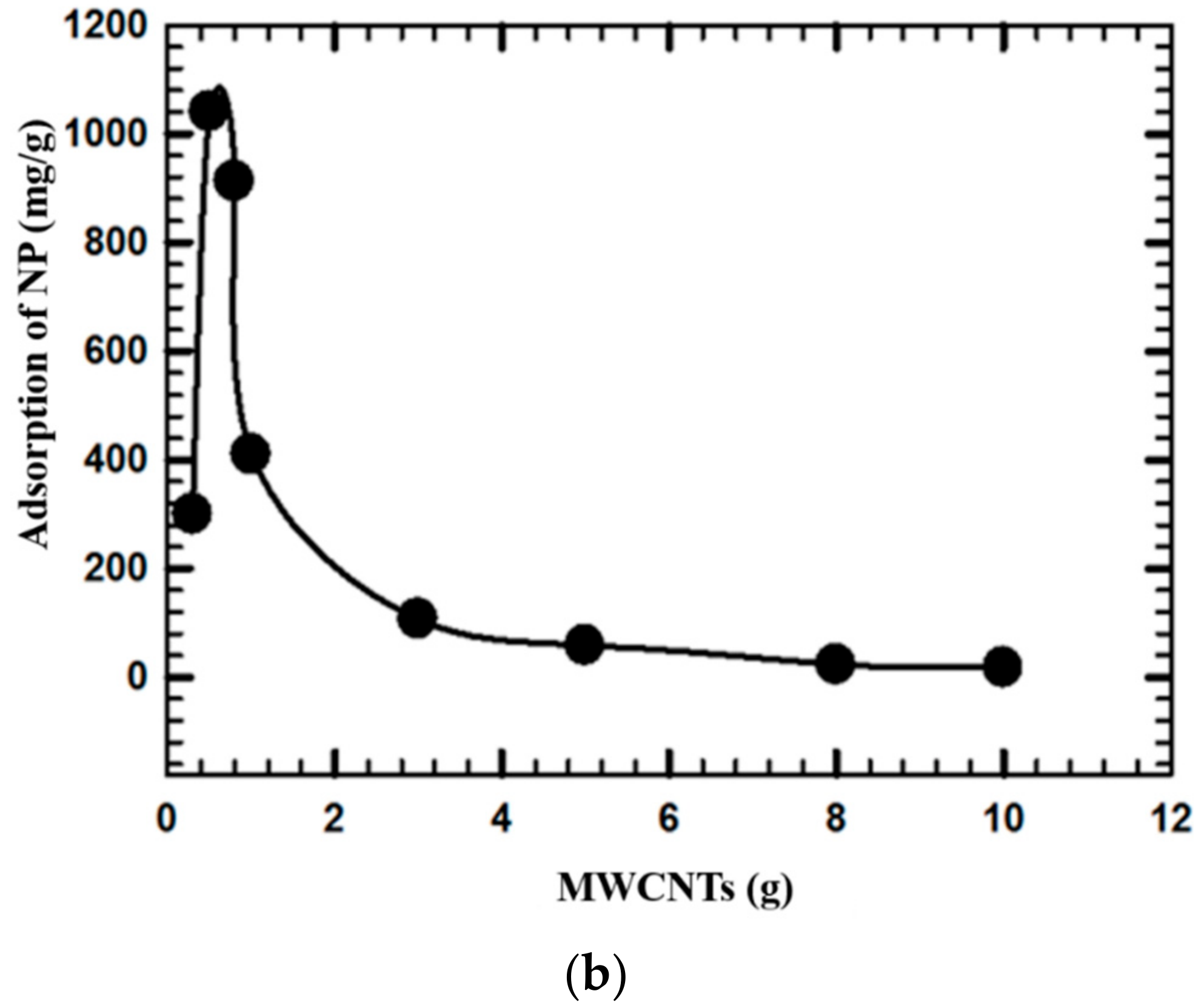
3.3. Effects of pH and MWCNTs Concentration on NP Removal
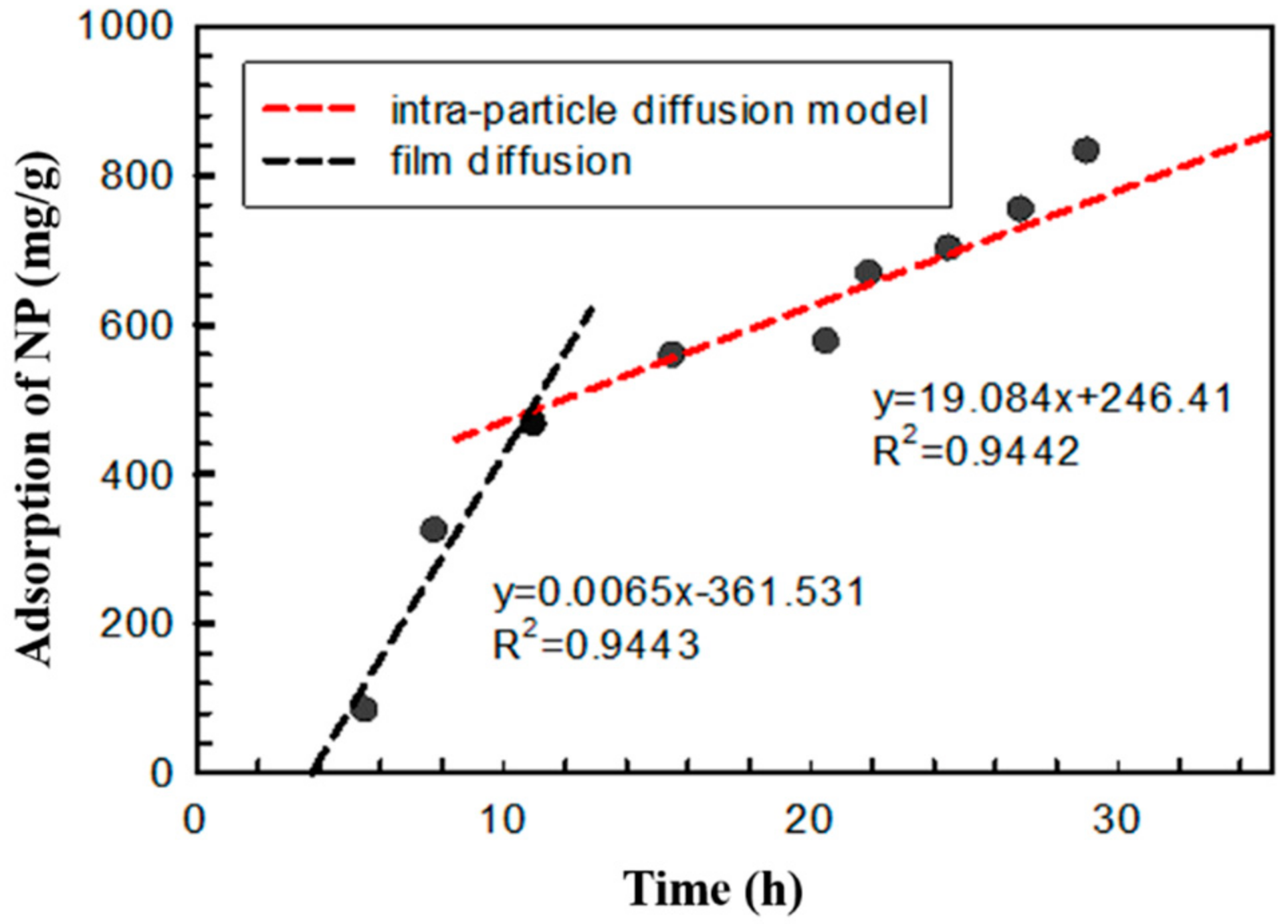
3.4. Adsorption Kinetics
3.5. Equilibrium Adsorption Isotherm
3.6. Thermodynamic Aspects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Soares, A.; Guieysse, B.; Jefferson, B.; Cartmell, E.; Lester, J.N. Nonylphenol in the environment: A critical review on oCcurrence, fate, toxicity and treatment in wastewaters. Environ. Int. 2008, 34, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Ademollo, N.; Delise, M.; Fabietti, F.; Funari, E. Alkylphenols and their ethoxylates in seafood from the Tyrrhenian sea. Chemosphere 2008, 72, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Groshart, C.P.; Okkerman, P.C.; Wassenberg, W.B.A.; Pijnenburg, A.M.C.M. Chemical study on alkylphenols. In RIKZ Report 2001.029; Nat. Inst. Coast and Marine Management (RIKZ): The Hague, The Netherlands, 2001. [Google Scholar]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol a, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouki, C.; Dvorakova, M.; Diamadopoulos, E. Adsorption of nonylphenol on activated sludge biomass under aseptic conditions. Clean Soil Air Water 2010, 38, 516–520. [Google Scholar] [CrossRef]
- Mao, Z.; Zheng, X.-F.; Zhang, Y.-Q.; Tao, X.-X.; Li, Y.; Wang, W. Occurrence and biodegradation of nonylphenol in the environment. Int. J. Mol. Sci. 2012, 13, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.N.; Ra, J.S.; Cho, J.; Kim, S.D.; Choi, H.K.; Park, J.H.; Kim, K.W.; Inam, E.; Kim, S.D. Estrogenic chemicals and estrogenicity in river waters of south Korea and seven Asian countries. Chemosphere 2010, 78, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Liang, C.H.; Wu, Z.M.; Chang, E.E.; Lin, T.F.; Chiang, P.C.; Wang, G.S. Occurrence, and assessment of treatment efficiency of nonylphenol, octylphenol and bisphenol-A in drinking water in Taiwan. Sci. Total Environ. 2013, 449, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Derbalah, A.S.H.; Nakatani, N.; Sakugawa, H. Distribution, seasonal pattern, flux and contamination source of pesticides and nonylphenol residues in Kurose River water, Higashi-Hiroshima, Japan. GeoChem. J. 2003, 37, 217–232. [Google Scholar] [CrossRef]
- Li, D.; Kim, M.; Oh, J.R.; Park, J. Distribution characteristics of nonylphenols in the artificial Lake Shihwa, and surrounding creeks in Korea. Chemosphere 2004, 56, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kim, M.; Shim, W.J.; Yim, U.H.; Oh, J.R.; Kwon, Y.J. Seasonal flux of nonylphenol in Han River, Korea. Chemosphere 2004, 56, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kawahata, H.; Ohta, H.; Inoue, M.; Suzuki, A. Endocrine disrupter nonylphenol and bisphenol A contamination in Okinawa and Ishigaki Islands, Japan—Within coral reefs and adjacent river mouths. Chemosphere 2004, 55, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Wu, C.Y.; Wang, C.H.; Ding, W.H. Determination and distribution characteristics of degradation products of nonylphenol polyethoxylates in the rivers of Taiwan. Chemosphere 2006, 65, 2275–2281. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, P.; Guo, W.; Dong, J.; Wang, L.; Dai, S. Seasonal and spatial distribution of nonylphenol in Lanzhou Reach of Yellow River in China. Chemosphere 2006, 65, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Li, Z.; Gao, H. Distribution characteristics of nonylphenol in Jiaozhou Bay of Qingdao and its adjacent rivers. Chemosphere 2007, 69, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Li, M.H. Acute Toxicity of 4-Nonylphenol to Aquatic Invertebrates in Taiwan. Bull. Environ. Contam. Toxicol. 2007, 78, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Motegi, M.; Nojiri, K.; Hosono, S.; Kawamura, K. Determination and evaluation of estrogenic contamination in an urban river basin. J. Environ. Chem. 2007, 17, 421–431, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Z.; Chen, S.; He, F.; Fu, G.; Liang, W. Nonylphenol and octylphenol in urban eutrophic lakes of the subtropical China. Fresenius Environ. Bull. 2007, 16, 227–234. [Google Scholar]
- Hong, S.; Won, E.J.; Ju, H.J.; Kim, M.S.; Shin, K.H. Current nonylphenol pollution and the past 30 years record in an artificial Lake Shihwa, Korea. Mar. Pollut. Bull. 2010, 60, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Shue, M.F.; Chen, F.A.; Chen, T.C. Total estrogenic activity and nonylphenol concentration in the Donggang River. Taiwan. Environ. Monit. Assess. 2010, 168, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Cherniaev, A.P.; Kondakove, A.S.; Zyk, E.N. Contents of 4-Nonylphenol in Surface Sea Water of Amur Bay (Japan/East Sea). Achiev. Life Sci. 2016, 10, 65–71. [Google Scholar] [CrossRef]
- Diao, P.; Chen, Q.; Wang, R.; Sun, D.; Cai, Z.; Wu, H.; Duan, S. Phenolic endocrine-disruptiong compounds in the Pearl River Estuary: Occurrence, bioaccumulation and risk assessment. Sci. Total Environ. 2017, 584–585, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Takano, T.; Nakamura, K.; Watanabe, S.; Seino, K. Water quality and concentration of alkylphenols in river used as source of drinking water and flowing through urban areas. Environ. Health Prev. Med. 2017, 12, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wang, L.; Xu, G.; Liu, N.; Tang, L.; Zheng, J.; Bu, T.; Lei, B. Seasonal and spatial distribution of 4-tert-oCtylphenol, 4-nonylphenol and bisphenol A in the Huangpu River and its tributaries, Shanghai, China. Environ. Monit. Assess. 2013, 185, 3149–3161. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.G.B.; Liu, S.; Ying, G.G.; Zheng, G.J.S.; Lee, J.H.W.; Leung, K.M.Y. The occurrence and ecological risks of endocrine disrupting chemicals in sewage effluents from three different sewage treatment plants, and in natural seawater from a marine reserve of Hong Kong. Mar. Pollut. Bull. 2014, 85, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Ren, N.Q.; Kannan, K.; Nan, J.; Liu, L.Y.; Ma, W.L.; Qi, H.; Li, Y.F. Occurrence of Endocrine-Disrupting Phenols and Estrogens in Water and Sediment of the Songhua River, Northeastern China. Arch. Environ. Contam. Toxicol. 2014, 66, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Yin, P.; Zhao, L. Nonylphenol and octylphenol in riverine waters and surface sediments of the Pearl River Estuaries, South China: Occurrence, ecological and human health risks. Water Sci. Technol. Water Supply 2017, 17, 1070–1079. [Google Scholar] [CrossRef]
- Cheng, J.R.; Wang, K.; Yu, J.; Yu, Z.X.; Yu, X.B.; Zhang, Z.Z. Distribution and fate modeling of 4-nonylphenol, 4-t-oCtylphenol, and bisphenol A in the Yong River of China. Chemosphere 2018, 195, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Tang, C.Y.; Song, X.F.; Li, F.D. Behavior and fate of alkylphenols in surface water of the Jialu river, Henan province, china. Chemosphere 2009, 77, 559–565. [Google Scholar] [CrossRef] [PubMed]
- CPAMI, Construction and Planning Agency Ministry of Interior. 2018. Available online: https://www.cpami.gov.tw/public-information/laws-regulations.html (accessed on 20 July 2018).
- Joss, A.; Andersen, H.; Ternes, T.; Richle, P.R.; Siegrist, H. Removal of estrogens in municipal wastewater treatment under aerobic and anaerobic conditions: Consequences for plant optimization. Environ. Sci. Technol. 2004, 38, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y.; Haneda, T.; Nishida, T. Removal of estrogenic activities of bisphenol a and nonylphenol by oxidative enzymes from lignin-degrading basidiomycetes. Chemosphere 2001, 42, 271–276. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F.; Puteh, M.H.; Rahman, M.A.; Jaafar, J. PhotoCatalytic degradation of nonylphenol by immobilized tio2 in dual layer hollow fibre membranes. Chem. Eng. J. 2015, 269, 255–261. [Google Scholar] [CrossRef]
- Ciorba, G.A.; Radovan, C.; Vlaicu, I.; Masu, S. Removal of nonylphenol ethoxylates by electrochemically-generated coagulants. J. Appl. Electrochem. 2002, 32, 561–567. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.E.; Frontana-Uribe, B.A.; Rodríguez-Peña, M.; Gomez-Palma, J.C.; Bilyeu, B. Integrated advanced oxidation process, ozonation-electrodegradation treatments, for nonylphenol removal in batch and continuous reactor. Catal. Today 2018, 305, 108–116. [Google Scholar] [CrossRef]
- Rashed, M.N. Adsorption technique for the removal of organic pollutants from water and wastewater. In Organic Pollutants-Monitoring, Risk and Treatment; InTech: London, UK, 2013; pp. 167–194. [Google Scholar]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A review of removal of pollutants from water/wastewater using different types of nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 1–24. [Google Scholar] [CrossRef]
- Ahel, M.; Giger, W. Aqueous solubility of alkylphenols and alkylphenol polyethoxylates. Chemosphere 1993, 26, 1461–1470. [Google Scholar] [CrossRef]
- Chokwe, T.B.; Okonkwo, J.O.; Sibali, L.L. Distribution, exposure pathways, sources and toxicity of nonylphenol and nonylphenol ethoxylates in the environment. Water SA 2017, 42, 529–542. [Google Scholar] [CrossRef]
- Lin, A.Y.-C.; Tsai, Y.-T. Occurrence of pharmaceuticals in taiwan’s surface waters: Impact of waste streams from hospitals and pharmaceutical production facilities. Sci. Total Environ. 2009, 407, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Aerni, H.R.; Kobler, B.; Rutishauser, B.V.; Wettstein, F.E.; Fischer, R.; Giger, W.; Hungerbuhler, A.; Marazuela, M.D.; Peter, A.; Schonenberger, R.; et al. Combined biological and chemical assessment of estrogenic activities in wastewater treatment plant effluents. Anal. Bioanal. Chem. 2004, 378, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.; Liu, X.W.; Gong, W.X.; Nie, W.; Gao, B.Y.; Yue, Q.Y. Adsorption of fulvic acids from aqueous solutions by carbon nanotubes. J. Chem. Technol. Biotechnol. 2007, 82, 698–704. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhou, F.P.; Yu, H.; Chen, L.F. Adsorption characteristic of acidified carbon nanotubes for heavy metal Pb(II) in aqueous solution. Mater. Sci. Eng. A 2007, 466, 201–206. [Google Scholar] [CrossRef]
- Liao, Q.; Sun, J.; Gao, L. The adsorption of resorcinol from water using multi-walled carbon nanotubes. Colloids Surf. A 2008, 312, 160–165. [Google Scholar] [CrossRef]
- Reinert, L.; Lasserre, F.; Gachot, C.; Grutzmacher, P.; MacLucas, T.; Souza, M.; Mucklich, F.; Suarez, S. Long-lasting solid lubrication by CNT-coated patterned surfaces. Sci. Rep. 2017, 42873, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Chung, Y.-L.; Chang, K.-F. Adsorption of trihalomethanes from water with carbon nanotubes. Water Res. 2005, 39, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, A.; Peng, F.; Yu, H.; Yang, J. Mechanism study on adsorption of acidified multiwalled carbon nanotubes to Pb(II). J. Colloide Interface Sci. 2007, 316, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.J.; Pan, S.-Y.; Shukla, A.D.; Shah, D.O.; Chiang, P.C. Mechanism of organic pollutant sorption from aqueous solution by cationic tunable organoClays. J. Colloid Interface Sci. 2018, 529, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shah, K.J.; Shi, L.; Chiang, P.C. Removal of cd(ii) and pb(ii) ions from aqueous solutions by synthetic mineral adsorbent: Performance and mechanisms. Appl. Surf. Sci. 2017, 409, 296–305. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solutions. J. Sanit. Eng. Div. 1963, 89, 30. [Google Scholar]
- RoCher, V.; Paffoni, C.; Gonçalves, A.; Guérin, S.; Azimi, S.; Gasperi, J.; Moilleron, R.; Pauss, A. Municipal wastewater treatment by biofiltration: Comparisons of various treatment layouts. Part 1: Assessment of carbon and nitrogen removal. Water Sci. Technol. 2012, 65, 1705. [Google Scholar] [CrossRef] [PubMed]
- Ersan, G.; Kaya, Y.; Apul, O.G.; Karanfil, T. Adsorption of organic contaminants by graphene nanosheets, carbon nanotubes and granular activated carbons under natural organic matter preloading conditions. Sci. Total Environ. 2016, 565, 811–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elovich, S.Y.; Larionov, O.G. Theory of adsorption from nonelectrolyte solutions on solid adsorbents. Bull. Acad. Sci. USSR Div. Chem. Sci. 1962, 11, 198–203. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids part i solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Oberflächeneinflüsse beim bler und bei der bierbereitung. Zeitschrift für Chemie und Industrie der Kolloide 1906, 1, 152. [Google Scholar] [CrossRef]
- Jovanović, D.S. Physical adsorption of gases. Kolloid-Zeitschrift Zeitschrift für Polymere 1969, 235, 1214–1225. [Google Scholar] [CrossRef]
- Isik, M. Biosorption of ni(ii) from aqueous solutions by living and non-living ureolytic mixed culture. Colloids Surf. B Biointerfaces 2008, 62, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.; Murthy, Z.V.P.; Jha, B. Sorption of hg(ii) from aqueous solutions ontoCarica papaya: Application of isotherms. Ind. Eng. Chem. Res. 2008, 47, 980–986. [Google Scholar] [CrossRef]

| Nonylphenol [39] | CNTs | ||
|---|---|---|---|
| Characteristics | Characteristics | ||
| CAS registry number | 84852-15-3 | Type | MWCNT |
| Synonyms | p-nonylphenol, 4-nonylphenol, | Length | 5–15 µm |
| Molecular formula | C9H19-C6H5O | Purity | ≥95% (v/v) |
| Molecular weight (g/mol) | 220.35 | Amorphous carbon | <3% |
| pKa | 10.7 | Ash | ≤0.2% (w/w) |
| log Kow | 4.8–5.3 | Brunauer–Emmett–Teller (BET) Surface Area | 122.2 m2/g |
| Henry’s law constant (Pa m3/mol) | 11.2 | Interlayer distance | 0.34 nm |
| Solubility (mg/L) | 5.4–8 | ||
| Adsorbent | SBET (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) | References |
|---|---|---|---|---|
| MWCNTs | 122 | 15.1 | 0.44 | |
| Modified MWCNTs (Acidified) | 211 | 15.5 | 0.81 | |
| Acidified MWCNTs | 237 | 15.7 | 0.0042 | [44] |
| Acidified MWCNTs | 121 | 0.49 | [45] | |
| Acidified MWCNTs | 295 | 20–40 | 0.21 | [47] |
| Acidified MWCNTs | 254 | [48] |
| Kinetic model | Parameter | Value | R2 | |
|---|---|---|---|---|
| Pseudo-first-order | qe (mg/g) | 2.1 × 101 | 0.93 | |
| k1 (/min) | 2.7 × 10−1 | |||
| Pseudo-second-order | qe (mg/g) | 3 × 102 | 0.46 | |
| k2 (g/mg/min) | 1.1 × 10−3 | |||
| Elovich | a | 4.3 × 10−3 | 0.96 | |
| α (g/mg/min) | 4.3 × 102 | |||
| Double exponential model (DEM) | D1 (mg/L) | 8.4 × 10−3 | -- | |
| Dk1 (/s) | 1.4 × 10−3 | |||
| D2 (mg/L) | 8.5 × 10−3 | |||
| Dk2 (/s) | 1.4 × 10−3 | |||
| Isotherm model | Parameter | Value | R2 | Reference | |
|---|---|---|---|---|---|
| Langmuir | qm (mg/g) | 1.11 × 104 | 0.9763 | [55] | |
| KL (L/mg) | 1.5 | ||||
| Freundlich | Kf ((mg/g) (mg/L)1/n) | 33.20 | 0.994 | [56] | |
| nf | 0.89 | ||||
| Temkin | Ce (g/mg) | 6.03 × 104 | 0.9986 | [57,59] | |
| AT (L/mg) | 0.60 | ||||
| Elovich | qm (mg/g) | 2.5 × 104 | 0.9996 | [53] | |
| KE (L/mg) | 0.38 | ||||
| Jovanovic | Kj (L/mg) | 0.22 | 0.9984 | [57] | |
| qm (g/mg) | 47.37 | ||||
| Temp. (°C) | K (L/mol) | ∆G° (kJ/mol) | ∆H° (kJ/mol) | ∆S° (J/K mol) |
|---|---|---|---|---|
| 15 | 1.47 | −0.93 | 11.18 | 42.02 |
| 25 | 1.72 | −1.35 | ||
| 35 | 2.00 | −1.77 | ||
| 45 | 2.29 | −2.19 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.-D.; Shah, K.J.; Huang, C.P.; Kim, H.; Chiang, P.-C. Adsorption of Nonylphenol to Multi-Walled Carbon Nanotubes: Kinetics and Isotherm Study. Appl. Sci. 2018, 8, 2295. https://doi.org/10.3390/app8112295
Dai Y-D, Shah KJ, Huang CP, Kim H, Chiang P-C. Adsorption of Nonylphenol to Multi-Walled Carbon Nanotubes: Kinetics and Isotherm Study. Applied Sciences. 2018; 8(11):2295. https://doi.org/10.3390/app8112295
Chicago/Turabian StyleDai, Yung-Dun, Kinjal J. Shah, Ching P. Huang, Hyunook Kim, and Pen-Chi Chiang. 2018. "Adsorption of Nonylphenol to Multi-Walled Carbon Nanotubes: Kinetics and Isotherm Study" Applied Sciences 8, no. 11: 2295. https://doi.org/10.3390/app8112295






