1.1. Background
Fluidized bed (FB) boilers are commercially attractive as a technology used for combustion of solid fuels. For biomass and waste fuels, the fluidized bed usually consists of silica sand, in addition to char and ash, which are continuously added to the boiler via the fuel. The bed material is important for: (i) propagating heat transfer throughout the boiler; (ii) equalizing temperature in space and time; (iii) the hydrodynamic performance of the boiler; and (iv) absorption of certain ash elements that otherwise would affect performance with respect to emissions, corrosion and sintering. While silica sand is currently the bed material of choice for biomass combustion, switching to other types of bed material is feasible and could potentially also provide certain advantages. In this study, the use of a chemically active bed material is demonstrated in a large-scale combustion facility. The material used is Linz-Donawitz (LD)-slag, which is the second most abundant by-product in a typical integrated steel mill. LD-slag has properties which could potentially improve the performance of fluidized bed boilers. Notably, it contains significant fractions of iron and manganese oxides. Under conditions relevant to combustion, iron and manganese oxides can be expected to be subject to oxidation in oxygen-rich zones of the boiler and reduction in fuel-rich zones. Thus, LD-slag exhibits properties similar to the solid Oxygen Carrier (OC) materials used in Chemical Looping Combustion (CLC), a potential future technology for carbon capture. The concept of replacing silica sand with an oxygen carrier in fluidized bed combustion will here be referred to as Oxygen Carrier Aided Combustion (OCAC).
1.2. Chemical Looping Combustion and Oxygen Carriers
The concept of oxidizing fuels with oxygen provided by solid oxygen carrier materials, rather than with oxygen from air, has several possible applications [
1,
2]. Chemical Looping Combustion (CLC) [
1,
2,
3,
4] is a combustion technology that would enable carbon capture without significant costs for gas separation [
5]. In CLC, fuel is oxidized with oxygen from a solid Oxygen Carrier (OC) in particle form, exemplified below by oxidation of methane with iron (III) oxide, see reaction (1).
The products are carbon dioxide, steam and iron (II, III) oxide, of which the latter is oxidized to its initial state with air in a separate air reactor, see reaction (2).
The oxidized material is then be returned to the first reactor vessel ready to oxidize more fuel. Since fuel and air are not mixed, the flue gas will be undiluted by N
2. The sum of reaction (1) and reaction (2) is reaction (3), that is, combustion of the fuel with oxygen.
Some oxygen carriers, such as, for example, manganese (III) oxide, are capable of releasing gas phase oxygen directly into an atmosphere with low partial pressure of oxygen, see reaction (4).
The mechanism described in reaction (4) is typically referred to as Chemical Looping with Oxygen Uncoupling (CLOU) [
6]. The oxygen that is released can react directly with fuels, in accordance with reaction (3). A chemical looping process could involve fuel oxidation both via reaction (1) and via reactions (4+3).
As of this moment, Chemical Looping Combustion is not a commercially available technology. Further, there is no clear consensus in the scientific community about reactor design and choice of oxygen carrier material. However, fluidized bed reactors with oxygen carrier as the bed material is currently the most examined design principle, and there are more than 10,000 h of operational experience with such pilot reactors [
7]. A large majority of studies about solid fuel applications utilizes cheap and readily available oxygen carriers such as naturally occurring minerals or industrial by-products [
4].
1.3. Oxygen Carrier Aided Combustion
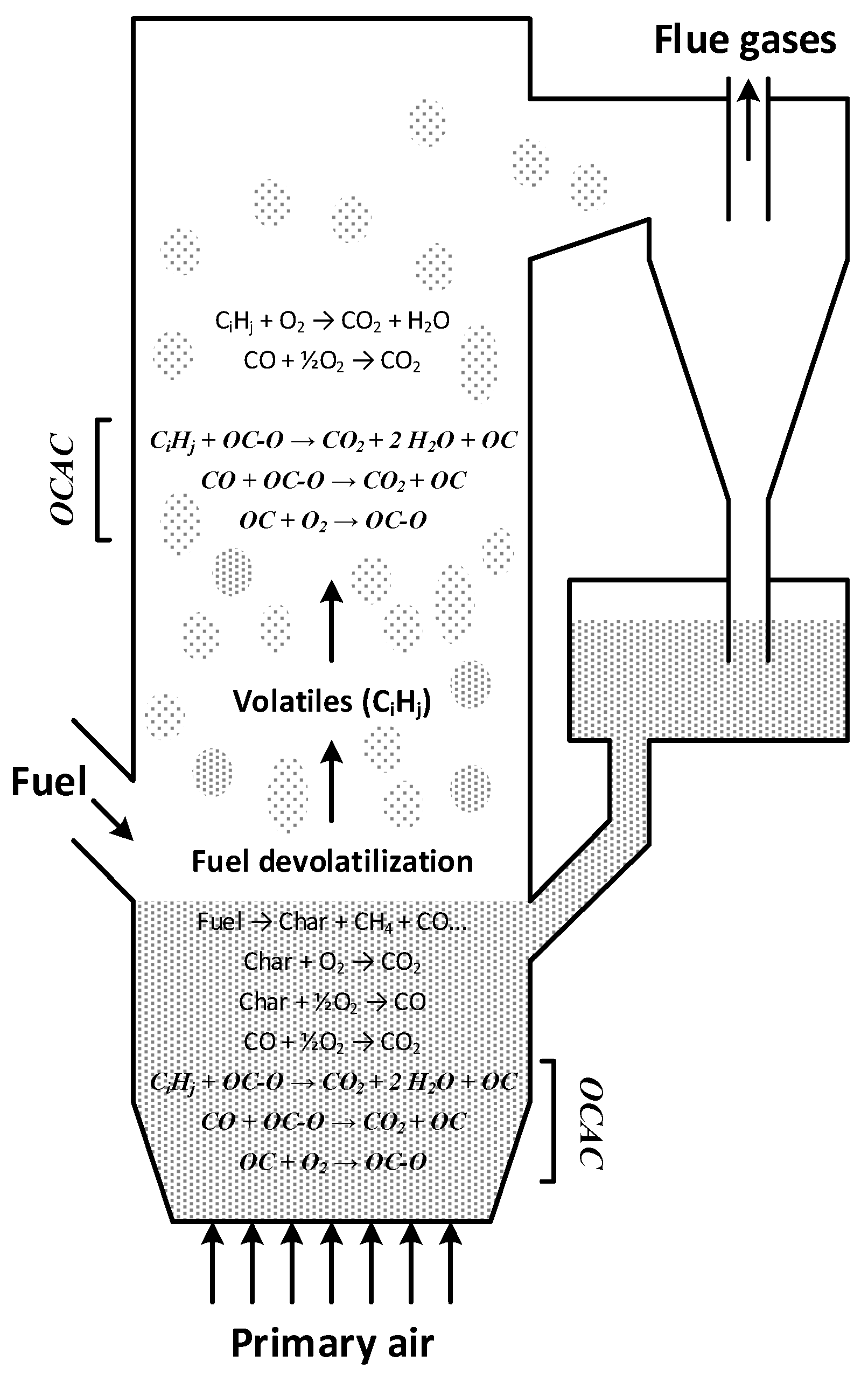
Oxygen Carrier Aided Combustion (OCAC) is a concept strongly related to Chemical Looping Combustion which can be directly implemented in existing fluidized bed boilers. It is realized by partial or complete substitution of the standard semi-inert bed material with an active oxygen carrier material. The active bed material will be reduced in fuel rich parts of the combustion chamber in accordance with reactions such as reaction (1), as well as by reaction with other products of pyrolysis such as CO and H
2. In oxygen rich parts it will be oxidized in accordance with reaction (2). Materials capable of releasing gas phase oxygen, such as described in reaction (4), would release oxygen in parts of the combustion chamber where it is lacking, see
Figure 1.
In OCAC several interesting effects could potentially be achieved:
Gas phase fuel components can be oxidized not only by homogenous reactions with oxygen, but also by heterogeneous reactions with the oxygen carrier, as described in reaction (1).
New mechanisms for oxygen transport in the boiler will be introduced, thus minimizing the presence of reducing zones and potentially reducing the emissions of CO, H2 and unburnt hydrocarbons.
The bed of oxygen carrier material will act as an oxygen buffer, possibly thwarting negative effects of uneven fuel feeding and load changes.
Enhanced fuel conversion in the dense bottom bed. In ordinary fluidized bed boilers the more stable fuel components (such as for example CH
4) do not burn rapidly in the bottom bed since the moderate temperature (≈800–850 °C) and thermal inertia of the bed inhibits formation of hot flames. However, it has been shown in CLC studies [
3] that CH
4 is readily oxidized by oxygen carrying solids. The apparent reason would be that the heterogeneous reaction between CH
4 and oxygen carrier is not hampered by temperature to the same extent as the homogeneous reaction. Consequently, in OCAC the conversion of CH
4 should proceed more rapidly also inside the dense bottom bed, a phenomena that has also been demonstrated experimentally [
8].
OCAC may offer opportunities to reduce traditional problems in biomass combustion. This includes sintering, agglomeration, fouling and corrosion issues connected to combustion of biomass in fluidized beds [
9].
OCAC may allow for the use of less excess air than what is needed in conventional boilers.
The last point is important. Mixing of oxygen and fuel is an important aspect affecting the performance of thermal power plants. Insufficient contact between fuel and oxygen results in emissions of carbon monoxide, unburnt hydrocarbons and char particles. For solid fuels, good mixing is sometimes difficult to achieve. In order to avoid poor fuel conversion and high emission levels, operation with a significant excess of air, compared to what is needed for stoichiometric combustion, is often necessary. This increase the total volumetric gas flow and results in: (i) increased boiler size, cost, and footprint; (ii) increased gas velocity and wear on heat transferring surfaces; (iii) increased heat losses associated with hot flue gas exiting the stack; and (iv) increased power consumption of support equipment, such as fans.
OCAC was first demonstrated in a campaign in Chalmers 12 MW
th Circulating Fluidized Bed (CFB) Research Boiler in 2012 with promising results [
10] (up to 80% reduction of CO emissions and significantly altered temperature profile with 40 wt% substitution of silica sand with oxygen carrier). In that campaign the mineral ilmenite (titanium-iron ore) was used as the bed material. In the past few years OCAC with ilmenite has been subject to commercialization in Sweden by the company Improbed AB (a subsidy of the global corporation E.ON). As part of this effort, ilmenite has been used as a bed material in an undisclosed number of commercial fluidized bed boilers in Sweden burning biomass and waste fuels. An analysis of ilmenite bed samples extracted during operation show interesting phenomena, such as diffusion of ash components, such as potassium, into the core of ilmenite particles [
11]. The oxygen buffering capability of ilmenite during rapid load change of large boilers has been verified [
12]. The performance of sand and rock ilmenite has been compared [
13]. An experimental campaign has also been conducted in Chalmers research boiler using a manganese-based bed material rather than ilmenite [
14]. The OCAC concept has been examined also in small lab scale experiments [
15].
1.4. LD-Slag as Oxygen Carrier
While it would be possible to produce synthetic oxygen carrier particles for OCAC, this would likely be a costly procedure. This is because ash elements present in biomass and waste fuels (K, Na, Ca, Si etc.) are expected to interact with the bed material in a potentially irreversible manner. For silica sand ash components are absorbed on the particle surface. For ilmenite it has been established that the ash component most central for operability during combustion of woody biomass, which is potassium, diffuses into the particle core resulting in formation of KTi
8O
16 [
11]. It is well established that alkali metals are central to several undesired phenomena such as sintering, agglomeration, corrosion and emissions [
9].
Regardless of mechanism, perpetual enrichment of ash components in the bed material is not possible. Thus, the bed material will need to be replaced in regular intervals. In a biomass boiler it is common to replace one third of the sand bed each day, a procedure commonly referred to as regeneration. For waste incineration the whole sand inventory can very well need to be replaced on daily basis. An industrially accepted rule of thumb for the consumption of silica sand during biomass combustion is 3 kg/MWhth. The corresponding number for waste incineration is 6 kg/MWhth, but it can sometimes need to be considerably higher.
While it is not obvious that regeneration will have to be done in precisely the same way for oxygen carrying materials as for sand, it seems reasonable to believe that the use of synthetic particles will be inconvenient from a cost and resource perspective. High-performing synthetic oxygen carriers are likely to be at least one order of magnitude more expensive compared to virgin minerals, which in turn very well could be one order of magnitude more expensive per ton than silica sand. Silica sand already represents a significant cost for biomass fired boilers. For a fluidized bed boiler at the scale of 100 MWhth (a common size in Swedish district heating system), the annual cost for silica sand is in the order of 1–5 MSEK annually, depending on fuel and annual hours of operation. Hence the use of considerably more expensive bed materials is not favored by plant operators, unless there are considerable practical gains. However, the use of bed materials with the potential to be even cheaper than silica sand and which also could provide certain technical advantages is met with considerable interest. Industrial by-products, for which there currently is limited demand, are likely the cheapest category of bed materials available.
The theoretical performance of oxide materials can quite easily be determined by means of thermodynamic equilibrium calculations [
16]. Iron oxides are viable oxygen carriers [
1,
2,
3,
4,
16]. However, iron oxides are incapable of releasing significant amounts of gas phase O
2 via oxygen uncoupling [
6]. Manganese oxides have higher oxygen transfer capacity by weight (7–10 wt% depending on temperature of operation) compared to iron oxide (3.3 wt%) [
16]. This is because complete conversion of fuel is possible both for reduction of Mn
2O
3 → Mn
3O
4 and for Mn
3O
4 → MnO, while iron is limited to the reaction Fe
2O
3 → Fe
3O
4. Manganese oxides also have the ability to release oxygen directly in gaseous form in inert atmosphere at temperatures around 800 °C [
6]. For mixed manganese oxides, which are not pure but also contain elements such as iron and silicon, other more favorable mechanisms for oxygen release which work at higher temperatures could also be available [
17,
18].
LD-slag is the second largest by-product in steelmaking. It is formed by reactions of slag formers (e.g., burned lime), silica, iron and other components during conversion of carbon-rich molten pig iron into steel in the basic oxygen-blown converter process (aka the Linz-Donawitz process). LD-slag and steel are generated during the converter process, the steel is then tapped from the converter and the slag remains in the converter vessel. The remaining molten slag is then poured into a slag pot which subsequently is discharged outside. Large slag lumps are then formed during cooling and solidification of the melt. The resulting lumps have high skeletal density (3500 kg/m3), but its particulate bulk density in crushed form (1500–2000 kg/m3) is not dissimilar to sand. A typical steel-mill may generate 100–200 kton/year. Depending on the level of impurities, parts of it can often be recirculated internally to replace limestone in the blast furnace. In Sweden the total production of LD-slag is >300 kton/year, of which only about half can be internally recirculated due to the size fractions in which the slag occurs. There is limited demand for the remaining part, however work is ongoing to find other usages for the material, for example through utilisation of the vanadium content in the slag as a raw material for vanadium production. To some extent, LD-slag is also used in low-value applications (e.g., construction, raw material in mineral-wool plants), but tens of thousands of tonnes is still being put into storage each year, in addition to the very large stock which is already available.
The chemical composition of LD-slag makes it highly interesting as oxygen carrier as it is rich in iron and manganese oxides. The precise composition will depend on the raw material fed to the steel converter. Guideline values are 22 wt% Fe
2O
3, 3 wt% MnO, 10 wt% SiO
2, 43 wt% CaO, 9 wt% MgO with oxides of aluminium, titanium and vanadium being the main impurities. It should be noted that combined Fe-Mn-Si oxides are among the more interesting combined oxide systems for oxygen carrying purposes [
17,
19].