Visible and Near-Infrared Hyperspectral Imaging for Cooking Loss Classification of Fresh Broiler Breast Fillets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Quality Attributes Measurements
2.3. Image Acquisition and Calibration
2.4. Image Pretreatment and Spectra Extraction
2.5. Reference Measurements Statistics and Spectral Data Processing
2.6. Visualization of CL Classification Results
3. Results and Discussion
3.1. Threshold Determination and Quality Characteristics of High-CL and Low-CL Broiler Breast Fillets
3.2. Spectral Features
3.3. Classification Models Using Full-Wavelength Range
3.4. Establishment of a Multi-Spectral Classification Model by PLS-DA
3.5. Classification Map of CL Categories
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barbut, S. Poultry Products Processing: An Industry Guide; CRC Press: Boca Raton, FL, USA, 2016; pp. 1–26. [Google Scholar]
- Bertram, H.C.; Andersen, H.J.; Karlsson, A.H.; Horn, P.; Hedegaard, J.; Nørgaard, L.; Engelsen, S.B. Prediction of technological quality (cooking loss and Napole Yield) of pork based on fresh meat characteristics. Meat Sci. 2003, 65, 707–712. [Google Scholar] [CrossRef]
- Tornberg, E. Effects of heat on meat proteins—Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Aaslyng, M.D.; Bejerholm, C.; Ertbjerg, P.; Bertram, H.C.; Andersen, H.J. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual. Preference 2003, 14, 277–288. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Andrés, S.; Murray, I.; Navajas, E.A.; Fisher, A.V.; Lambe, N.R.; Bünger, L. Prediction of sensory characteristics of lamb meat samples by near infrared reflectance spectroscopy. Meat Sci. 2007, 76, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Kapper, C.; Klont, R.E.; Verdonk, J.; Urlings, H. Prediction of pork quality with near infrared spectroscopy (NIRS): 1. Feasibility and robustness of NIRS measurements at laboratory scale. Meat Sci. 2012, 91, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Bowker, B.; Hawkins, S.; Zhuang, H. Measurement of water-holding capacity in raw and freeze-dried broiler breast meat with visible and near-infrared spectroscopy. Poult. Sci. 2014, 93, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Leroy, B.; Lambotte, S.; Dotreppe, O.; Lecocq, H.; Istasse, L.; Clinquart, A. Prediction of technological and organoleptic properties of beef longissimus thoracis from near-infrared reflectance and transmission spectra. Meat Sci. 2003, 66, 45–54. [Google Scholar] [CrossRef]
- Liu, Y.; Lyon, B.G.; Windham, W.R.; Lyon, C.E.; Savage, E.M. Prediction of physical, color, and sensory characteristics of broiler breasts by visible/near infrared reflectance spectroscopy. Poult. Sci. 2004, 83, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Prieto, N.; Andrés, S.; Giráldez, F.J.; Mantecón, A.R.; Lavín, P. Ability of near infrared reflectance spectroscopy (NIRS) to estimate physical parameters of adult steers (oxen) and young cattle meat samples. Meat Sci. 2008, 79, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Prevolnik, M.; Čandek-Potokar, M.; Škorjanc, D. Predicting pork water-holding capacity with NIR spectroscopy in relation to different reference methods. J. Food Eng. 2010, 98, 347–352. [Google Scholar] [CrossRef]
- Elmasry, G.M.; Nakauchi, S. Image analysis operations applied to hyperspectral images for non-invasive sensing of food quality—A comprehensive review. Biosyst. Eng. 2016, 142, 53–82. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Fisk, I.D. Protein content prediction in single wheat kernels using hyperspectral imaging. Food Chem. 2017, 240, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lawrence, K.C.; Ni, X.; Yoon, S.C.; Heitschmidt, G.W.; Feldner, P. Near-infrared hyperspectral imaging for detecting aflatoxin B1, ofmaize kernels. Food Control 2015, 51, 347–355. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Fisk, I.D. Application of calibrations to hyperspectral images of food grains: Example for wheat falling number. J. Spectr. Imaging 2017, 6, 1–15. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, D.; Li, L.; Wang, Z. How to predict the sugariness and hardness of melons: A near-infrared hyperspectral imaging method. Food Chem. 2017, 218, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Valenzuela, G.A.; Lu, R.; Aguilera, J.M. Prediction of firmness and soluble solids content of blueberries using hyperspectral reflectance imaging. J. Food Eng. 2013, 115, 91–98. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.R.; Wang, C.Y.; Chan, D.E.; Kim, M.S. Development of a simple algorithm for the detection of chilling injury in cucumbers from visible/near-infrared hyperspectral imaging. Appl. Spectrosc. 2005, 59, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lorente, D.; Aleixos, N.; Gómez-Sanchis, J.; Cubero, S.; García-Navarrete, O.L.; Blasco, J. Recent advances and applications of hyperspectral imaging for fruit and vegetable quality assessment. Food Bioprocess Technol. 2012, 5, 1121–1142. [Google Scholar] [CrossRef]
- Liu, L.; Ngadi, M.O. Detecting fertility and early embryo development of chicken eggs using near-infrared hyperspectral imaging. Food Bioprocess Technol. 2013, 6, 2503–2513. [Google Scholar] [CrossRef]
- Qu, J.H.; Cheng, J.H.; Sun, D.W.; Pu, H.; Wang, Q.J.; Ma, J. Discrimination of shelled shrimp (Metapenaeus ensis) among fresh, frozen-thawed and cold-stored by hyperspectral imaging technique. LWT-Food Sci. Technol. 2015, 62, 202–209. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Grebby, S.; Fisk, I.D. Non-destructive analysis of sucrose, caffeine and trigonelline on single green coffee beans by hyperspectral imaging. Food Res. Int. 2018, 106, 193–203. [Google Scholar] [CrossRef]
- Barbin, D.F.; Elmasry, G.M.; Sun, D.W.; Allen, P. Non-destructive determination of chemical composition in intact and minced pork using near-infrared hyperspectral imaging. Food Chem. 2013, 138, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Barbin, D.F.; Elmasry, G.M.; Sun, D.W.; Allen, P. Near-infrared hyperspectral imaging for grading and classification of pork. Meat Sci. 2012, 90, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Barbin, D.F.; Sun, D.W.; Su, C. NIR hyperspectral imaging as non-destructive evaluation tool for the recognition of fresh and frozen–thawed porcine longissimus dorsi, muscles. Innov. Food Sci. Emerg. Technol. 2013, 18, 226–236. [Google Scholar] [CrossRef]
- Elmasry, G.M.; Sun, D.W.; Allen, P. Chemical-free assessment and mapping of major constituents in beef using hyperspectral imaging. J. Food Eng. 2013, 117, 235–246. [Google Scholar] [CrossRef]
- Elmasry, G.M.; Sun, D.W.; Allen, P. Non-destructive determination of water-holding capacity in fresh beef by using NIR hyperspectral imaging. Food Res. Int. 2011, 44, 2624–2633. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Elmasry, G.M.; Sun, D.W.; Allen, P. Non-destructive assessment of instrumental and sensory tenderness of lamb meat using NIR hyperspectral imaging. Food Chem. 2013, 141, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Sun, D.W. Application of visible and near infrared hyperspectral imaging for non-invasively measuring distribution of water-holding capacity in salmon flesh. Talanta 2013, 116, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Sun, D.W.; Dai, Q.; Han, Z.; Zeng, X.A.; Wang, L. Application of visible hyperspectral imaging for prediction of springiness of fresh chicken meat. Food Anal. Methods 2015, 8, 380–391. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, D.W.; Pu, H.; Xie, A.; Han, Z.; Luo, M. Non-destructive prediction of thiobarbituricacid reactive substances (TBARS) value for freshness evaluation of chicken meat using hyperspectral imaging. Food Chem. 2015, 179, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Z.; Sun, D.W. Near-infrared hyperspectral imaging in tandem with partial least squares regression and genetic algorithm for non-destructive determination and visualization of Pseudomonas loads in chicken fillets. Talanta 2013, 109, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Z.; ElMasry, G.M.; Sun, D.W.; Scannell, A.G.; Walsh, D.; Morcy, N. Near-infrared hyperspectral imaging and partial least squares regression for rapid and reagentless determination of Enterobacteriaceae on chicken fillets. Food Chem. 2013, 138, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.C.; Park, B.; Lawrence, K.C.; Windham, W.R.; Heitschmidt, G.W. Line-scan hyperspectral imaging system for real-time inspection of poultry carcasses with fecal material and ingesta. Comput. Electron. Agric. 2011, 79, 159–168. [Google Scholar] [CrossRef]
- Xiong, Z.; Sun, D.W.; Xie, A.; Han, Z.; Wang, L. Potential of hyperspectral imaging for rapid prediction of hydroxyproline content in chicken meat. Food Chem. 2015, 175, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Savage, E.M. Postmortem aging and freezing and thawing storage enhance ability of early deboned chicken pectoralis major muscle to hold added salt water. Poult. Sci. 2012, 91, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Savage, E.M. Variation and Pearson correlation coefficients of Warner-Bratzler shear force measurements within broiler breast fillets. Poult. Sci. 2009, 88, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.C.; Lawrence, K.C.; Siragusa, G.R.; Line, J.E.; Park, B.; Feldner, P.W. Hyperspectral reflectance imaging for detecting a foodborne pathogen: Campylobacter. Trans. ASABE 2009, 52, 651–662. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Fang, C.H.; Wang, D. Feasibility study on identification of green, black and Oolong teas using near-infrared reflectance spectroscopy based on support vector machine (SVM). Spectrochim. Acta Part A 2007, 66, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Centner, V.; Massart, D.L.; De Noord, O.E.; De, J.S.; Vandeginste, B.M.; Sterna, C. Elimination of uninformative variables for multivariate calibration. Anal. Chem. 1996, 68, 3851–3858. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Y.; Xu, Q.; Cao, D. Key wavelengths screening using competitive adaptive reweighted sampling method for multivariate calibration. Anal. Chim. Acta 2009, 648, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bowker, B.; Zhuang, H. Relationship between water-holding capacity and protein denaturation in broiler breast meat. Poult. Sci. 2015, 94, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Fletcher, D.L.; Smith, D.P.; Northcutt, J.K. Effects of raw broiler breast meat color variation on marination and cooked meat quality. Poult. Sci. 2002, 81, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Chen, Y.R. Two-dimensional correlation spectroscopy study of visible and near-infrared spectral variations of chicken meats in cold storage. Appl. Spectrosc. 2000, 54, 1458–1470. [Google Scholar] [CrossRef]
- Samuel, D.; Park, B.; Sohn, M.; Wicker, L. Visible-near-infrared spectroscopy to predict water-holding capacity in normal and pale broiler breast meat. Poult. Sci. 2011, 90, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, M.; Barbin, D.; Elmasry, G.M.; Sun, D.W.; Allen, P. Potential of hyperspectral imaging and pattern recognition for categorization and authentication of red meat. Innov. Food Sci. Emerg. Technol. 2012, 16, 316–325. [Google Scholar] [CrossRef]
- Pu, H.; Sun, D.W.; Ma, J.; Cheng, J.H. Classification of fresh and frozen-thawed pork muscles using visible and near infrared hyperspectral imaging and textural analysis. Meat Sci. 2015, 99, 81–88. [Google Scholar] [CrossRef] [PubMed]





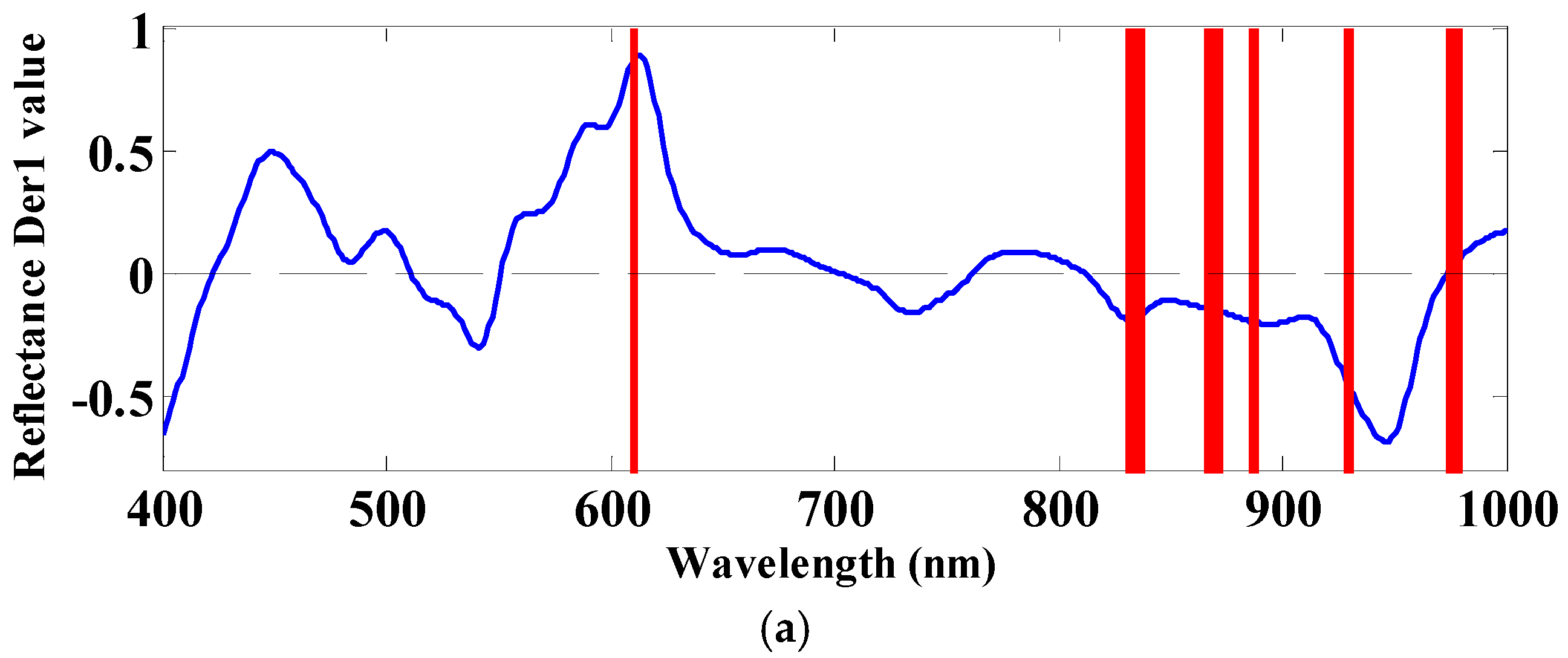



| Trait | High-CL Fillets (n = 37) | Low-CL Fillets (n = 37) | ||||
|---|---|---|---|---|---|---|
| Min. | Max. | Mean ± SD | Min. | Max. | Mean ± SD | |
| L* | 47.29 | 63.76 | 57.35 ± 3.26 a | 48.39 | 61.60 | 56.45 ± 2.79 a |
| a* | −1.51 | 1.71 | 0.20 ± 0.84 a | −1.39 | 1.96 | −0.11 ± 0.84 a |
| b* | 8.88 | 16.32 | 12.61 ± 1.78 a | 8.55 | 17.48 | 12.17 ± 1.90 a |
| pH | 5.75 | 6.24 | 6.01 ± 0.11 b | 5.84 | 6.27 | 6.06 ± 0.12 a |
| WBSF (kgf) | 1.66 | 12.08 | 5.06 ± 2.43 a | 2.64 | 8.24 | 4.27 ± 1.26 b |
| CL (%) | 20.02 | 36.18 | 22.05 ± 2.96 a | 15.17 | 19.76 | 18.15 ± 1.25 b |
| Method | Preprocessing | LVs | Calibration Set CCR | Prediction Set CCR |
|---|---|---|---|---|
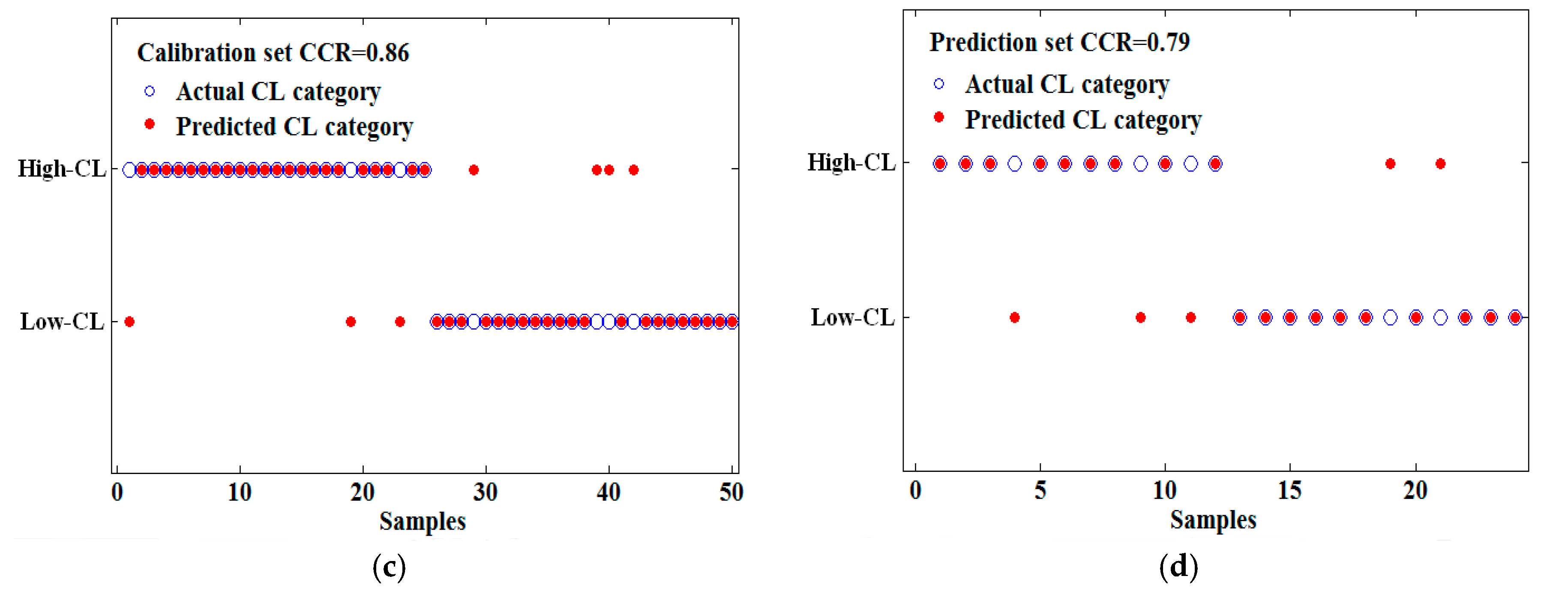
| PLS-DA | Der1 | 11 | 0.90 | 0.79 |
| RBF-SVM | Der2 | - | 1.00 | 0.79 |
| Methods | Wavelengths (nm) | Number | LVs | Calibration CCR | Prediction CCR |
|---|---|---|---|---|---|
| CARS | 434, 435, 436, 469, 557, 576, 578, 654, 656, 679, 681, 686, 687, 747, 750, 960, 978, 979 | 18 | 13 | 0.86 | 0.79 |
| UVE | 610, 611, 831, 832, 833, 835, 836, 837, 838, 866, 867, 868, 870, 871, 872, 886, 888, 929, 930, 931, 974, 976, 977, 978, 979 | 25 | 12 | 0.78 | 0.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Wang, W.; Zhuang, H.; Yoon, S.; Li, Y.; Yang, Y. Visible and Near-Infrared Hyperspectral Imaging for Cooking Loss Classification of Fresh Broiler Breast Fillets. Appl. Sci. 2018, 8, 256. https://doi.org/10.3390/app8020256
Jiang H, Wang W, Zhuang H, Yoon S, Li Y, Yang Y. Visible and Near-Infrared Hyperspectral Imaging for Cooking Loss Classification of Fresh Broiler Breast Fillets. Applied Sciences. 2018; 8(2):256. https://doi.org/10.3390/app8020256
Chicago/Turabian StyleJiang, Hongzhe, Wei Wang, Hong Zhuang, Seungchul Yoon, Yufeng Li, and Yi Yang. 2018. "Visible and Near-Infrared Hyperspectral Imaging for Cooking Loss Classification of Fresh Broiler Breast Fillets" Applied Sciences 8, no. 2: 256. https://doi.org/10.3390/app8020256





