Molecular Characterization and Theoretical Calculation of Plant Growth Regulators Based on Terahertz Time-Domain Spectroscopy
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Spectral Acquisition
2.3. Data Processing
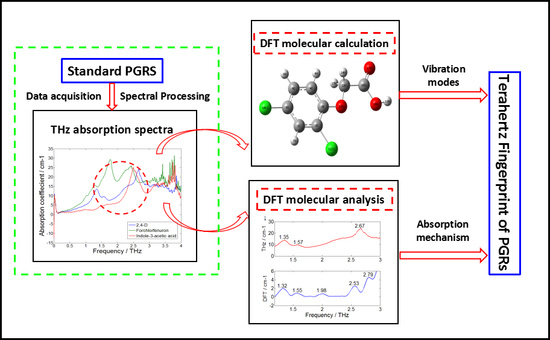
3. Results and Discussion
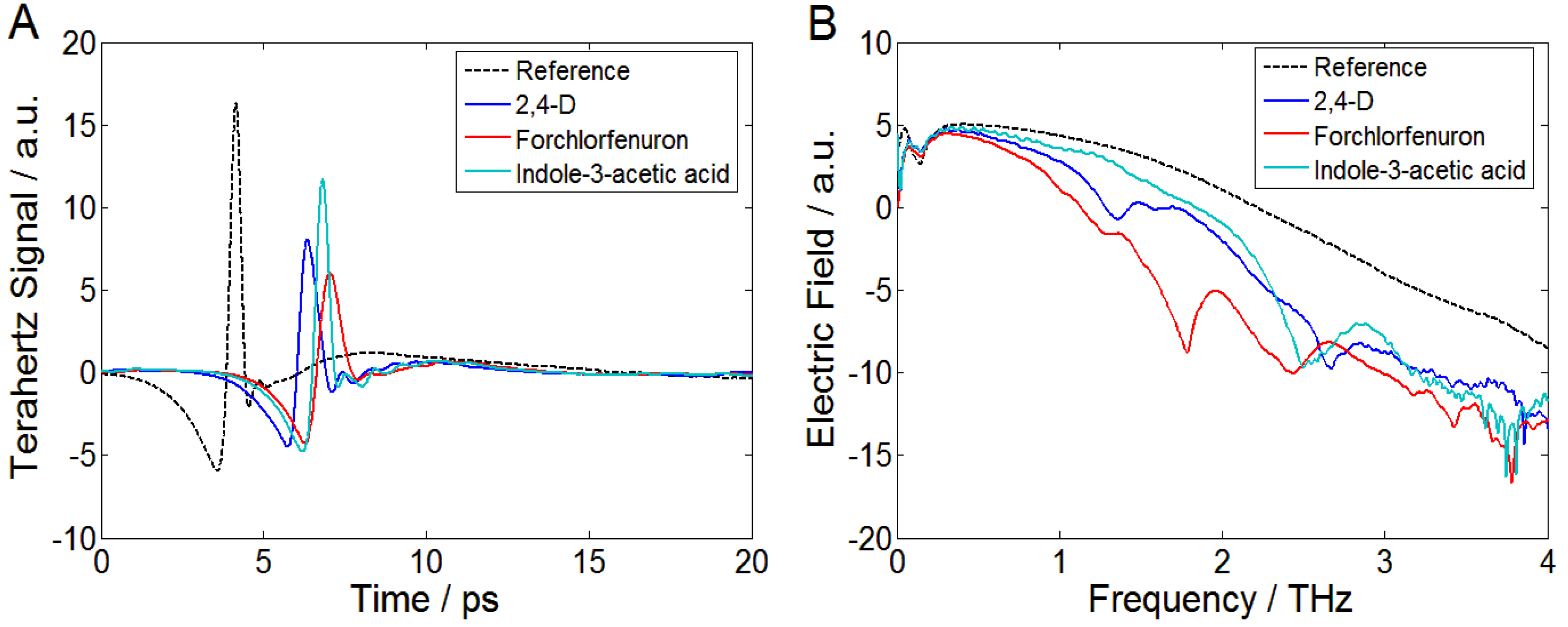
3.1. THz Time-Domain and Frequency-Domain Spectra of PGRs
3.2. Spectral Processing by Window Functions
3.3. Absorption Coefficient and Refractive Index of PGRs
3.4. Theoretical Calculation of THz Spectra by DFT
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, K.; Deol, J.S.; Dass, A.; Choudhary, A.K. Possibilities of Improving Performance of Direct Seeded Rice Using Plant Growth Regulators: A Review. Proc. Natl. Acad. Sci. India 2015, 85, 909–922. [Google Scholar] [CrossRef]
- Oruç, E.O.; Uner, N. Combined effects of 2,4-D and azinphosmethyl on antioxidant enzymes and lipid peroxidation in liver of Oreochromis niloticus. Comp. Biochem. Physiol. Part C 2000, 127, 291–296. [Google Scholar] [CrossRef]
- Stachler, J.M.; Kells, J.J.; Penner, D. Resistance of wild carrot (Daucus carota) to 2,4-D in Michigan. Weed Technol. 2000, 14, 734–739. [Google Scholar] [CrossRef]
- Kruger, G.R.; Davis, V.M.; Weller, S.C.; Johnson, W.G. Growth and Seed Production of Horseweed (Conyza canadensis) Populations after Exposure to Postemergence 2,4-D. Weed Sci. 2010, 58, 413–419. [Google Scholar] [CrossRef]
- Valverde, A.; Piedra, L.; Aguilera, A.; Boulaid, M.; Camacho, F. Analysis and residue levels of forchlorfenuron (CPPU) in watermelons. J. Environ. Sci. Health. Part B 2007, 42, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Peppi, M.C.; Fidelibus, M.W. Effects of Forchlorfenuron and Abscisic Acid on the Quality of ‘Flame Seedless’ Grapes. HortScience 2008, 43, 173–176. [Google Scholar]
- Idris, E.S.E.; Iglesias, D.J.; Talon, M.; Borriss, R. Tryptophan-Dependent Production of Indole-3-Acetic Acid (IAA) Affects Level of Plant Growth Promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Koca, N.; Karaman, Ş. The effects of plant growth regulators and L-phenylalanine on phenolic compounds of sweet basil. Food Chem. 2015, 166, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Satish, L.; Rency, A.S.; Rathinapriya, P.; Ceasar, S.A.; Pandian, S.; Rameshkumar, R.; Rao, T.B.; Balachandran, S.M.; Ramesh, M. Influence of plant growth regulators and spermidine on somatic embryogenesis and plant regeneration in four Indian genotypes of finger millet (Eleusine coracana (L.) Gaertn). Plant Cell Tissue Organ Cult. 2016, 124, 15–31. [Google Scholar] [CrossRef]
- Kelley, K.B.; Zhang, Q.; Lambert, K.N.; Riechers, D.E. Evaluation of auxin-responsive genes in soybean for detection of off-target plant growth regulator herbicides. Weed Sci. 2006, 54, 220–229. [Google Scholar] [CrossRef]
- Liu, S.J.; Fang, C.G.; Cui, Y.; Jiang, N. Advances in detection technologies of plant growth regulator residue in plant foods. J. Food Saf. Qual. 2016, 7, 8–13. [Google Scholar]
- Erban, T.; Trojakova, L.; Kamler, M.; Titera, D. Detection of the desiccant and plant growth regulator chlormequat in honeybees and comb pollen. Vet. Med. 2017, 62, 596–603. [Google Scholar] [CrossRef]
- Cui, G.; Rina, S.U.; Song, G.; Ren, X.; Hou, X. Research and Progress of the Pretreatment Choices for Detection of Plant Growth Regulator. Forensic Sci. Technol. 2017, 42, 231–235. [Google Scholar]
- Siegel, P.H. Terahertz technology in biology and medicine. IEEE Trans. Microwave Theory Tech. 2004, 52, 2438–2447. [Google Scholar] [CrossRef]
- Fischer, B.; Hoffmann, M.; Helm, H.; Modjesch, G.; Jepsen, P.U. Chemical recognition in terahertz time-domain spectroscopy and imaging. Semicond. Sci. Technol. 2005, 20, S246. [Google Scholar] [CrossRef]
- Nie, P.; Qu, F.; Lin, L.; Dong, T.; He, Y.; Shao, Y.; Zhang, Y. Detection of Water Content in Rapeseed Leaves Using Terahertz Spectroscopy. Sensors 2017, 17, 2830. [Google Scholar] [CrossRef] [PubMed]
- Walther, M.; Plochocka, P.; Fischer, B.; Helm, H.; Uhd, J.P. Collective vibrational modes in biological molecules investigated by terahertz time-domain spectroscopy. Biopolymers 2002, 67, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Cen, H.; Weng, H.; Yao, J.; He, M.; Lv, J.; Hua, S.; Li, H.; He, Y. Chlorophyll Fluorescence Imaging Uncovers Photosynthetic Fingerprint of Citrus Huanglongbing. Front. Plant Sci. 2017, 8, 1509. [Google Scholar] [CrossRef] [PubMed]
- Siegel, P.H. Terahertz technology. IEEE Trans. Microwave Theory Tech. 2002, 50, 910–928. [Google Scholar] [CrossRef]
- Naftaly, M.; Miles, R.E. Terahertz Time-Domain Spectroscopy for Material Characterization. Proc. IEEE 2007, 95, 1658–1665. [Google Scholar] [CrossRef]
- Duvillaret, L.; Garet, F.; Coutaz, J.L. A reliable method for extraction of material parameters in terahertz time-domain spectroscopy. IEEE J. Sel. Top. Quantum Electron. 2002, 2, 739–746. [Google Scholar] [CrossRef]
- Yan, Z.G.; Hou, D.B.; Cao, B.H.; Zhang, G.X.; Zhou, Z.K. Terahertz spectroscopic investigation of organophosphorous pesticide acephate. J. Zhejiang Univ. 2008, 12, 2207–2211. [Google Scholar]
- Baek, S.H.; Lim, H.B.; Chun, H.S. Detection of melamine in foods using terahertz time-domain spectroscopy. J. Agric. Food Chem. 2014, 62, 5403–5407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, H.W.; Tominaga, K.; Hayashi, M.; Hasunuma, T.; Kondo, A. Application of THz Vibrational Spectroscopy to Molecular Characterization and the Theoretical Fundamentals: An Illustration Using Saccharide Molecules. Chem. Asian J. 2017, 12, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.; Yang, H.; Lee, S.H.; Kim, Y.A.; Son, J.H. Terahertz molecular resonance of cancer DNA. Sci. Rep. 2016, 6, 37103. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hibino, K.; Sugita, N.; Mitsuishi, M. Simultaneous measurement of surface shape and optical thickness using wavelength tuning and a polynomial window function. Opt. Express 2015, 23, 32869–32880. [Google Scholar] [CrossRef] [PubMed]
- Bailoni, A.; Mancini, A.S.; Amendola, L. Improving the galaxy clustering Fisher matrix: window function, bin cross-correlation, and bin redshift uncertainty. arXiv, 2016; arXiv:1608.00458. [Google Scholar]
- Traficante, D.D.; Rajabzadeh, M. Optimum window function for sensitivity enhancement of NMR signals. Concepts Magn. Reson. Part A 2015, 12, 83–101. [Google Scholar] [CrossRef]
- Agarwal, P.; Singh, S.P.; Pandey, V.K. Mathematical analysis of blackman window function in fractional Fourier transform domain. In Proceedings of the 2014 International Conference on Medical Imaging, m-Health and Emerging Communication Systems (MedCom), Greater Noida, India, 7–8 November 2015. [Google Scholar] [CrossRef]
- Liang, L.; Tong, M.M.; Wei, M.S.; Meng, L.I.; Dong, H.B. Research on Scattering in the THz Quantitative Analysis of Granulated Coal. Spectrosc. Spectr. Anal. 2017, 8, 2365–2370. [Google Scholar]



| NO. | Plant Growth Regulators | Molecular Formula | Molecular Mass | Melting Point | Water Solubility |
|---|---|---|---|---|---|
| 1 | 2,4-Dichlorophenoxyacetic acid | C8H6CL2O3 | 221.04 | 137–141 °C | 0.890 g/L |
| 2 | Forchlorfenuron | C12H10ClN3O | 247.68 | 170–172 °C | 0.039 g/L |
| 5 | Indole-3-acetic acid | C10H9NO2 | 175.18 | 165–169 °C | 1.500 g/L |
| Calculation (THz) | Experiment (THz) | Deviation (THz) | Assignment |
|---|---|---|---|
| 2,4-Dichlorophenoxyacetic acid (2,4-D) | |||
| 1.32 (vs) | 1.35 | +0.03 | υ(C-C)ip |
| 1.55 (vw) | 1.57 | +0.02 | δ(C-H)oop |
| 1.98 (vw) | - | - | υring+δ(C-C-C)ip |
| 2.53 (w) | 2.67 | +0.14 | δ(C-O-C)ip+υ(C-CL)ip |
| 2.79 (w) | - | - | υring+υ(C-C)ip |
| Forchlorfenuron (CPPU) | |||
| 1.70 (m) | 1.77 | +0.07 | υ(C=O)ip+δ(N-C-N)oop |
| 2.53 (m) | 2.44 | −0.09 | δ(C-N)oop |
| Indole-3-acetic acid (IAA) | |||
| 0.58 (w) | - | - | δ(C-O)oop |
| 2.5 (w) | 2.5 | ±0 | υ(C-C-C)ip |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, F.; Lin, L.; Cai, C.; Dong, T.; He, Y.; Nie, P. Molecular Characterization and Theoretical Calculation of Plant Growth Regulators Based on Terahertz Time-Domain Spectroscopy. Appl. Sci. 2018, 8, 420. https://doi.org/10.3390/app8030420
Qu F, Lin L, Cai C, Dong T, He Y, Nie P. Molecular Characterization and Theoretical Calculation of Plant Growth Regulators Based on Terahertz Time-Domain Spectroscopy. Applied Sciences. 2018; 8(3):420. https://doi.org/10.3390/app8030420
Chicago/Turabian StyleQu, Fangfang, Lei Lin, Chengyong Cai, Tao Dong, Yong He, and Pengcheng Nie. 2018. "Molecular Characterization and Theoretical Calculation of Plant Growth Regulators Based on Terahertz Time-Domain Spectroscopy" Applied Sciences 8, no. 3: 420. https://doi.org/10.3390/app8030420
APA StyleQu, F., Lin, L., Cai, C., Dong, T., He, Y., & Nie, P. (2018). Molecular Characterization and Theoretical Calculation of Plant Growth Regulators Based on Terahertz Time-Domain Spectroscopy. Applied Sciences, 8(3), 420. https://doi.org/10.3390/app8030420