Ca/Si and Si/Al Ratios of Metakaolinite-Based Wastes: Their Influence on Mineralogy and Mechanical Strengths
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and calcined clays as pozzolans for concrete: A review. Cem. Concr. Compos. 2001, 441, 423–454. [Google Scholar] [CrossRef]
- Samet, B.; Mnif, T.; Chaabouni, M. Use of a kaolinitic clay as a pozzolanic material for cements: Formulation of blended cement. Cem. Concr. Compos. 2007, 741, 729–749. [Google Scholar] [CrossRef]
- Rodríguez, N.H.; Granados, R.J.; Blanco-Varela, M.T.; Cortina, J.L.; Martínez-Ramírez, S.; Marsal, M.; Guillem, M.; Puig, J.; Fos, C.; Larrotcha, E.; et al. Evaluation of a lime-mediated sewage sludge stabilisation process. Product characterization and technological validation for its use in the cement industry. Waste Manag. 2012, 32, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Mas, M.A.; Monzo, J.; Payá, J.; Reig, L.; Borrachero, M.V. Ceramic tiles waste as replacement material in Portland cement. Adv. Cem. Res. 2016, 28, 221–232. [Google Scholar] [CrossRef]
- Morales, E.V.; Villar-Cociña, E.; Frías, M. Effects of calcining conditions on the microstructure of sugar cane waste ashes: Influence in the pozzolanic activation. Cem. Concr. Compos. 2009, 31, 22–28. [Google Scholar] [CrossRef]
- Composition, Specification and Conformity Criteria for Common Cements; European Standard EN 197-1; European Standard: Madrid, Spain, 2011.
- Rios, C.A.; Williams, C.D.; Fullen, M.A. Hydrogarnet and Tobermorite at 175 °C from Kaolinite and Metakaolinite in the CaO-Al2O3-SiO2-H2O System: A Comparative Study. Appl. Clay Sci. 2009, 43, 228–237. [Google Scholar] [CrossRef]
- Vejmelkova, E.; Pavlikova, M.; Kepprt, M.; Kersner, Z.; Rovnanikova, P.; Ondracek, M.; Sedlmayer, M.; Cerny, R. High Performance Concrete with Czech Metakaolin: Experimental Analysis of Strength, Toughness and Durability Characteristics. Constr. Build. Mater. 2010, 24, 1404–1411. [Google Scholar] [CrossRef]
- Janotka, I.; Puertas, F.; Palacios, M.; Kuliffayova, M.; Varga, C. Metakaolin sand Blended Cement Pastes: Rheology, Hydration Process and Mechanical Properties. Constr. Build. Mater. 2010, 24, 791–802. [Google Scholar] [CrossRef]
- Ptacek, P.; Frajkorova, F.; Soukal, F.; Opravil, T. Kinetics and mechanism of three stages of thermal transformation of kaolinite to metakaolinite. Powder Technol. 2014, 264, 439–445. [Google Scholar] [CrossRef]
- Pesce, G.L.; Bowen, C.R.; Rocha, J.; Sardo, M.; Allen, G.C.; Walker, P.J.; Denuault, G.; Serrapede, M.; Ball, R.J. Monitoring hydration in lime-MK composites using electrochemical impedance spectroscopy and nuclear magnetic resonance spectroscopy. Clay Minecraft 2014, 4, 341–358. [Google Scholar] [CrossRef] [Green Version]
- Frías, M.; de la Villa, R.V.; de Soto, I.; Garcia, R.; Baloa, T.A. Influence of activated drinking-water treatment waste on binary cement-based composite behavior: Characterization and properties. Compos. Part B 2014, 60, 14–20. [Google Scholar] [CrossRef]
- Tironi, A.; Castellano, C.C.; Bonavetti, V.L.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Kaolinitic calcined clays—Portland cement system: Hydration and properties. Constr. Build. Mater. 2014, 64, 215–221. [Google Scholar] [CrossRef]
- Habert, G.; Choupay, N.; Montel, J.M.; Guillaume, D.; Escadeillas, G. Effects of the secondary minerals of the natural pozzolans on their pozzolanic activity. Cem. Concr. Res. 2008, 38, 963–975. [Google Scholar] [CrossRef]
- Habert, G.; Choupay, N.; Escadeillas, G.; Guillaume, D.; Montel, J.M. Clay content of argillites: Influence on cement based mortars. Appl. Clay Sci. 2009, 322, 330–343. [Google Scholar] [CrossRef]
- De la Villa, R.V.; Fernández, R.; Rodríguez, O.; García, R.; Villar-Cociña, E.; Frías, M. Evolution of the pozzolanic activity of a thermally treated zeolite. J. Mater. Sci. 2013, 48, 3213–3224. [Google Scholar] [CrossRef]
- Frías, M.; de la Villa, R.V.; García, R.; de Soto, I.; Medina, C.; de Rojas, M.S. Scientific and technical aspects of blended cement matrices containing activated slate waste. Cem. Concr. Compos. 2014, 348, 19–25. [Google Scholar] [CrossRef]
- García, R.; de la Villa, R.V.; Frías, M.; Rodríguez, O.; Martínez-Ramírez, S.; Fernández-Carrasco, L.; de Soto, I.S.; Villar-Cociña, E. Mineralogical study of calcined coal waste in a pozzolan/Ca(OH)2 system. Appl. Clay Sci. 2015, 108, 45–54. [Google Scholar] [CrossRef]
- Panagiotopoulou, C.H.; Kontori, E.; Perraki, T.H.; Kakali, G. Dissolution of aluminosilicate minerals and by-products in alkaline media. J. Mater. Sci. 2007, 42, 2967–2973. [Google Scholar] [CrossRef]
- Vigil, R.; Frías, M.; de Rojas, M.S.; Vegas, I.; García, R. Mineralogical and Morphological Changes of Calcined Paper Sludge at Different Temperatures and Retention in Furnace. Appl. Clay Sci. 2007, 36, 279–286. [Google Scholar] [CrossRef]
- Frías, M.; Rodríguez, O.; García, R.; Vegas, I. Influence of Activation Temperature on Reaction Kinetics in Recycled Clay Waste—Calcium Hydroxide Systems. J. Am. Ceram. Soc. 2008, 91, 4044–4051. [Google Scholar] [CrossRef]
- Chakchouk, A.; Trifi, L.; Samet, B.; Bouaziz, S. Formulation of blended cement: Effect of process variables on clay pozzolanic activity. Constr. Build. Mater. 2009, 23, 1365–1373. [Google Scholar] [CrossRef]
- Bai, J.; Chaipanich, A.; Kinuthia, J.M.; O’Farrell, M.; Sabir, B.B.; Wild, S.; Lewis, M.H. Compressive Strength and Hydration of Waste Paper Sludge Ash Ground Granulated Blast furnace Slag Blended Pastes. Cem. Concr. Res. 2003, 33, 1189–1202. [Google Scholar] [CrossRef]
- Vegas, I.; Frias, M.; Urreta, J.; San Jose, T. Obtaining a Pozzolanic Addition from the Controlled Calcinations of Paper Mill Sludge. Performance in Cement Matrices. Mater. Constr. 2006, 56, 49–60. [Google Scholar]
- Rodríguez, O.; de Rojas, M.S.; García, R.; Vigil, R. Effect of thermally activated paper sludge on the mechanical properties and porosity of cement pastes. Mater. Constr. 2009, 59, 17–28. [Google Scholar]
- Rodríguez, O.; de la Villa, R.V.; de Rojas, M.S.; Frías, M. Novel use of kaolin wastes in blended cements. J. Am. Ceram. Soc. 2009, 92, 2443–2446. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Ruan, C.D.; Ward, C.R. Quantitative X-ray powder diffraction analysis of clay minerals in Australian coals using Rietveld methods. Appl. Clay Sci. 2002, 21, 227–240. [Google Scholar] [CrossRef]
- Methods of Testing Cement—Part 5: Pozzolanicity Test for Pozzolanic Cement; UNE-EN 196-5; European Standard: Madrid, Spain, 2011.
- Koch, A.; Steinegger, H. A Rapid Method for Testing the Resistance of Cements to Sulphate Attack. Zem. Kalk Gips 1960, 13, 317–324. [Google Scholar]
- García, R.; Vigil, R.; Rodríguez, O.; Frías, M. Study of hydrated phases present in calcined paper sludge (metakaolinite)/saturated CaO dissolution system cured at 40 °C and 28 days of reaction. Mater. Sci. Eng. A 2010, 527, 3936–3941. [Google Scholar] [CrossRef]
- Frías, M.; Vigil, R.; García, R.; Rodríguez, O.; Goñi, S.; Vegas, I. Evolution of mineralogical phases produced during the pozzolanic reaction of different metakaolinite by-products: Influence of the activation process. Appl. Clay Sci. 2012, 56, 48–52. [Google Scholar] [CrossRef]
- Cementos: Cálculo de la Composición Potencial del Clinker Portland; Norma española UNE 80304:2006; European Standard: Madrid, Spain, 2006.
- Frías, M.; de Rojas, M.S.; Rodriguez, O.; García, R.; Vigil, R. Characterization of calcined paper sludge as an environmentally friendly source of MK for manufacture of cementitious materials. Adv. Cem. Res. 2008, 20, 23–30. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Ltd.: London, UK, 1997. [Google Scholar]
- Tajuelo-Rodriguez, E.; Richardson, I.G.; Black, L.; Boehm-Courjault, P.; Nonat, A.; Skibsted, J. Composition, silicate anion structure and morphology of calcium silicate hydrates (C-S-H) synthesised by silica-lime reaction and by controlled hydration of tricalcium silicate (C3S). Adv. Appl. Certif. 2015, 114, 362–371. [Google Scholar] [CrossRef]
- Grangeon, S.; Fernandez-Martinez, A.; Baronnet, A.; Marty, N.; Poulain, A.; Elkäm, E.; Roosz, C.; Gaboreau, S.; Henocq, P.; Claret, F. Quantitative X-ray pair distribution function analysis of nanocrystalline calcium silicate hydrates: A contribution to the understanding of cement chemistry. J. Appl. Cryst. 2017, 50, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, M.; Kim, H.S.; Basu, S.; Radovic, M. Mechanical properties of sodium and potassium activated metakaolin-based geopolymers. J. Mater. Sci. 2012, 47, 2607–2616. [Google Scholar] [CrossRef]
- Richardson, I.G. Model structures for C-(A)-S-H (I). Acta Cryst. B 2014, 70, 903–923. [Google Scholar] [CrossRef] [PubMed]
- L’Hôpital, E.; Lothenbach, B.; Kulik, D.A.; Scrivener, K. Influence of calcium to silica ratio on aluminium uptake in calcium silicate hydrate. Cem. Concr. Res. 2016, 85, 111–121. [Google Scholar] [CrossRef]
- Kani, E.N.; Allahverdi, A. Effects of curing time and temperature on strength development of inorganic polymeric binder based on natural pozzolan. J. Mater. Sci. 2009, 44, 3088–3097. [Google Scholar] [CrossRef]
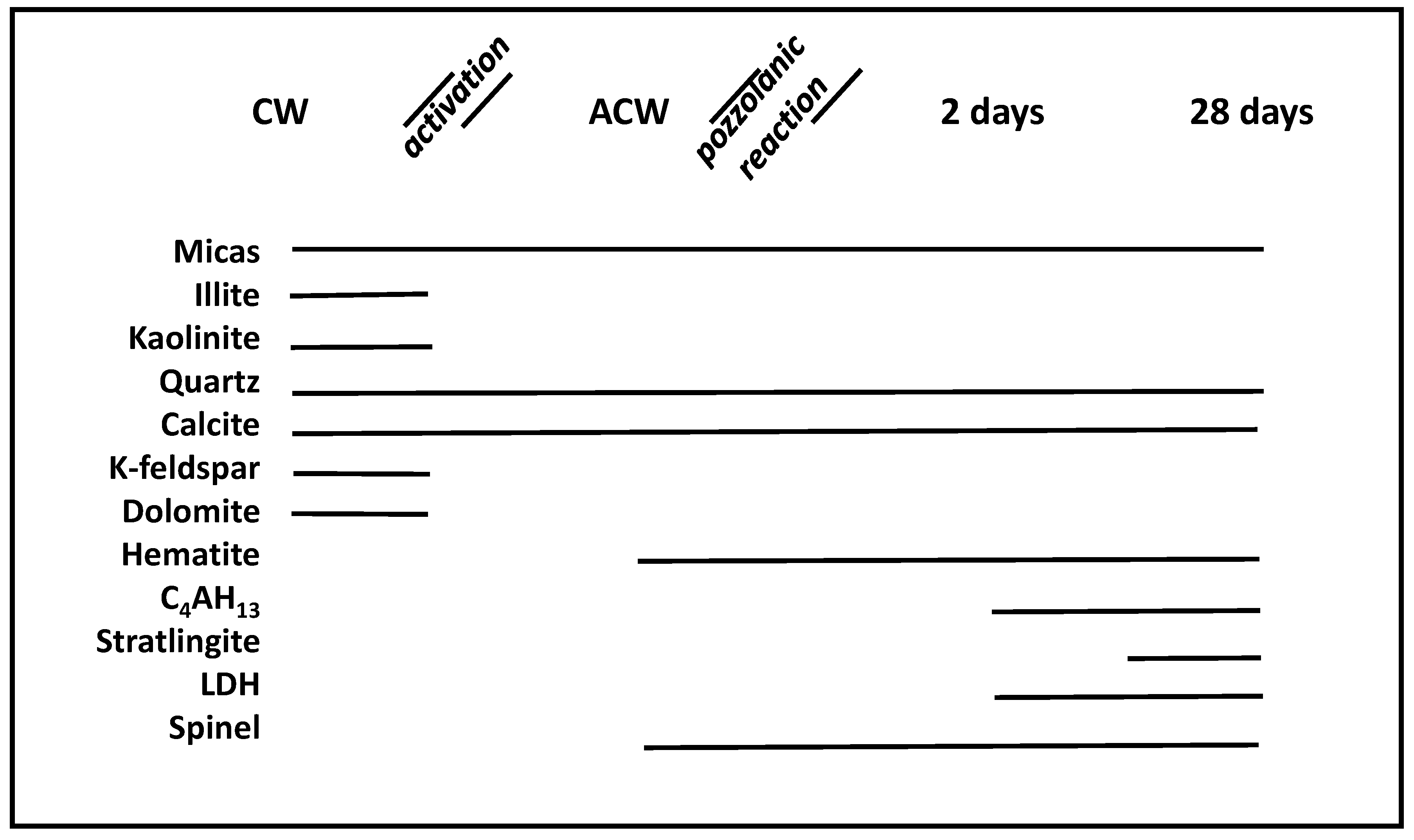
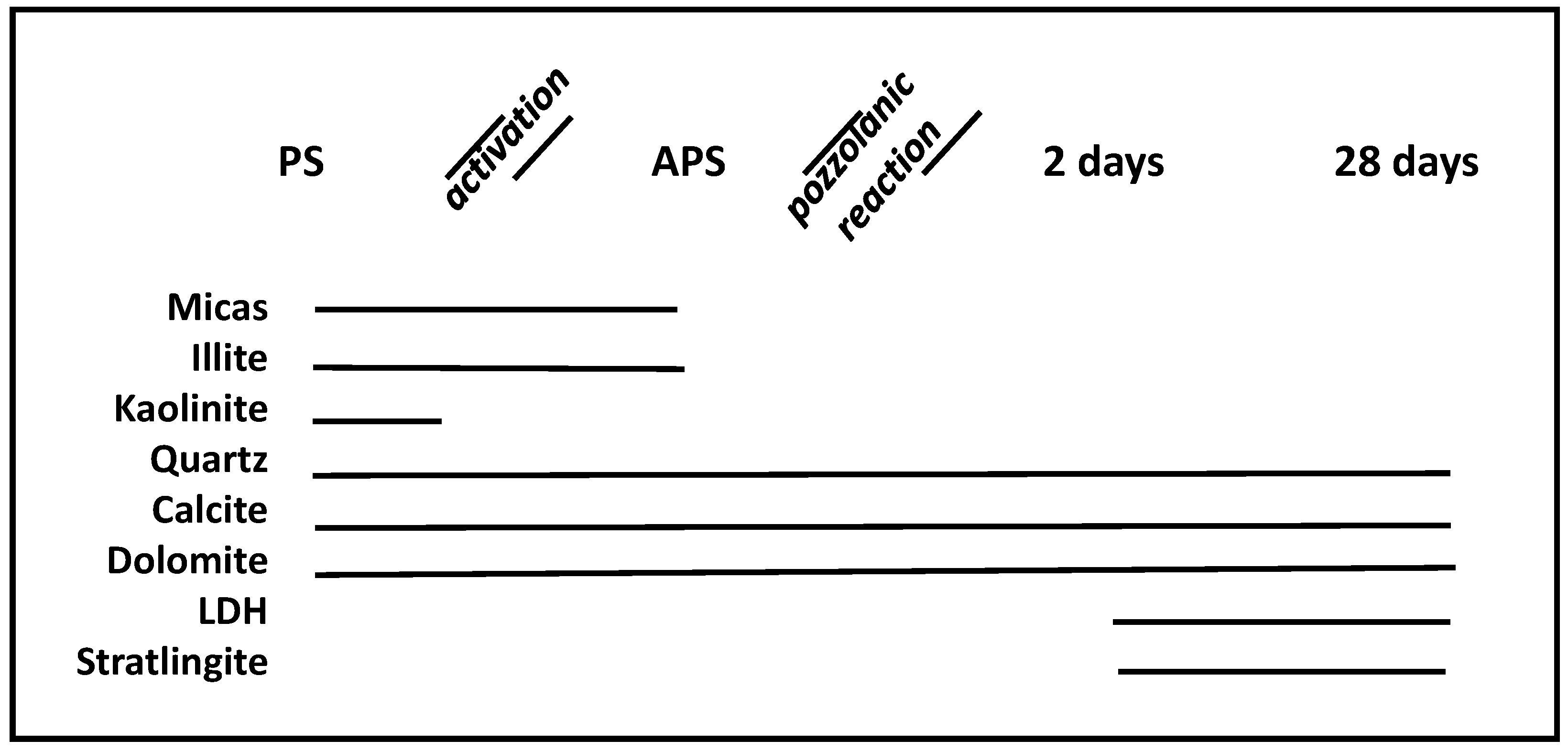
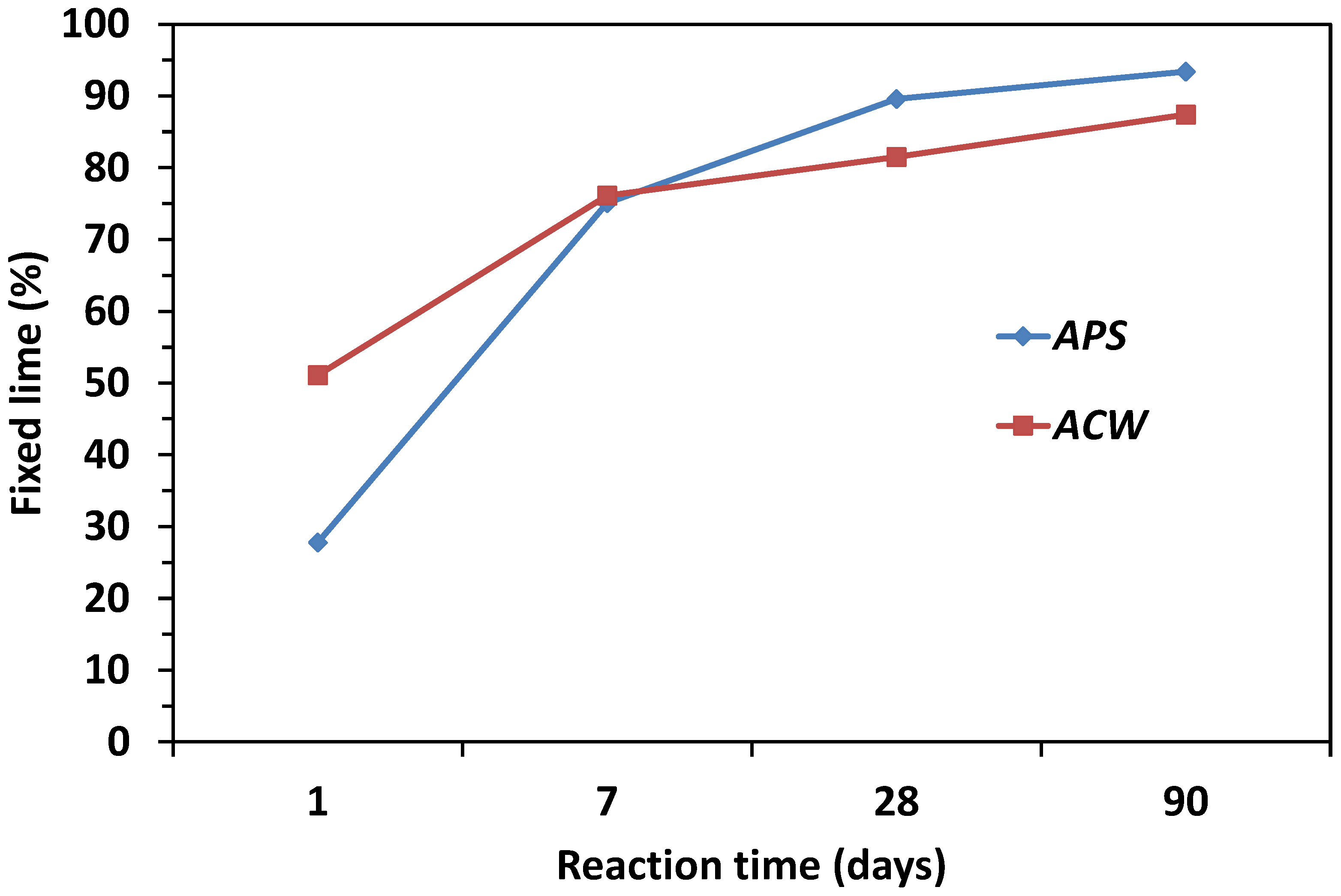

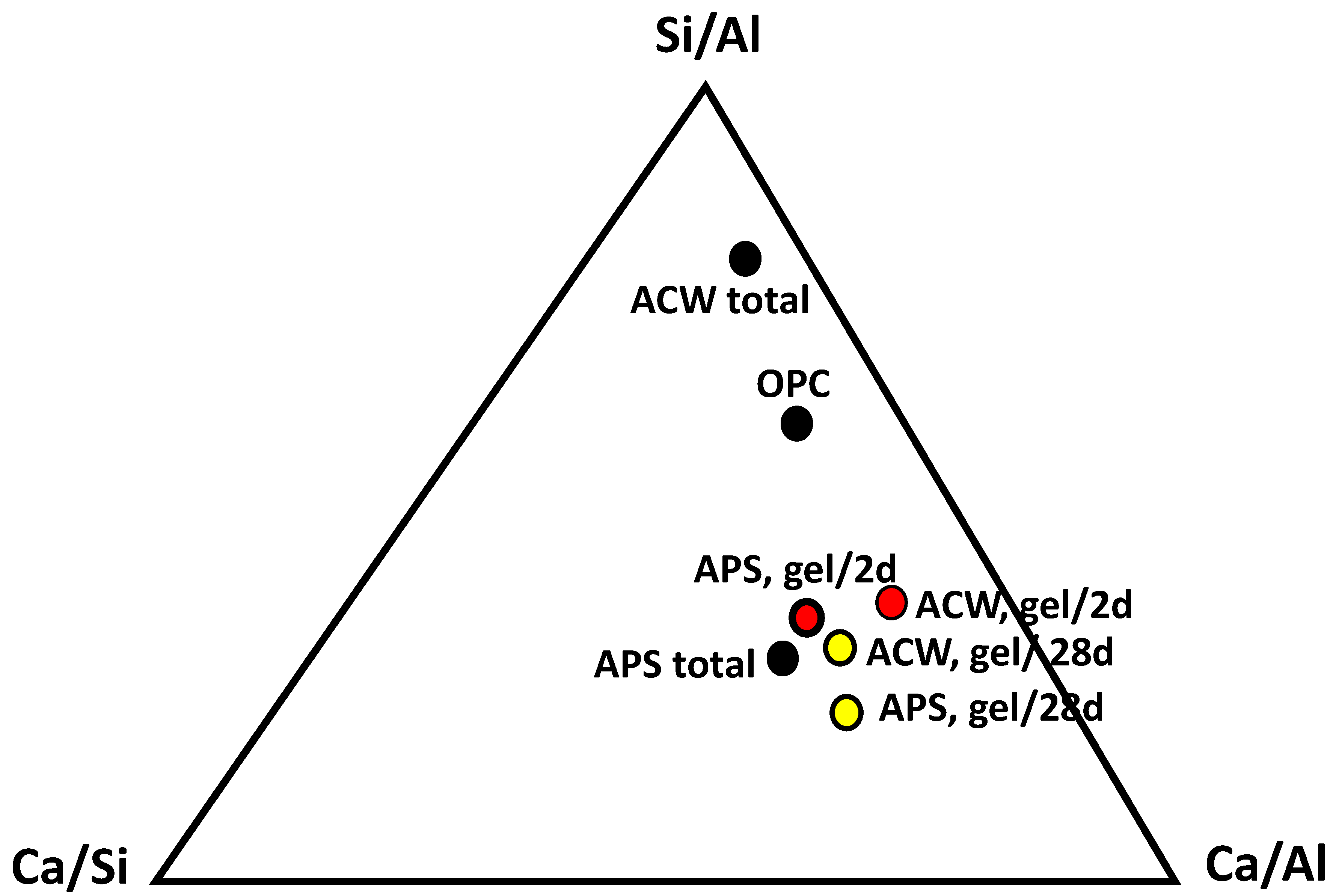
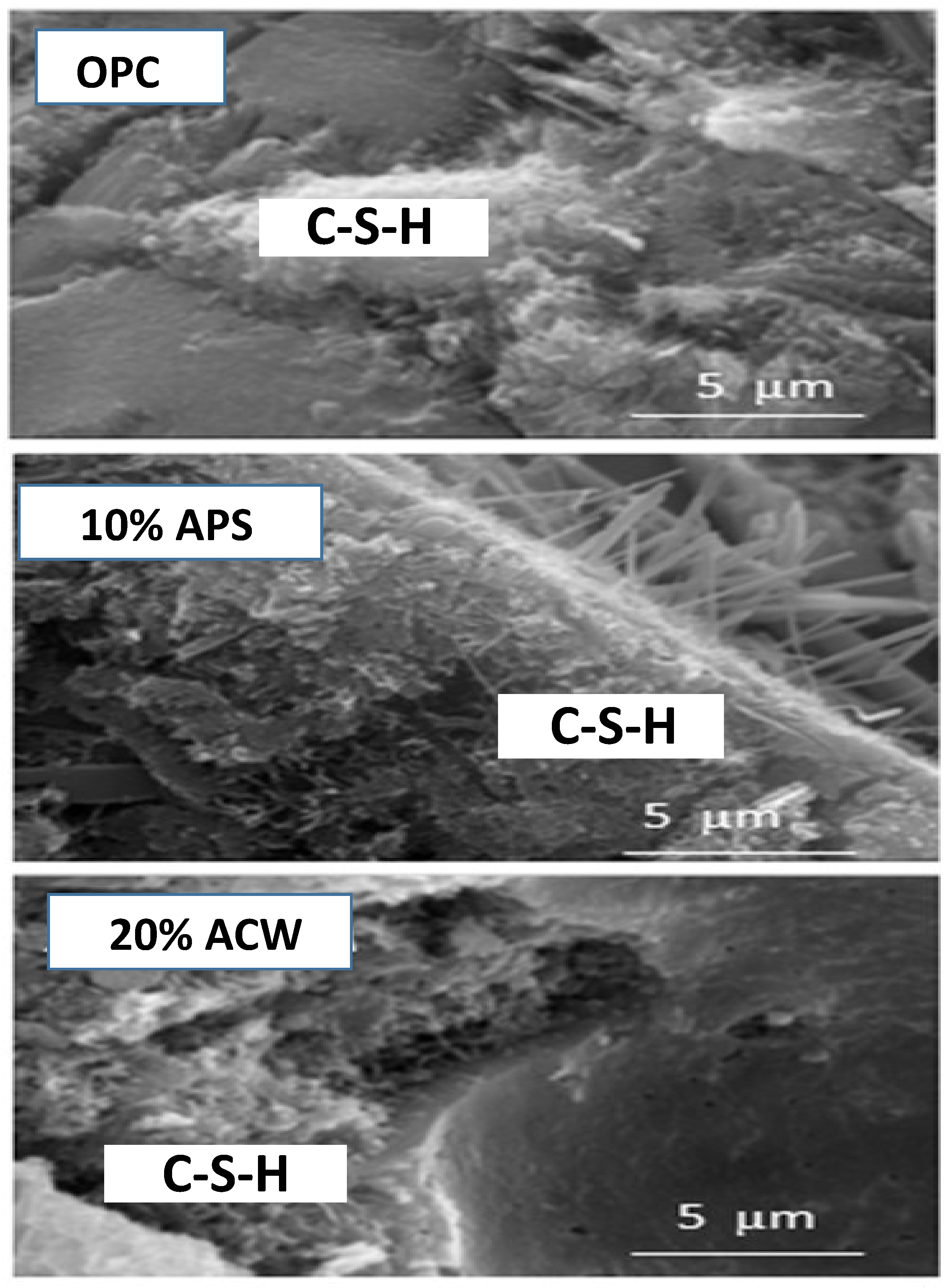
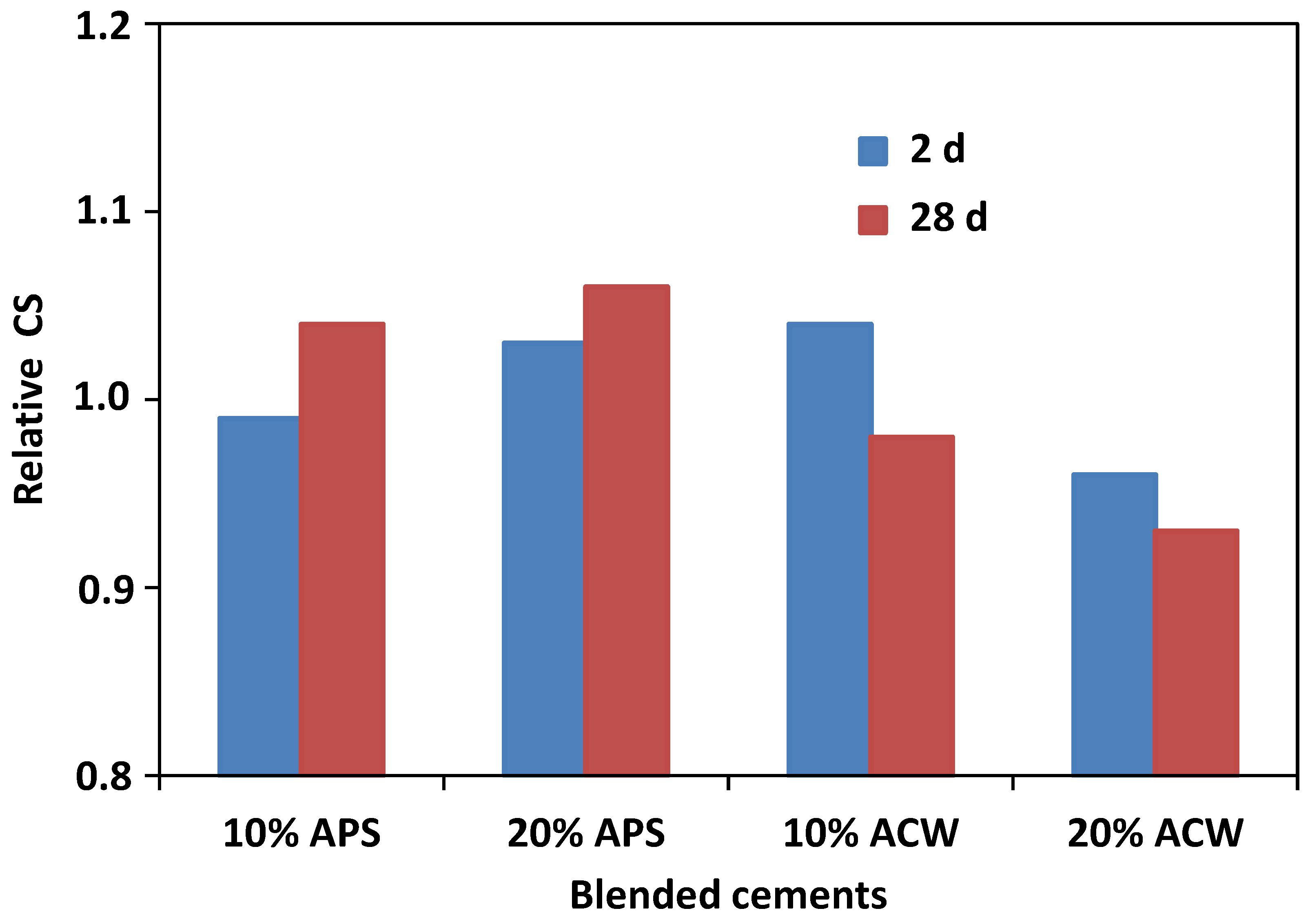
| Oxide (%) | PS | APS | CW | ACW | OPC |
|---|---|---|---|---|---|
| SiO2 | 10.69 | 20.24 | 49.79 | 56.63 | 20.16 |
| Al2O3 | 6.74 | 3.11 | 21.77 | 25.29 | 4.36 |
| Fe2O3 | 0.41 | 0.52 | 4.07 | 4.64 | 2.52 |
| CaO | 24.15 | 36.39 | 3.84 | 4.20 | 63.41 |
| MgO | 0.96 | 2.15 | 0.64 | 0.77 | 2.21 |
| SO3 | 0.30 | 0.28 | 0.27 | 0.27 | 3.57 |
| K2O | 0.22 | 0.34 | 2.74 | 3.09 | 0.91 |
| Na2O | 0.24 | 0.08 | 0.13 | 0.17 | 0.35 |
| TiO2 | 0.21 | 0.24 | 1.07 | 1.17 | 0.21 |
| P2O5 | 0.16 | 0.17 | 0.13 | 0.14 | 0.14 |
| LOI | 55.71 | 26.24 | 15.18 | 3.09 | 1.99 |
| Oxides (%) | C-S-H Gels (at 2 Days) in ACW | C-S-H Gels (at 28 Days) in ACW | C-S-H Gels (at 2 Days) in APS | C-S-H Gels (at 28 Days) in APS |
|---|---|---|---|---|
| Na2O | 0.22 ± 0.10 | 0.78 ± 0.12 | n.d. | n.d. |
| MgO | 0.43 ± 0.08 | 0.60 ± 0.28 | n.d. | n.d. |
| Al2O3 | 19.49 ± 1.32 | 22.91 ± 0.42 | 21.87 ± 0.47 | 18.20 ± 3.28 |
| SiO2 | 38.28 ± 0.99 | 31.02 ± 2.39 | 30.05 ± 0.84 | 31.07 ± 0.06 |
| SO3 | 0.97 ± 0.52 | 2.50 ± 0.61 | n.d. | n.d. |
| K2O | n.d. | 1.19 ± 0.27 | n.d. | n.d. |
| CaO | 40.23 ± 1.27 | 39.33 ± 0.86 | 48.08 ± 1.12 | 50.72 ± 0.37 |
| Fe2O3 | 0.12 ± 0.05 | 1.66 ± 0.32 | n.d. | n.d. |
| Ca/Si ratio | 1.07 | 1.26 | 1.60 | 1.63 |
| Number of analysis | 10 | 10 | 10 | 10 |
| Ratio | APS Gels (2 Days) | APS Gels (28 Days) | ACW Gels (2 Days) | ACW Gels (28 Days) |
|---|---|---|---|---|
| Ca/Si | 1.60 | 1.63 | 1.05 | 1.27 |
| Si/Al | 1.37 | 1.71 | 1.97 | 1.35 |
| Al/Ca | 0.45 | 0.35 | 0.48 | 0.58 |
| Oxides (%) | C-S-H Gels (at 28 Days) in OPC | C-S-H Gels (at 28 Days) in OPC 10% APS | C-S-H Gels (at 28 Days) in OPC 20% APS | C-S-H Gels (at 28 Days) in OPC 10% ACW | C-S-H Gels (at 28 Days) in OPC 20% ACW |
|---|---|---|---|---|---|
| Na2O | n.d | n.d. | n.d. | 0.90 ± 0.31 | 0.98 ± 0.52 |
| MgO | n.d | n.d. | n.d.- | 0.90 ± 0.28 | 0.95 ± 0.33 |
| Al2O3 | 10.60 ± 1.21 | 16.12 ± 1.32 | 18.74 ± 1.36 | 1.57 ± 0.64 | 2.12 ± 0.33 |
| SiO2 | 20.91 ± 1.49 | 28.26 ± 1.74 | 25.63 ± 2.45 | 31.25 ± 1.37 | 28.15 ± 2.16 |
| SO3 | n.d | n.d. | n.d. | 2.10 ± 0.62 | 2.007 ± 0.40 |
| K2O | n.d | n.d. | n.d. | 1.84 ± 0.35 | 1.93 ± 0.42 |
| CaO | 68.49 ± 2.25 | 52.38 ± 2.37 | 47.87 ± 2.39 | 60.31 ± 1.16 | 60.15 ± 1.19 |
| Fe2O3 | n.d | n.d. | n.d. | 1.05 ± 0.11 | 1.11 ± 0.23 |
| Ca/Si | 3.28 | 1.85 | 1.88 | 1.93 | 2.14 |
| Si/Al | 1.97 | 1.75 | 1.37 | 19.90 | 13.28 |
| Al/Ca | 0.15 | 0.31 | 0.39 | 0.03 | 0.03 |
| Number of analyses | 10 | 10 | 10 | 10 | 10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Giménez, R.; Frias, M.; Vigil de la Villa, R.; Martínez-Ramírez, S. Ca/Si and Si/Al Ratios of Metakaolinite-Based Wastes: Their Influence on Mineralogy and Mechanical Strengths. Appl. Sci. 2018, 8, 480. https://doi.org/10.3390/app8040480
García-Giménez R, Frias M, Vigil de la Villa R, Martínez-Ramírez S. Ca/Si and Si/Al Ratios of Metakaolinite-Based Wastes: Their Influence on Mineralogy and Mechanical Strengths. Applied Sciences. 2018; 8(4):480. https://doi.org/10.3390/app8040480
Chicago/Turabian StyleGarcía-Giménez, Rosario, Moisés Frias, Raquel Vigil de la Villa, and Sagrario Martínez-Ramírez. 2018. "Ca/Si and Si/Al Ratios of Metakaolinite-Based Wastes: Their Influence on Mineralogy and Mechanical Strengths" Applied Sciences 8, no. 4: 480. https://doi.org/10.3390/app8040480





