Thermally Activated Delayed Fluorescence Emitters for Deep Blue Organic Light Emitting Diodes: A Review of Recent Advances
Abstract
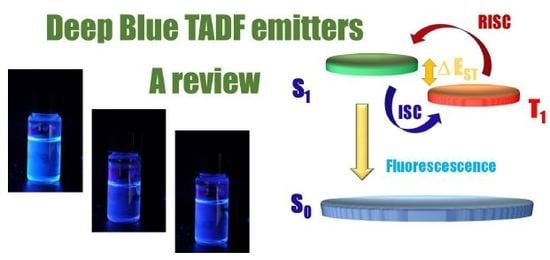
:1. Introduction
2. Results and Discussion
2.1. Molecular Design of Deep Blue TADF Emitters
2.2. Diphenylsulfone Based Emitters
2.3. Triazine-Based TADF Light-Emitting Materials
2.4. Phosphine Oxide Derivatives
2.5. Cyanide Based TADF Emitter
2.6. Triarylborane Emitters
2.7. TADF Exciplex Emitters
2.8. Lifetime of Blue TADF Emitters
3. Conclusions
- ■
- DFT calculations proved to be useful to investigate the photophysical properties of the materials prior to its synthesis.
- ■
- The maximum decrease of the π-conjugation through the whole molecule by introducing a large twist angle between the electron-donating and withdrawing parts is an effective way to reduce the electronic communication between the two parts and ΔEST. However, the donor should be in proximity of the acceptor to avoid a complete isolation of the electro-donating and the accepting groups. A careful selection of the π-bridge connecter introduced between the electron donor/acceptor moieties is of crucial importance. This has been exemplified throughout this review and unequal performances were observed for materials simply differing by the substitution pattern.
- ■
- A small value of ∆EST optimizes the rate constant of the reverse intersystem crossing by the small energy gap.
- ■
- Elongation of the π-conjugated system of both the donor and/or the acceptor maximizes the oscillator strength, and thus increases the PLQY.
- ■
- To address the excited states annihilation issue, lifetimes of the delayed component should be shortened.
Author Contributions
Conflicts of Interest
References
- Wang, H.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. AIE luminogens: Emission brightened by aggregation. Mater. Today 2015, 18, 365–377. [Google Scholar] [CrossRef]
- Minaev, B.; Baryshnikov, G.; Agren, H. Principles of phosphorescent organic light emitting devices. Phys. Chem. Chem. Phys. 2014, 16, 1719–1758. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.-P.; Li, Y.-Q.; Tang, J.-X. Recent advances in flexible organic light-emitting diodes. J. Mater. Chem. C 2016, 4, 9116–9142. [Google Scholar] [CrossRef]
- Luo, D.; Li, X.-L.; Zhao, Y.; Gao, Y.; Liu, B. High-Performance Blue Molecular Emitter-Free and Doping-Free Hybrid White Organic Light-Emitting Diodes: An Alternative Concept To Manipulate Charges and Excitons Based on Exciplex and Electroplex Emission. ACS Photonics 2017, 4, 1566–1575. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.-L.; Luo, D.; Xiao, P.; Xiao, W.; Song, Y.; Ang, Q.; Liu, B. Strategies to Achieve High-Performance White Organic Light-Emitting Diodes. Materials 2017, 10, 1378. [Google Scholar] [CrossRef] [PubMed]
- Shimotsu, R.; Takumi, T.; Vohra, V. All solution-processed micro-structured flexible electrodes for low-cost light-emitting pressure sensors fabrication. Sci. Rep. 2017, 7, 6921. [Google Scholar] [CrossRef] [PubMed]
- Hippola, C.; Kaudal, R.; Manna, E.; Xiao, T.; Peer, A.; Biswas, R.; Slafer, W.D.; Trovato, T.; Shinar, J.; Shinar, R. Enhanced Light Extraction from OLEDs Fabricated on Patterned Plastic Substrates. Adv. Opt. Mater. 2018, 6, 1701244. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, M.; Chen, X.; Nie, S.; Lai, W.-Y.; Su, W.; Cui, Z.; Huang, W. Screen-Printed Poly(3,4-Ethylenedioxythiophene):Poly(Styrenesulfonate) Grids as ITO-Free Anodes for Flexible Organic Light-Emitting Diodes. Adv. Funct. Mater. 2018, 28, 1705955. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Zhou, G. Recent advances of the emitters for high performance deep-blue organic light-emitting diodes. J. Mater. Chem. C 2015, 3, 913–944. [Google Scholar] [CrossRef]
- Farinola, G.M.; Ragni, R. Electroluminescent materials for white organic light emitting diodes. Chem. Soc. Rev. 2011, 40, 3467. [Google Scholar] [CrossRef] [PubMed]
- Gather, M.C.; Köhnen, A.; Meerholz, K. White Organic Light-Emitting Diodes. Adv. Mater. 2011, 23, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.M.; Kang, J.-W.; Park, J.W.; Jung, S.O.; Lee, S.-H.; Park, H.-D.; Kim, Y.-H.; Shin, S.C.; Kim, J.-J.; Kwon, S.-K. Iridium Complexes with Cyclometalated 2-Cycloalkenyl-Pyridine Ligands as Highly Efficient Emitters for Organic Light-Emitting Diodes. Adv. Mater. 2008, 20, 2003–2007. [Google Scholar] [CrossRef]
- Park, T.J.; Jeon, W.S.; Park, J.J.; Kim, S.Y.; Lee, Y.K.; Jang, J.; Kwon, J.H.; Pode, R. Efficient simple structure red phosphorescent organic light emitting devices with narrow band-gap fluorescent host. Appl. Phys. Lett. 2008, 92, 113308. [Google Scholar] [CrossRef]
- Polo, F.; Rizzo, F.; Veiga-Gutierrez, M.; De Cola, L.; Quici, S. Efficient Greenish Blue Electrochemiluminescence from Fluorene and Spirobifluorene Derivatives. J. Am. Chem. Soc. 2012, 134, 15402–15409. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Jeong, H.-C.; Kim, S.-H.; Yang, K.; Kwon, S.-K. High-Purity-Blue and High-Efficiency Electroluminescent Devices Based on Anthracene. Adv. Funct. Mater. 2005, 15, 1799–1805. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Zhao, W.-M.; Wang, Z.-Q.; Huang, D.; Ye, J.; Ou, X.-M.; Zhang, X.-H.; Lee, C.-S.; Lee, S.-T. Highly efficient non-doped deep-blue organic light-emitting diodes based on anthracene derivatives. J. Mater. Chem. 2010, 20, 1560. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, S.; Lam, J.W.Y.; Lu, P.; Zhong, Y.; Wong, K.S.; Kwok, H.S.; Tang, B.Z. Creation of highly efficient solid emitter by decorating pyrene core with AIE-active tetraphenylethene peripheries. Chem. Commun. 2010, 46, 2221. [Google Scholar] [CrossRef] [PubMed]
- Justin Thomas, K.R.; Velusamy, M.; Lin, J.T.; Tao, Y.-T.; Chuen, C.-H. Cyanocarbazole Derivatives for High-Performance Electroluminescent Devices. Adv. Funct. Mater. 2004, 14, 387–392. [Google Scholar] [CrossRef]
- Fleetham, T.; Li, G.; Wen, L.; Li, J. Efficient “Pure” Blue OLEDs Employing Tetradentate Pt Complexes with a Narrow Spectral Bandwidth. Adv. Mater. 2014, 26, 7116–7121. [Google Scholar] [CrossRef] [PubMed]
- Fleetham, T.; Ecton, J.; Wang, Z.; Bakken, N.; Li, J. Single-Doped White Organic Light-Emitting Device with an External Quantum Efficiency Over 20%. Adv. Mater. 2013, 25, 2573–2576. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-L.; Chi, L.-C.; Hung, W.-Y.; Chen, W.-J.; Lin, Y.-C.; Wu, H.; Mondal, E.; Zhou, G.-J.; Wong, K.-T.; Wong, W.-Y. Carbazole-based coplanar molecule (CmInF) as a universal host for multi-color electrophosphorescent devices. J. Mater. Chem. 2012, 22, 215–224. [Google Scholar] [CrossRef]
- Hudson, Z.M.; Sun, C.; Helander, M.G.; Chang, Y.-L.; Lu, Z.-H.; Wang, S. Highly Efficient Blue Phosphorescence from Triarylboron-Functionalized Platinum(II) Complexes of N -Heterocyclic Carbenes. J. Am. Chem. Soc. 2012, 134, 13930–13933. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.-O.; Shin, H.; Yun, H.-J.; Yang, K.; Kwon, S.-K.; Kim, J.-J.; Kim, Y.-H. Deep-Blue Phosphorescence from Perfluoro Carbonyl-Substituted Iridium Complexes. J. Am. Chem. Soc. 2013, 135, 14321–14328. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Zhao, Z.; Wang, L.; Yang, H.; Chang, Q.; Jiang, N.; Liu, Z.; Bian, Z.; Liu, W.; et al. Deep Blue Phosphorescent Organic Light-Emitting Diodes with CIE y Value of 0.11 and External Quantum Efficiency up to 22.5%. Adv. Mater. 2018, 1705005. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, Y.; Ding, J.; Wang, Y.; Wang, L. Highly Efficient Phosphorescent Furo[3,2-c]pyridine Based Iridium Complexes with Tunable Emission Colors over the Whole Visible Range. ACS Appl. Mater. Interfaces 2018, 10, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Bai, Q.; Shan, T.; Li, J.; Gao, Y.; Liu, F.; Liu, H.; Peng, Q.; Yang, B.; Li, F.; et al. Efficient Nondoped Blue Fluorescent Organic Light-Emitting Diodes (OLEDs) with a High External Quantum Efficiency of 9.4% @ 1000 cd m −2 Based on Phenanthroimidazole−Anthracene Derivative. Adv. Funct. Mater. 2018, 28, 1705813. [Google Scholar] [CrossRef]
- Xing, X.; Zhang, L.; Liu, R.; Li, S.; Qu, B.; Chen, Z.; Sun, W.; Xiao, L.; Gong, Q. A Deep-Blue Emitter with Electron Transporting Property to Improve Charge Balance for Organic Light-Emitting Device. ACS Appl. Mater. Interfaces 2012, 4, 2877–2880. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.J.; D’Andrade, B.W.; Forrest, S.R.; Ren, X.; Li, J.; Thompson, M.E. Efficient, deep-blue organic electrophosphorescence by guest charge trapping. Appl. Phys. Lett. 2003, 83, 3818–3820. [Google Scholar] [CrossRef]
- Ren, X.; Li, J.; Holmes, R.J.; Djurovich, P.I.; Forrest, S.R.; Thompson, M.E. Ultrahigh Energy Gap Hosts in Deep Blue Organic Electrophosphorescent Devices. Chem. Mater. 2004, 16, 4743–4747. [Google Scholar] [CrossRef]
- Lin, S.-L.; Chan, L.-H.; Lee, R.-H.; Yen, M.-Y.; Kuo, W.-J.; Chen, C.-T.; Jeng, R.-J. Highly Efficient Carbazole- π -Dimesitylborane Bipolar Fluorophores for Nondoped Blue Organic Light-Emitting Diodes. Adv. Mater. 2008, 20, 3947–3952. [Google Scholar] [CrossRef]
- Kumar Konidena, R.; Justin Thomas, K.R.; Kumar Dubey, D.; Sahoo, S.; Jou, J.-H. A new molecular design based on hybridized local and charge transfer fluorescence for highly efficient (>6%) deep-blue organic light emitting diodes. Chem. Commun. 2017, 53, 11802–11805. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Puttaraju, B.; Patil, S. A Deep-Blue Electroluminescent Device Based on a Coumarin Derivative. Chempluschem 2016, 81, 384–390. [Google Scholar] [CrossRef]
- Chien, C.-H.; Chen, C.-K.; Hsu, F.-M.; Shu, C.-F.; Chou, P.-T.; Lai, C.-H. Multifunctional Deep-Blue Emitter Comprising an Anthracene Core and Terminal Triphenylphosphine Oxide Groups. Adv. Funct. Mater. 2009, 19, 560–566. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Venkatakrishnan, P.; Natarajan, P.; Lin, Z.; Chow, T.J. Nondoped Pure-Blue OLEDs Based on Amorphous Phenylenevinylene-Functionalized Twisted Bimesitylenes. J. Org. Chem. 2010, 75, 2599–2609. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.J.; Forrest, S.R.; Sajoto, T.; Tamayo, A.; Djurovich, P.I.; Thompson, M.E.; Brooks, J.; Tung, Y.-J.; D’Andrade, B.W.; Weaver, M.S.; et al. Saturated deep blue organic electrophosphorescence using a fluorine-free emitter. Appl. Phys. Lett. 2005, 87, 243507. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, Y.; Niu, Z.-Q.; Zhou, Q.-F.; Ma, Y.; Pei, J. Three-Dimensional Architectures for Highly Stable Pure Blue Emission. J. Am. Chem. Soc. 2007, 129, 11314–11315. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhao, F.; Zhang, Z.; Han, C.; Xu, H.; Li, J.; Ma, D.; Yan, P. Insulated donor–π–acceptor systems based on fluorene-phosphine oxide hybrids for non-doped deep-blue electroluminescent devices. Chem. Commun. 2012, 48, 6157. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, Z.; Yang, C.; Zhong, C.; Qin, J.; Yu, G.; Liu, Y. Multifunctional Fluorene-Based Oligomers with Novel Spiro-Annulated Triarylamine: Efficient, Stable Deep-Blue Electroluminescence, Good Hole Injection, and Transporting Materials with Very High Tg. Adv. Funct. Mater. 2009, 19, 3987–3995. [Google Scholar] [CrossRef]
- Huang, J.; Sun, N.; Chen, P.; Tang, R.; Li, Q.; Ma, D.; Li, Z. Largely blue-shifted emission through minor structural modifications: Molecular design, synthesis, aggregation-induced emission and deep-blue OLED application. Chem. Commun. 2014, 50, 2136. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Sun, N.; Dong, Y.; Tang, R.; Lu, P.; Cai, P.; Li, Q.; Ma, D.; Qin, J.; Li, Z. Similar or Totally Different: The Control of Conjugation Degree through Minor Structural Modifications, and Deep-Blue Aggregation-Induced Emission Luminogens for Non-Doped OLEDs. Adv. Funct. Mater. 2013, 23, 2329–2337. [Google Scholar] [CrossRef]
- Zhan, X.; Sun, N.; Wu, Z.; Tu, J.; Yuan, L.; Tang, X.; Xie, Y.; Peng, Q.; Dong, Y.; Li, Q.; et al. Polyphenylbenzene as a Platform for Deep-Blue OLEDs: Aggregation Enhanced Emission and High External Quantum Efficiency of 3.98%. Chem. Mater. 2015, 27, 1847–1854. [Google Scholar] [CrossRef]
- Endo, A.; Ogasawara, M.; Takahashi, A.; Yokoyama, D.; Kato, Y.; Adachi, C. Thermally Activated Delayed Fluorescence from Sn4+-Porphyrin Complexes and Their Application to Organic Light Emitting Diodes—A Novel Mechanism for Electroluminescence. Adv. Mater. 2009, 21, 4802–4806. [Google Scholar] [CrossRef] [PubMed]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Mamada, M.; Inada, K.; Komino, T.; Potscavage, W.J., Jr.; Nakanotani, H.; Adachi, C. Highly efficient thermally activated delayed fluorescence from an excited-state intramolecular proton transfer system. ACS Cent. Sci. 2017, 3, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Z.; Hu, T.; Zhang, Y.; Wang, Y.; Yi, Y.; Guo, F.; Zhao, L. Highly efficient blue organic light-emitting diodes from pyrimidine-based thermally activated delayed fluorescence emitters. J. Mater. Chem. C, 2018, 6, 2351–2359. [Google Scholar] [CrossRef]
- Yu, L.; Wu, Z.; Xie, G.; Zhong, C.; Zhu, Z.; Ma, D.; Yang, C. An efficient exciton harvest route for high-performance OLEDs based on aggregation-induced delayed fluorescence. Chem. Commun. 2018, 54, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Han, H.-B.; Yan, Z.-P.; Liu, L.; Zheng, Y.-X.; Meng, H.; Huang, W. Versatile functionalization of trifluoromethyl based deep blue thermally activated delayed fluorescence materials for organic light emitting diodes. New J. Chem. 2018, 42, 4317–4323. [Google Scholar] [CrossRef]
- Grybauskaite-Kaminskiene, G.; Ivaniuk, K.; Bagdziunas, G.; Turyk, P.; Stakhira, P.; Baryshnikov, G.; Volyniuk, D.; Cherpak, V.; Minaev, B.; Hotra, Z.; et al. Contribution of TADF and exciplex emission for efficient “warm-white” OLEDs. J. Mater. Chem. C 2018, 6, 1543–1550. [Google Scholar] [CrossRef]
- Bouzrati, M.; Noirbent, G.; Goubard, F.; Bui, T.-T.; Villotte, S.; Dietlin, C.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.P.; Dumur, F.; et al. A novel class of photoinitiators for polymerization reactions: Metal-Based and Metal-Free Photoredox Catalysts with a Thermally Activated Delayed Fluorescence (TADF) property. New J. Chem. 2018. [Google Scholar] [CrossRef]
- Mousawi, A.A.; Garra, P.; Dumur, F.; Bui, T.-T.; Goubard, F.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; et al. Novel carbazole skeleton-based photoinitiators for led polymerization and LED projector 3D printing. Molecules 2017, 22, 2143. [Google Scholar] [CrossRef] [PubMed]
- Al Mousawi, A.; Lara, D.M.; Noirbent, G.; Dumur, F.; Toufaily, J.; Hamieh, T.; Bui, T.-T.; Goubard, F.; Graff, B.; Gigmes, D.; et al. Carbazole Derivatives with Thermally Activated Delayed Fluorescence Property as Photoinitiators/Photoredox Catalysts for LED 3D Printing Technology. Macromolecules 2017, 50, 4913–4926. [Google Scholar] [CrossRef]
- Wong, M.Y.; Zysman-Colman, E. Purely Organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.-T.; Goubard, F.; Ibrahim-Ouali, M.; Gigmes, D.; Dumur, F. Recent Advances on Organic Blue Thermally Activated Delayed Fluorescence (TADF) Emitters for Organic Light-Emitting Diodes (OLEDs). Beilstein J. Org. Chem. 2018, 14, 282–308. [Google Scholar] [CrossRef] [PubMed]
- Adachi, C. Third-generation organic electroluminescence materials. Jpn. J. Appl. Phys. 2014, 53, 60101. [Google Scholar] [CrossRef]
- Shizu, K.; Tanaka, H.; Uejima, M.; Sato, T.; Tanaka, K.; Kaji, H.; Adachi, C. Strategy for Designing Electron Donors for Thermally Activated Delayed Fluorescence Emitters. J. Phys. Chem. C 2015, 119, 1291–1297. [Google Scholar] [CrossRef]
- Berberan-Santos, M.N.; Garcia, J.M.M. Unusually Strong Delayed Fluorescence of C70. J. Am. Chem. Soc. 1996, 118, 9391–9394. [Google Scholar] [CrossRef]
- Toptygin, D. Effects of the Solvent Refractive Index and Its Dispersion on the Radiative Decay Rate and Extinction Coefficient of a Fluorescent Solute. J. Fluoresc. 2003, 13, 201–219. [Google Scholar] [CrossRef]
- Manzhos, S.; Segawa, H.; Yamashita, K. A model for recombination in Type II dye-sensitized solar cells: Catechol–thiophene dyes. Chem. Phys. Lett. 2011, 504, 230–235. [Google Scholar] [CrossRef]
- Manzhos, S.; Segawa, H.; Yamashita, K. Derivative coupling constants of NK1, NK7 dyes and their relation to excited state dynamics in solar cell applications. Chem. Phys. Lett. 2011, 501, 580–586. [Google Scholar] [CrossRef]
- Dos Santos, P.L.; Ward, J.S.; Bryce, M.R.; Monkman, A.P. Using Guest–Host Interactions To Optimize the Efficiency of TADF OLEDs. J. Phys. Chem. Lett. 2016, 7, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; Monkman, A.P.; Penfold, T.J. The Importance of Vibronic Coupling for Efficient Reverse Intersystem Crossing in Thermally Activated Delayed Fluorescence Molecules. ChemPhysChem 2016, 17, 2956–2961. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.K.; Kim, D.; Coropceanu, V.; Brédas, J.-L. Up-Conversion Intersystem Crossing Rates in Organic Emitters for Thermally Activated Delayed Fluorescence: Impact of the Nature of Singlet vs Triplet Excited States. J. Am. Chem. Soc. 2017, 139, 4042–4051. [Google Scholar] [CrossRef] [PubMed]
- Etherington, M.K.; Franchello, F.; Gibson, J.; Northey, T.; Santos, J.; Ward, J.S.; Higginbotham, H.F.; Data, P.; Kurowska, A.; Dos Santos, P.L.; et al. Regio- and conformational isomerization critical to design of efficient thermally-activated delayed fluorescence emitters. Nat. Commun. 2017, 8, 14987. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.S.; dos Santos, P.L.; Ward, J.S.; Data, P.; Okazaki, M.; Takeda, Y.; Minakata, S.; Bryce, M.R.; Monkman, A.P. An optical and electrical study of full thermally activated delayed fluorescent white organic light-emitting diodes. Sci. Rep. 2017, 7, 6234. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Li, X.; Xie, G.; Chen, D.; Wang, Y.-F.; Lo, C.-C.; Lien, A.; Peng, J.; Cao, Y.; et al. Highly Efficient Spiro[fluorene-9,9′-thioxanthene] Core Derived Blue Emitters and Fluorescent/Phosphorescent Hybrid White Organic Light-Emitting Diodes. Chem. Mater. 2015, 27, 1100–1109. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Shizu, K.; Huang, S.; Hirata, S.; Miyazaki, H.; Adachi, C. Design of Efficient Thermally Activated Delayed Fluorescence Materials for Pure Blue Organic Light Emitting Diodes. J. Am. Chem. Soc. 2012, 134, 14706–14709. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Aonuma, M.; Zhang, Q.; Huang, S.; Nakagawa, T.; Kuwabara, K.; Adachi, C. High-efficiency deep-blue organic light-emitting diodes based on a thermally activated delayed fluorescence emitter. J. Mater. Chem. C 2014, 2, 421–424. [Google Scholar] [CrossRef]
- Mei, L.; Hu, J.; Cao, X.; Wang, F.; Zheng, C.; Tao, Y.; Zhang, X.; Huang, W. The inductive-effect of electron withdrawing trifluoromethyl for thermally activated delayed fluorescence: Tunable emission from tetra- to penta-carbazole in solution processed blue OLEDs. Chem. Commun. 2015, 51, 13024–13027. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Qi, Q.; Jiang, W.; Tang, J.; Liu, Y.; Fan, W.; Yin, Z.; Shi, F.; Ban, X.; Xu, H.; et al. Thermally activated delayed fluorescence materials based on 3,6-di-tert-butyl-9-((phenylsulfonyl)phenyl)-9H-carbazoles. Dyes Pigment. 2014, 111, 135–144. [Google Scholar] [CrossRef]
- Wang, H.; Xie, L.; Peng, Q.; Meng, L.; Wang, Y.; Yi, Y.; Wang, P. Novel Thermally Activated Delayed Fluorescence Materials-Thioxanthone Derivatives and Their Applications for Highly Efficient OLEDs. Adv. Mater. 2014, 26, 5198–5204. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-Y.; Pu, Y.-J.; Satoh, F.; Kawata, S.; Katagiri, H.; Sasabe, H.; Kido, J. Bisanthracene-Based Donor-Acceptor-type Light-Emitting Dopants: Highly Efficient Deep-Blue Emission in Organic Light-Emitting Devices. Adv. Funct. Mater. 2014, 24, 2064–2071. [Google Scholar] [CrossRef]
- Li, J.; Liao, X.; Xu, H.; Li, L.; Zhang, J.; Wang, H.; Xu, B. Deep-blue thermally activated delayed fluorescence dendrimers with reduced singlet-triplet energy gap for low roll-off non-doped solution-processed organic light-emitting diodes. Dyes Pigment. 2017, 140, 79–86. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, L.; Zhou, Y.; Cui, Y.-X.; Wang, J.; Cao, Y.; Pei, J. π-Conjugated Dendrimers as Stable Pure-Blue Emissive Materials: Photophysical, Electrochemical, and Electroluminescent Properties. Chem. Asian J. 2009, 4, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D. Dendrimers for organic light-emitting diodes. J. Mater. Chem. 2009, 19, 7584. [Google Scholar] [CrossRef]
- Luo, J.; Gong, S.; Gu, Y.; Chen, T.; Li, Y.; Zhong, C.; Xie, G.; Yang, C. Multi-carbazole encapsulation as a simple strategy for the construction of solution-processed, non-doped thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2016, 4, 2442–2446. [Google Scholar] [CrossRef]
- Albrecht, K.; Matsuoka, K.; Fujita, K.; Yamamoto, K. Carbazole Dendrimers as Solution-Processable Thermally Activated Delayed-Fluorescence Materials. Angew. Chem. Int. Ed. 2015, 54, 5677–5682. [Google Scholar] [CrossRef] [PubMed]
- Ban, X.; Jiang, W.; Lu, T.; Jing, X.; Tang, Q.; Huang, S.; Sun, K.; Huang, B.; Lin, B.; Sun, Y. Self-host thermally activated delayed fluorescent dendrimers with flexible chains: An effective strategy for non-doped electroluminescent devices based on solution processing. J. Mater. Chem. C 2016, 4, 8810–8816. [Google Scholar] [CrossRef]
- Li, Y.; Xie, G.; Gong, S.; Wu, K.; Yang, C. Dendronized delayed fluorescence emitters for non-doped, solution-processed organic light-emitting diodes with high efficiency and low efficiency roll-off simultaneously: Two parallel emissive channels. Chem. Sci. 2016, 7, 5441–5447. [Google Scholar] [CrossRef]
- Sun, J.W.; Baek, J.Y.; Kim, K.-H.; Huh, J.-S.; Kwon, S.-K.; Kim, Y.-H.; Kim, J.-J. Azasiline-based thermally activated delayed fluorescence emitters for blue organic light emitting diodes. J. Mater. Chem. C 2017, 5, 1027–1032. [Google Scholar] [CrossRef]
- Hirata, S.; Sakai, Y.; Masui, K.; Tanaka, H.; Lee, S.Y.; Nomura, H.; Nakamura, N.; Yasumatsu, M.; Nakanotani, H.; Zhang, Q.; et al. Highly efficient blue electroluminescence based on thermally activated delayed fluorescence. Nat. Mater. 2015, 14, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Seino, Y.; Chen, D.; Inomata, S.; Su, S.-J.; Sasabe, H.; Kido, J. Blue thermally activated delayed fluorescence materials based on bis(phenylsulfonyl)benzene derivatives. Chem. Commun. 2015, 51, 16353–16356. [Google Scholar] [CrossRef] [PubMed]
- Jürgensen, N.; Kretzschmar, A.; Höfle, S.; Freudenberg, J.; Bunz, U.H.F.; Hernandez-Sosa, G. Sulfone-Based Deep Blue Thermally Activated Delayed Fluorescence Emitters: Solution-Processed Organic Light-Emitting Diodes with High Efficiency and Brightness. Chem. Mater. 2017, 29, 9154–9161. [Google Scholar] [CrossRef]
- Sun, J.W.; Baek, J.Y.; Kim, K.-H.; Moon, C.-K.; Lee, J.-H.; Kwon, S.-K.; Kim, Y.-H.; Kim, J.-J. Thermally Activated Delayed Fluorescence from Azasiline Based Intramolecular Charge-Transfer Emitter (DTPDDA) and a Highly Efficient Blue Light Emitting Diode. Chem. Mater. 2015, 27, 6675–6681. [Google Scholar] [CrossRef]
- Cui, L.-S.; Nomura, H.; Geng, Y.; Kim, J.U.; Nakanotani, H.; Adachi, C. Controlling Singlet-Triplet Energy Splitting for Deep-Blue Thermally Activated Delayed Fluorescence Emitters. Angew. Chem. Int. Ed. 2017, 56, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Li, J.; Han, C.; Ding, D.; Yang, H.; Wei, Y.; Xu, H. Multi-dipolar Chromophores Featuring Phosphine Oxide as Joint Acceptor: A New Strategy toward High-Efficiency Blue Thermally Activated Delayed Fluorescence Dyes. Chem. Mater. 2016, 28, 5667–5679. [Google Scholar] [CrossRef]
- Cho, Y.J.; Jeon, S.K.; Lee, S.-S.; Yu, E.; Lee, J.Y. Donor Interlocked Molecular Design for Fluorescence-like Narrow Emission in Deep Blue Thermally Activated Delayed Fluorescent Emitters. Chem. Mater. 2016, 28, 5400–5405. [Google Scholar] [CrossRef]
- Pan, K.-C.; Li, S.-W.; Ho, Y.-Y.; Shiu, Y.-J.; Tsai, W.-L.; Jiao, M.; Lee, W.-K.; Wu, C.-C.; Chung, C.-L.; Chatterjee, T.; et al. Efficient and Tunable Thermally Activated Delayed Fluorescence Emitters Having Orientation-Adjustable CN-Substituted Pyridine and Pyrimidine Acceptor Units. Adv. Funct. Mater. 2016, 26, 7560–7571. [Google Scholar] [CrossRef]
- Chan, C.-Y.; Cui, L.-S.; Kim, J.U.; Nakanotani, H.; Adachi, C. Rational Molecular Design for Deep-Blue Thermally Activated Delayed Fluorescence Emitters. Adv. Funct. Mater. 2018, 28, 1706023. [Google Scholar] [CrossRef]
- Kinoshita, M.; Kita, H.; Shirota, Y. A Novel Family of Boron-Containing Hole-Blocking Amorphous Molecular Materials for Blue- and Blue–Violet-Emitting Organic Electroluminescent Devices. Adv. Funct. Mater. 2002, 12, 780–786. [Google Scholar] [CrossRef]
- Nagai, A.; Kobayashi, S.; Nagata, Y.; Kokado, K.; Taka, H.; Kita, H.; Suzuri, Y.; Chujo, Y. Luminescent alternating boron quinolate–fluorene copolymers exhibiting high electron mobility. J. Mater. Chem. 2010, 20, 5196. [Google Scholar] [CrossRef]
- Kitamoto, Y.; Namikawa, T.; Ikemizu, D.; Miyata, Y.; Suzuki, T.; Kita, H.; Sato, T.; Oi, S. Light blue and green thermally activated delayed fluorescence from 10H-phenoxaborin-derivatives and their application to organic light-emitting diodes. J. Mater. Chem. C 2015, 3, 9122–9130. [Google Scholar] [CrossRef]
- Kitamoto, Y.; Namikawa, T.; Suzuki, T.; Miyata, Y.; Kita, H.; Sato, T.; Oi, S. Dimesitylarylborane-based luminescent emitters exhibiting highly-efficient thermally activated delayed fluorescence for organic light-emitting diodes. Org. Electron. 2016, 34, 208–217. [Google Scholar] [CrossRef]
- Lee, Y.H.; Park, S.; Oh, J.; Shin, J.W.; Jung, J.; Yoo, S.; Lee, M.H. Rigidity-Induced Delayed Fluorescence by Ortho Donor-Appended Triarylboron Compounds: Record-High Efficiency in Pure Blue Fluorescent Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2017, 9, 24035–24042. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Yasuda, T.; Adachi, C. High efficiency pure blue thermally activated delayed fluorescence molecules having 10H-phenoxaborin and acridan units. Chem. Commun. 2015, 51, 9443–9446. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Shiren, K.; Nakajima, K.; Nomura, S.; Nakatsuka, S.; Kinoshita, K.; Ni, J.; Ono, Y.; Ikuta, T. Ultrapure Blue Thermally Activated Delayed Fluorescence Molecules: Efficient HOMO-LUMO Separation by the Multiple Resonance Effect. Adv. Mater. 2016, 28, 2777–2781. [Google Scholar] [CrossRef] [PubMed]
- Poitras, D.; Kuo, C.-C.; Py, C. Design of high-contrast OLEDs with microcavity effect. Opt. Express 2008, 16, 8003. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.-Y.; Lin, C.-L.; Wu, C.-C. Microcavity two-unit tandem organic light-emitting devices having a high efficiency. Appl. Phys. Lett. 2006, 88, 111106. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.; Chu, B.; Su, Z.; Bi, D.; Zhang, D.; Zhu, J.; Yan, F.; Chen, Y.; Tsuboi, T. Highly efficient green organic light-emitting diodes from single exciplex emission. Appl. Phys. Lett. 2008, 92, 53304. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.-K.; Zheng, C.-J.; Ye, J.; Liu, C.-L.; Li, F.; Ou, X.-M.; Lee, C.-S.; Zhang, X.-H. High Performance Exciplex-Based Fluorescence–Phosphorescence White Organic Light-Emitting Device with Highly Simplified Structure. Chem. Mater. 2015, 27, 5206–5211. [Google Scholar] [CrossRef]
- Goushi, K.; Yoshida, K.; Sato, K.; Adachi, C. Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nat. Photonics 2012, 6, 253–258. [Google Scholar] [CrossRef]
- Park, Y.-S.; Kim, K.-H.; Kim, J.-J. Efficient triplet harvesting by fluorescent molecules through exciplexes for high efficiency organic light-emitting diodes. Appl. Phys. Lett. 2013, 102, 153306. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, C.; Li, K.F.; Tam, H.L.; Chan, K.L.; Cheah, K.W. Efficient Organic Light-Emitting Diode through Triplet Exciton Reharvesting by Employing Blended Electron Donor and Acceptor as the Emissive Layer. ACS Appl. Mater. Interfaces 2015, 7, 24983–24986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, B.; Chu, B.; Li, W.; Su, Z.; Wang, L.; Wang, J.; Jin, F.; Yan, X.; Gao, Y.; et al. Blue exciplex emission and its role as a host of phosphorescent emitter. Org. Electron. 2015, 24, 1–6. [Google Scholar] [CrossRef]
- Zhang, T.; Chu, B.; Li, W.; Su, Z.; Peng, Q.M.; Zhao, B.; Luo, Y.; Jin, F.; Yan, X.; Gao, Y.; et al. Efficient Triplet Application in Exciplex Delayed-Fluorescence OLEDs Using a Reverse Intersystem Crossing Mechanism Based on a ΔES−T of around Zero. ACS Appl. Mater. Interfaces 2014, 6, 11907–11914. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nomura, H.; Miyazaki, H.; Adachi, C. Highly efficient exciplex organic light-emitting diodes incorporating a heptazine derivative as an electron acceptor. Chem. Commun. 2014, 50, 6174–6176. [Google Scholar] [CrossRef] [PubMed]
- Goushi, K.; Adachi, C. Efficient organic light-emitting diodes through up-conversion from triplet to singlet excited states of exciplexes. Appl. Phys. Lett. 2012, 101, 23306. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Zhu, J.; Wu, P.; Shen, B.; Dou, D.; Wei, B. Manipulation of Thermally Activated Delayed Fluorescence of Blue Exciplex Emission: Fully Utilizing Exciton Energy for Highly Efficient Organic Light Emitting Diodes with Low Roll-Off. ACS Appl. Mater. Interfaces 2017, 9, 21346–21354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Cai, M.; Zhang, Y.; Zhang, D.; Duan, L. Sterically shielded blue thermally activated delayed fluorescence emitters with improved efficiency and stability. Mater. Horiz. 2016, 3, 145–151. [Google Scholar] [CrossRef]
- Kim, M.; Jeon, S.K.; Hwang, S.-H.; Lee, J.Y. Stable Blue Thermally Activated Delayed Fluorescent Organic Light-Emitting Diodes with Three Times Longer Lifetime than Phosphorescent Organic Light-Emitting Diodes. Adv. Mater. 2015, 27, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, B.; Huang, S.; Nomura, H.; Tanaka, H.; Adachi, C. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photonics 2014, 8, 326–332. [Google Scholar] [CrossRef]










| Emitter | ΔEST (eV) | Td (µs) | PE (Lm/W) | EQE (%) | CIE (x, y) | Reference |
|---|---|---|---|---|---|---|
| 1 | 0.54 | 90.0 | n/a | 2.9 | n/a | [66] |
| 2 | 0.45 | 140.0 | n/a | 5.6 | n/a | [66] |
| 3 | 0.32 | 270.0 | n/a | 9.9 | (0.15, 0.07) | [66] |
| 4 | 0.21 | 93.0 | n/a | 14.5 | (0.16, 0.16) | [67] |
| 5 | 0.31 | 50.0 | n/a | 2.0 | (0.12, 0.13) | [67] |
| 6 | 0.25 | 464.0 | 1.6 | n/a | (0.15, 0.12) | [72] |
| 7 | 0.17 | 814.0 | 0.49 | n/a | (0.19, 0.15) | [72] |
| 8 | 0.07 | n/a | n/a | 2.3 | (0.15, 0.11) | [79] |
| 9 | 0.05 | 1.23 | n/a | 11.7 | (0.18, 0.19) | [81] |
| 10 | 0.24 | 1.16 | n/a | 5.5 | (0.15, 0.08) | [81] |
| 11 | 0.42 | 70.0 | 3.2 | 8.5 | (0.16, 0.08) | [82] |
| 12 | 0.14 | 25.4 | 30.4 | 22.3 | (0.15, 0.20) | [83] |
| 13 | 0.04 | n/a | n/a | 4.7 | (0.15, 0.09) | [79] |
| 14 | 0.43 | n/a | n/a | 7.2 | n/a | [84] |
| 15 | 0.07 | 3.5 | n/a | 22 | n/a | [84] |
| 16 | 0.17 | 13.0 | n/a | 19.2 | (0.15,0.10) | [84] |
| 17 | 0.15 | 10.3 | n/a | 18.3 | (0,15, 0.10) | [84] |
| 18 | 0.26 | 95.0 | 7.6 | 6.3 | (0.16, 0.12) | [85] |
| 19 | 0.19 | 31.0 | 16.9 | 10.6 | (0.16, 0.20) | [85] |
| 20 | 0.11 | 17.0 | 23.6 | 15.3 | (0.17, 0.20) | [85] |
| 21 | 0.27 | 24.34 | n/a | 4.8 | n/a | [86] |
| 22 | 0.27 | 48.22 | n/a | 14.0 | (0.14, 0.12) | [86] |
| 23 | 0.403 | 0.01 | 0.8 | 1.6 | (0.16, 0.06) | [87] |
| 24 | 0.31 | 18 | 0.6 | 2.5 | (0.15, 0.05) | [88] |
| 25 | 0.22 | 11.2 | 2.7 | 7.7 | (0.15, 0.07) | [88] |
| 26 | 0.36 | 13.5 | 3.5 | 10.3 | (0.16, 0.06) | [88] |
| 27 | 0.14 | 5.5 | 19.0 | 19.0 | (0.16, 0.23) | [88] |
| 28 | 0.013 | 93.7 | 8.3 | 13.5 | (0.13, 0.09) | [95] |
| 29 | 0.041 | 65.3 | 15.1 | 20.2 | (0.12, 0.13) | [95] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, T.-T.; Goubard, F.; Ibrahim-Ouali, M.; Gigmes, D.; Dumur, F. Thermally Activated Delayed Fluorescence Emitters for Deep Blue Organic Light Emitting Diodes: A Review of Recent Advances. Appl. Sci. 2018, 8, 494. https://doi.org/10.3390/app8040494
Bui T-T, Goubard F, Ibrahim-Ouali M, Gigmes D, Dumur F. Thermally Activated Delayed Fluorescence Emitters for Deep Blue Organic Light Emitting Diodes: A Review of Recent Advances. Applied Sciences. 2018; 8(4):494. https://doi.org/10.3390/app8040494
Chicago/Turabian StyleBui, Thanh-Tuân, Fabrice Goubard, Malika Ibrahim-Ouali, Didier Gigmes, and Frédéric Dumur. 2018. "Thermally Activated Delayed Fluorescence Emitters for Deep Blue Organic Light Emitting Diodes: A Review of Recent Advances" Applied Sciences 8, no. 4: 494. https://doi.org/10.3390/app8040494