1. Introduction
The oligomerization of ethylene is an important industrial reaction, and megatons of α-olefins are produced in this way every year. Depending on the chain length of the alkene, these materials are used for manufacturing various products, ranging from plastics to lubricants and linear low-density polyethylene (LLDPE). Among the industrial processes used for ethylene oligomerization using homogeneous catalysis, many processes were developed by companies such as Shell (Shell High Olefins Process (SHOP)) [
1] and the IFP—Institut Français du Pétrole (Dimersol and Alfabutol) [
2,
3]. These systems allow the production of products with high activities and selectivities; however, the separation of the products from the reaction medium makes catalyst recycling a challenge. The use of heterogeneous catalytic systems may be an interesting way to address the issue of separating the catalyst from the reaction products, increase the catalysts’ resistance and facilitate its reuse [
4].
Currently, a wide variety of molecular sieves are available, including zeolites, and these materials have become important as catalysts and adsorbents [
5,
6,
7]. Molecular sieves are most commonly used in processes that employ relatively small molecules due to the sizes of their pores, which are approximately 8 Å [
8]. The β-zeolite described by Mobil Oil Corporation is an example of a zeolite structure that is applicable in such a situation. The zeolite is a β-zeolite, which has a three-dimensional system of channels and micropores (diameters of 7.5 Å) that are circumscribed by rings of 12 tetrahedra that can be directly synthesized with a considerably high Si/Al ratio. Its high acidity, thermal stability, and hydrothermal stability and the ease by which relatively large molecules can diffuse through the channels make this a very interesting material from the perspective of developing new catalytic zeolites.
Other supports used with the same propose are the mesoporous MCM-41 (Mobil Composition of Matter 41) solids. These supports represent a new platform because their morphological characteristics and pore diameters of between 2 and 50 nm allow the introduction of compounds with greater steric hindrance into the cavities. The solids [Si]-MCM-41 and [Si,Al]-MCM-41 [
9] have high thermal stability and a hexagonal arrangement of pores with linear and parallel channels and are constructed with a beehive-type silica matrix. Use of MCM-41 as support of several metal complexes that are catalysts of ethylene, propylene or 1-hexene oligomerization reactions was described in the literature [
6,
10,
11,
12,
13,
14]. In all cases, the reported oligomer selectivity was affected by the presence of MCM-41.
We have used ionic liquids as solvents in several types of catalytic reactions (biphasic catalysis) [
15,
16]. Recently, a new type of tether was described in the literature in which the complex is anchored to the ionic liquid that is supported on mesoporous silica; this technique has been employed in hydroformylation [
17,
18,
19], carbonylation [
20], hydrogenation [
21], Heck [
22] and epoxidation [
23] reactions.
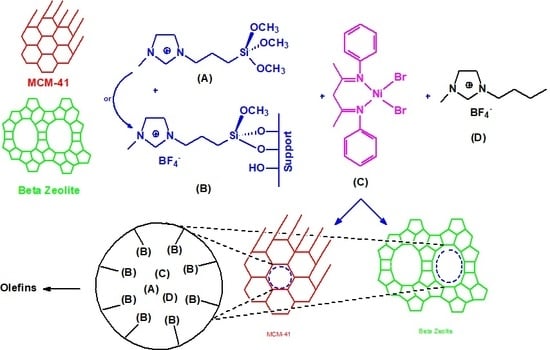
In this work, a nickel-β-diimine complex was immobilized on β-zeolite, [Si]-MCM-41 and [Si,Al]-MCM-41 modified with the tetrafluoroborate of 1-(3-trimetoxisililpropil)-3-methylimidazolium ionic liquid in the presence of the tetrafluoroborate of 1-butyl-3-methylimidazolium (BMI.BF4). The catalyst obtained was used in ethylene oligomerization reactions.
2. Materials and Methods
All syntheses were performed under an argon atmosphere using standard Schlenk techniques. Ethylene (White Martins 99.99%) was used for the catalytic tests. The cocatalyst ethylaluminum sesquichloride (EASC) was used after being diluted in toluene (10% v/v). Characterization of the compounds was done using gas chromatography (GC), nuclear magnetic resonance (NMR), elemental analysis, N2 adsorption, infrared (IR) spectroscopy, X-ray diffraction (XRD), and atomic absorption spectroscopy (AAS), as detailed in the following. GC was performed in a Varian Star 3400 CX chromatograph with a flame ionization detector, equipped with a Petrocol DH capillary column (methyl silicone, 100 m in length, 0.25 mm ID, 0.5 μm film thickness). The temperature was initially maintained at 36 °C for 15 min, followed by heating to 250 °C at a rate of 5 °C/min. 1H- and 13C-NMR spectroscopy employed a Varian 300 NMR spectrometer, operating at 300 MHz for 1H-NMR and 75 MHz for 13C-NMR, using CDCl3 as solvent. Elemental analysis used a Perkin Elmer M CHN/O Model 2400 system. N2 adsorption utilized a Quantachrome Nova 2200e model system in conjunction with the BET method to determine the specific surface area of samples previously treated at 80 °C by 3 h. Vibrational spectroscopy used a Shimadzu infrared spectrometer. XRD was performed using a diffractometer from Siemens (model D500) with CuKα radiation (λ = 1.54 Å). For AAS, done in a spectrometer from Perkin Elmer (A. Analyst 200), 20 mg of samples were prepared with 2 mL of HCl, 6 mL of HNO3, 5 mL of HF, adding the mixture to Teflon autoclaves, subsequently using a digester for 10 h at 150 °C. After cooling, the samples were diluted to 50 mL.
2.1. Ligand 2-(phenyl)amino-4-(phenyl)imino-2-pentene
An argon-filled glass flask was charged with 30 mL of toluene, 10 mL of acetylacetone (100 mmol) and 18 mL of aniline (200 mmol). This mixture was cooled in an ice bath and then 8.3 mL of concentrated hydrochloric acid was slowly added to the flask. After 24 h, a precipitate was obtained that was then isolated by filtration and washed with hexane. The solid was neutralized and extracted by adding 8 mL of dichloromethane, 50 mL of distilled water and 20 mL of saturated solution of sodium carbonate. Next, a pear-shaped separation flask was used to extract the organic phase. The solution was concentrated under reduced pressure, and the ligand was crystalized from methanol and left in the freezer. We obtained 9.13 g of the ligand (36.5 mmol, 36.5% yield) as a yellow solid. The ligand was characterized by 1H-NMR spectroscopy (CDCl3, 300 MHz, Room temperature, δ in ppm): 12.7 (s, 1H, H9), 7.3–6.9 (m, 10H, H4, H5, H6, H7, H8), 4.9 (s, 1H, H2), 2 (6H, H1, H3). Anal. Calc for C17H18N2: C, 81.56; H, 7.25; N, 11.19. Found: C, 81.24; H, 7.41; N, 11.35.
2.2. Synthesis of the Bis-(acetonitrile)dibromidonickel(II) Adduct [24]
To a 250 mL Schlenk tube, 120 mL of acetonitrile and 2.26 g of NiBr2 (anhydrous) were added. This suspension remained under reflux at 80 °C until a dark blue color was obtained. The final solution was concentrated to approximately 40 mL under reduced pressure, and the resulting solid was filtered through a fritted filter Schlenk tube, washed with 30 mL of acetonitrile and then dried under flowing argon.
2.3. Synthesis of the 1,5-Bisphenyl-2,4-pentanediimine-dibromidonickel(II) Complex
The complex was synthesized as described in the literature [
25]. To a 250 mL Schlenk tube, the diimine ligand (0.77 g, 3.08 mmol) (which was synthesized as described in
Section 3.1) and 40 mL of dichloromethane were added. To this solution, the
bis-(acetonitrile)dibromidonickel(II) adduct (0.914 g, 3.04 mmol) was added. The reaction mixture was magnetically stirred for 4 days at room temperature. After the reaction was complete, the solution was concentrated to approximately 15 mL and filtered through a fritted filter Schlenk tube before the solvent was evaporated under vacuum to obtain the resulting solid nickel complex (light purple). We isolated 0.48 g of the complex (33% yield). Anal. Calc for C
19H
24Br
2N
2Ni: C, 45.74; H, 4.85; N, 5.61 Found: C, 44.76; H, 4.12; N, 6.01.
2.4. Synthesis of 1-(3-Trimethoxysilylpropyl)-3-methylimidazolium Chloride
The procedure used for the preparation of the ionic liquid was similar to the methods described in the literature [
26]. 1-Methylimidazole (3.36 g, 40 mmol) and 7.92 g of 3-chloropropyltrimethoxysilane (40 mmol) were added to a Schlenk tube under argon. The reaction mixture was stirred for 24 h at 95 °C. A viscous oil formed after heating. This oil was used immediately in the next step. The assignments of the
1H-NMR and
13C-NMR signals of 1-(3-trimetoxisililpropil)-3-methylimidazoliumchloride are as follows:
1H-NMR (CDCl
3): δ (ppm) = 0.65 (t, 2H), 2.0 (m, 2H), 3.6 (s, 9H), 4.1 (s, 3H), 4.36 (t, 2H), 7.54 (s, 1H), 7.83 (s, 1H), 10.5 (s, 1H);
13C-NMR (CDCl
3): δ (ppm) = 5.5 (SiCH
2), 24.0 (CH
2), 36.4 (CH
3N), 51.2 (OCH
3), 76.9 (CH
2N), 121.7, 123.4, 137.2.
2.5. Synthesis of 1-(3-Trimethoxysilylpropyl)-3-methylimidazoliumhexafluoro-phosphate
To a solution of the above compound (3.2 g, 10 mmol) in 15 mL of acetone, sodium tetrafluoroborate (1.15 g, 10.5 mmol) was added. This mixture was stirred at room temperature for 3 days. The solid was then removed by filtration, and the solvent was evaporated. The assignments of the 1H-NMR and 13C-NMR signals of the tetrafluoroborate 1-(3-trimetoxisililpropil)-3-methylimidazolium are as follows: 1H-NMR (CDCl3), δ (ppm) = 0.65 (t, 2H), 2.0 (m, 2H), 3.6 (s, 9H), 3.9 (s, 3H), 4.2 (t, 2H), 7.34 (s, 1H), 7.39 (s, 1H), 8.90 (s, 1H); 13C-NMR (CDCl3), δ (ppm) = 6.1 (SiCH2), 24.3 (CH2), 36.7 (CH3N), 51.7 (CH3O), 52.1 (CH2N), 122.0, 124.2, 137.1.
2.6. Synthesis of the Supports
β-Zeolite, with a ratio Si/Al = 20, was synthesized as describe in the literature [
27]. Before use, the β-zeolite was calcined at 600 °C for 6 h at a heating rate of 3 °C/min to remove the ionic liquid, which had been used as a structure-directing agent and was present in the cavities.
The mesoporous supports, [Si]-MCM-41 and [Si,Al]-MCM-41, were synthesized according to the route proposed by Corma [
28]. Finally, the obtained samples were calcined in air under static conditions at 540 °C for 6 h at a heating rate of 0.5 °C/min, with 60 min holds at 150 and 350 °C.
2.7. Synthesis of Silylated Ionic Liquid-Modified Supports
Ionic liquid (6.5 mmol) (synthesized as described in
Section 2.4) was dissolved in chloroform (50 mL) and mixed with 1 g of support (previously dried under vacuum and heated to 180 °C overnight). The mixture was heated to 65 °C under reflux and stirred for 26 h. The mixture was cooled to room temperature and filtered, and the solid was washed with chloroform (50 mL) and diethyl ether (50 mL). Afterwards, the solid was dried under reduced pressure.
The synthesized materials were named IL-Zeoβ, IL-MCM-41 and IL-Al-MCM-41. The incorporation of the silylated ionic liquid onto the materials is outlined in
Figure 1.
2.8. Immobilization of the Nickel-β-Diimine Complex onto the Modified Supports
To a Schlenk tube, the nickel-β-diimine complex (0.30 mmol) dissolved in 10 mL of dichloromethane, 300 mg of ionic liquid BMI.BF4 and 1 g of modified support (synthesized in the previous step) was added. The mixture was stirred for 1 h at room temperature and then the solvent was evaporated. The synthesized materials are named NiIL-Zeoβ, NiIL-MCM-41 and NiIL-Al-MCM-41.
2.9. Catalytic Tests
Ethylene oligomerization reactions were performed in triplicate, under both homogeneous and heterogeneous conditions, and the results were compared. The catalytic reactions were performed in a 200 mL double-walled glass reactor, equipped with a magnetic stirrer, a thermocouple, and a continuous feed of ethylene. The reaction temperature was controlled by a thermostatic circulation bath. All reactions employed 30 mL of toluene as the solvent and a solution of EASC as the cocatalyst. The amounts of catalyst and cocatalyst, as well as temperature and pressure, were optimized. After each reaction, the reactor was cooled with a mixture of ethanol and liquid N
2 (to reach temperatures up to −40 °C). After cooling, the reaction mixture was poured into a vial for later analysis, and eventual quantification by gas chromatography using isooctane as an internal standard. The catalytic tests evaluated activity (expressed by turnover frequency (TOF), s
−1) and selectivity (% of butenes and % of 1-butene in fraction C
4) as shown in
Table 1.
Catalyst-recycling tests were performed with NiIL-Zeoβ. After the first reaction, the products were removed from the reactor through a cannula and collected in a cooled Schlenk tube. Through this technique, we could isolate the NiIL-Zeoβ catalyst from the reactor while maintaining the argon atmosphere. Recycling was accomplished by adding another 30 mL of toluene and re-adding the same volume of EASC cocatalyst as in the first cycle.
3. Results and Discussion
First, we present the results of the characterization of the materials; next, we discuss the results of the catalytic oligomerization tests in detail.
3.1. Materials Characterization
In the infrared spectra of the supported complexes (
Figure 2), there are specific bands at approximately 1098, 794 and 448 cm
−1 that can be attributed to the vibrations of the mesoporous structure (Si–O), a band at 547 cm
−1 that can be assigned to the characteristic absorption of β-zeolite and mesoporous materials, and a broad band at approximately 3409 cm
−1 that can be assigned to silanol groups (SiO–H). The bands at 3154 and 3098 cm
−1 are assigned to the CH vibrations of the imidazole aromatic ring and the CH stretching of the alkyl groups belonging to the silylating agent. The bands at 2960 and 2870 cm
−1 in the spectra of the supported complexes are assigned to symmetric and asymmetric stretches of the CH bonds present in the complexes, suggesting that the complexes were immobilized on the support.
Figure 3 shows the diffractograms that contain peaks characteristic of supports modified with an ionic liquid (IL-Zeoβ, IL-MCMC-41 and IL-Al-MCM-41) as well as β-zeolite, MCM-41 and Al-MCMC-41 (upper right insets). Based on a comparison of the sets of spectra, we conclude that the network organization did not change, which confirms that the structure of these supports did not changed even after the immobilization of the silylated ionic liquid in their cavities. The spectra in
Figure 3 are consistent with the XRD patterns reported in the literature [
19].
Evaluation of specific regions of the spectra can be used to confirm the results obtained by XRD wherein the characteristics of the materials were maintained, even after incorporation of the ionic liquid. Analysis of these data showed significant reductions in the specific surface areas of the IL-Zeoβ from 418 to 32 m2 g−1, the IL-MCM-41 from 1038 to 403 m2 g−1 and the IL-Al-MCM-41 from 860 to 465 m2 g−1 due to the incorporation of a substantial number of organic groups. These results suggest that the ionic liquid can be immobilized in the pores of the materials.
Based on the elemental analysis, one can estimate the amount of ionic liquid that was anchored to each support. We obtained 8.92% C, 1.39% H and 2.29% N on the IL-Zeoβ support; 7.28% C, 2.14% H and 2.31% N on the IL-MCM-41 support; and 10.57% C, 2.23% H and 2.76% N on the IL-Al-MCM-41 support. From these results, one can calculate the amount of ionic liquid incorporated into each medium; the relevant results were 22.0% (indicating that 41% of ionic liquid added was immobilized on the Zeoβ support), 22.2% (indicating that 47% of ionic liquid added was immobilized on IL-MCM-41) and 27.5% (indicating that 61% of ionic liquid added was immobilized on IL-Al-MCM-41).
The results of texture analysis from N
2 adsorption–desorption isotherms are shown in
Table 2.
The specific areas were calculated by the BET method, resulting in 1038 m2 g−1 for the MCM-41, 860 m2 g−1 for the Al-MCM-41 and 418 m2 g−1 for the β-zeolite, and 403, 465 and 32 m2 g−1 for IL-MCM-41, IL-Al-MCM-41 and IL-β-zeolite, respectively. As expected, a decrease in BET surface area is observed in the ionic liquid-modified materials.
To confirm the binding of the nickel complex to the β-zeolite, MCM-41 and Al-MCM-41, the heterogenized complexes were analyzed by flame atomic absorption. Analyses were done in triplicate, and the values are reported in
Table 3. The amount of nickel incorporated per gram of support is 75 ± 5% of added nickel, indicating the total immobilization of the complex. The NiIL-Zeoβ complex was also analyzed after an oligomerization reaction, and the results indicate that more than 50% of the immobilized complex in β-zeolite was lost during this reaction.
3.2. Catalytic Tests Results
3.2.1. Homogeneous Catalytic Reactions
Homogeneous reactions were conducted to assess the selectivity and activity of the nickel-β-diimine complex so that those parameters could be compared with those of the catalytic tests performed with the heterogenized catalyst probably due to decomposition.
Table 4 presents the results of the catalytic tests. The ratio of Al/Ni was varied between 25 and 100 (entries 1–4). The highest activity was achieved at an Al/Ni ratio of 50, and the highest selectivity in butenes at an Al/Ni ratio of 25. The increase in activity with the Al/Ni ratio may be explained by an increase in the number of active species in the reaction medium. The maximum reactivity occurs before a decrease in activity, indicating catalyst deactivation, probably due to decomposition.
When the amount of catalyst was changed from 5 to 10 μmol (entries 2 and 6), the activity and selectivity of the system decreased. This may be interpreted as resulting from an increase in the number of moles of catalyst precursor, which promotes an increase in the amount of active species in the reaction. Thus, the relative amount of ethylene reacting with the catalytic species is reduced, resulting in lower activity and selectivity in butenes. The reduction was mainly observed in 1-butene selectivity, due to parallel isomerization reaction. C
6 production is not significantly affected, and C
8 products slightly increase. This is consistent with the results reported by Busico et al. [
29].
By increasing the initial reaction temperature from 10 to 23 °C (entries 4 and 5), the activity of the system also increases. Dubois et al. and Souza et al. [
30,
31] studied the influences of reaction temperature on oligomerization when using nickel complexes and found that the 1-butene best selectivity is achieved at low temperatures. Therefore, the subsequent reactions were performed at 10 °C.
The last parameter to be studied was the pressure (entries 2 and 7). In the determination of the effects of pressure on the activity and selectivity in ethylene oligomerization, it can be observed that there are no meaningful changes in the selectivity of the system, likely because the system is close to ethylene saturation.
Comparing these results with the industrial Shell High Olefins Process (SHOP), our oligomerization process is more selective to light olefins (C4–C8), while SHOP results in a larger range (C4–C40).
3.2.2. Catalytic Tests in Heterogeneous Reactions
The catalytic properties of the β-zeolite-supported nickel-β-diimine complex were tested under different reaction conditions, as shown in
Table 5. These experiments were performed to find the conditions under which the reaction showed the best activity and selectivity. We used the same amount of heterogenized catalyst precursor as was determined in the optimization of the homogeneous catalysis (i.e., 5 μmol).
The first parameter studied was the Al/Ni molar ratio. Entries 8, 9 and 10 present the results of catalytic tests with different molar ratios of aluminum from the cocatalyst and nickel. These results show that as we increase the Al/Ni molar ratio, the activity of the catalyst also increases due to the increase in the concentration of active species present in the reaction mixture.
After assessing the effect of the Al/Ni molar ratio, we set out to determine the optimal temperature for the reaction to obtain the best activity and selectivity in the production of butenes. As shown in entry 10, when the reaction was conducted at 10 °C, the selectivity was approximately 74% for 1-butene. Based on entry 11, when the reaction was performed at 23 °C, there was a decrease in the selectivity for 1-butene (approximately 43%) compared to entry 10. Using the conditions described above, this system shows higher selectivity than the homogeneous system (entries 4 and 5,
Table 4), so the other reactions were performed at 10 °C.
The following studies were performed with pressures between 2.5, 5 and 10 atm to determine the effect of pressure on the activity of the oligomerization of ethylene. It can be observed from entries 9, 12 and 13 that the role of pressure in the system is very important. When comparing the results of reactions run at 2.5 and 5 atm, a slight difference in activity values can be observed, which can be explained by the difficulty of ethylene insertion into the cavities of zeolite due to the presence of the ionic liquid used as catalyst support. On the other hand, increasing the pressure from 5 to 10 atm increases the solubility of ethylene and, as a result, the ethylene enters the cavities, making it closer to the active species, which may explain the increased activity.
When comparing the homogeneous (entry 7,
Table 4) and heterogeneous (entry 13,
Table 5) reactions at 10 atm, it was observed that the heterogeneous reaction was more selective than the homogeneous reaction for 1-butene, which shows that the catalyst lies within the cavities of the β-zeolite and that the increase in selectivity is due to the confinement effect of the catalyst. However, based on the selectivities observed in hexenes and octenes, the reaction may also be occurring with the homogeneous catalyst, as demonstrated by atomic absorption analysis after the reaction of the nickel complex immobilized on β-zeolite. Our results show higher activity in heterogeneous media than is reported in the literature [
7].
The next step was to test the catalytic properties of the NiIL-MCM-41 (entries 17, 18 and 19,
Table 6) and NiIL-Al-MCM-41 (entry 20) catalysts under the best conditions obtained for the β-zeolite-supported β-diimine nickel complex: temperature (10 °C), moles of catalyst (5 μmol), pressure (10 atm) and reaction time (30 min). As shown in
Table 5, these catalysts are less active than the β-zeolite-supported system; however, these catalysts are 100% selective for butenes. When the Al/Ni ratio was increased from 25 to 100, it was found that, at an Al/Ni ratio of 50, the selectivity of the system did not vary significantly with respect to the activity; the catalytic system was more active, which indicates an increase in the active species present in the reaction medium.
In our analysis of the results of the catalytic test with NiIL-Al-MCM-41, we observed that the system was highly active (approximately 2.3 s−1) and reached 90% selectivity for 1-butene, which makes its activity superior to that of the NiIL-MCM-41 system.
Comparing the selectivities of the three heterogeneous and homogeneous systems, we observed that the effect of the confinement of the catalyst inside the MCM-41 pores was a determining factor in the high selectivity of the heterogeneous system, in this case. However, when comparing the supports used, we observed that the difference in the pore size of these materials was not a determining factor for the activity of the reactions, but rather the presence of acid sites in the structure of the β-zeolite and Al-MCM-41 was a determining factor. Hulea et al. [
32] reported that the content of both nickel and acid sites contribute to the activation of the oligomerization reaction.
4. Conclusions
In this work, we described synthesis and caractherization of nickel-β-diimine complexes immobilized on β-zeolite, [Si]-MCM-41 and [Si,Al]-MCM-41 modified with an ionic liquid. These catalytic systems were used in ethylene oligomerization reactions and compared with the same reactions in homogeneous media. Catalytic properties of synthesized materials were tested under different conditions, varying Al/Ni molar ratio, temperature, pressure and catalyst loading.
The results obtained in oligomerization reactions demonstrate the efficiency of heterogenized catalystic system. The techniques of characterization of the different complexes supported on β-zeolite or mesoporous materials of the MCM type corroborate that immobilization of the complex did not alter the structure of the supports of Si and Al.
Comparison between the heterogeneous systems indicates that differences in pore sizes of the support materials did not influence the activity, but the presence and strength of acid sites, occurring in β-zeolite and Al-MCM-41, affects reaction activity. We suggest that the effect of acid sites of the support on the metal site is to increase the strength of ethylene coordination to the metal, causing the system to be more active.
Comparison between homogeneous and heterogeneous systems containing the β-diimine-nickel complex showed that heterogenized systems are more selective in the formation of 1-butene. This higher selectivity indicates a positive relationship between porosity and confinement of the catalyst within channels of the support materials.