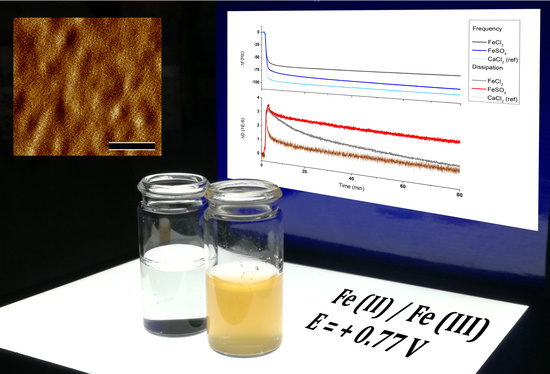
Electrochemical-QCMD Control over S-Layer (SbpA) Recrystallization with Fe2+ as Specific Ion for Self-Assembly Induction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of wtSbpA S-Layer Protein Solutions
2.2. Secondary Cell Wall Polymer (SCWP) Preparation
2.3. Recrystallization of S-Layer Proteins on Silicon wafers
2.4. Atomic Force Microscopy
2.5. Quartz Crystal Microbalance with Dissipation (QCM-D) Monitoring
2.6. Measurement of the Contact Angle
3. Results and Discussion
3.1. Sbpa Recrystallization in Fe (II): Chemical Control
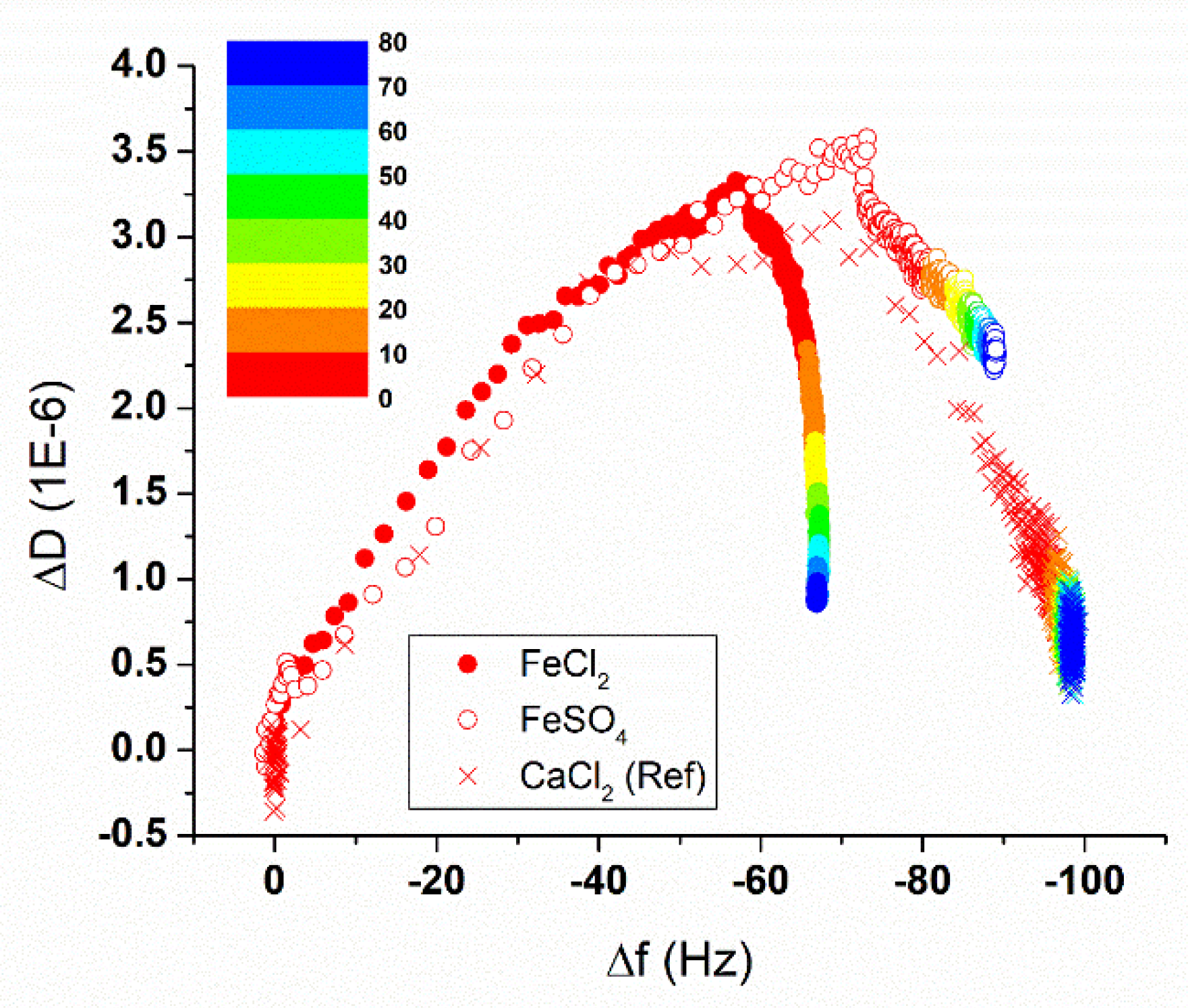
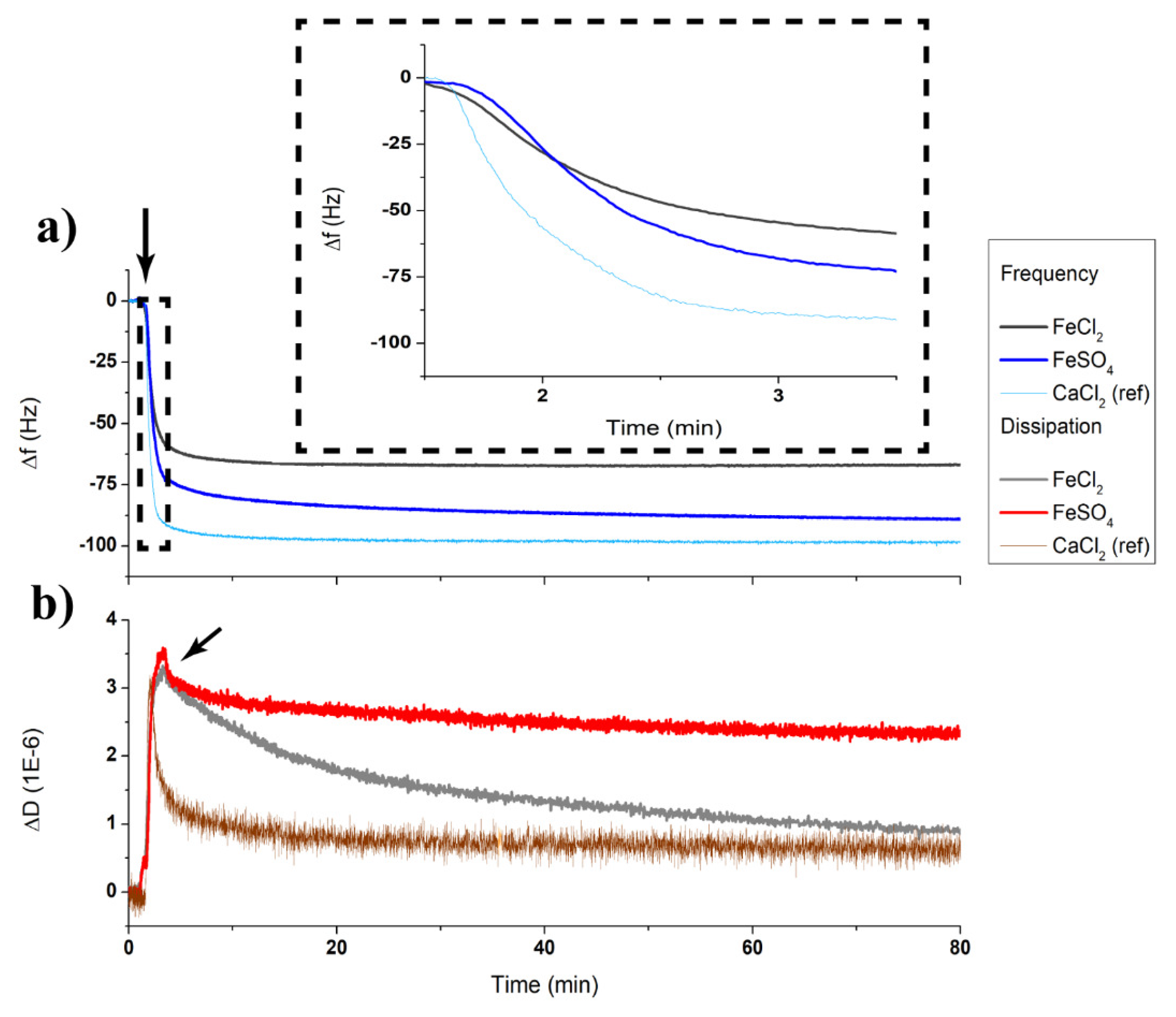
3.2. Sbpa Recrystallization in Fe (II): Electrical Control and Real Time Monitoring
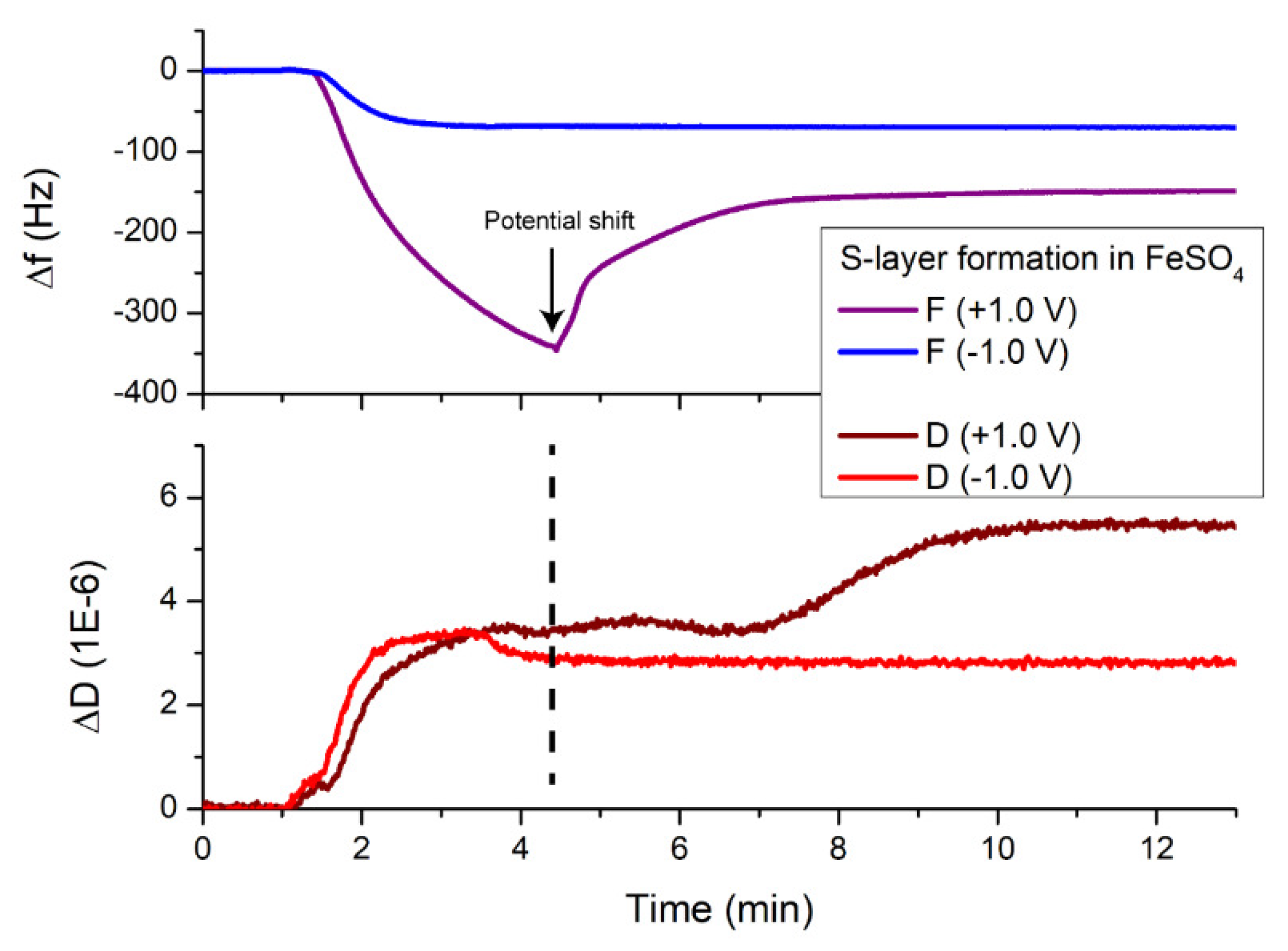
3.3. Electrical Stability of the Fe²+-Containing S-Layer
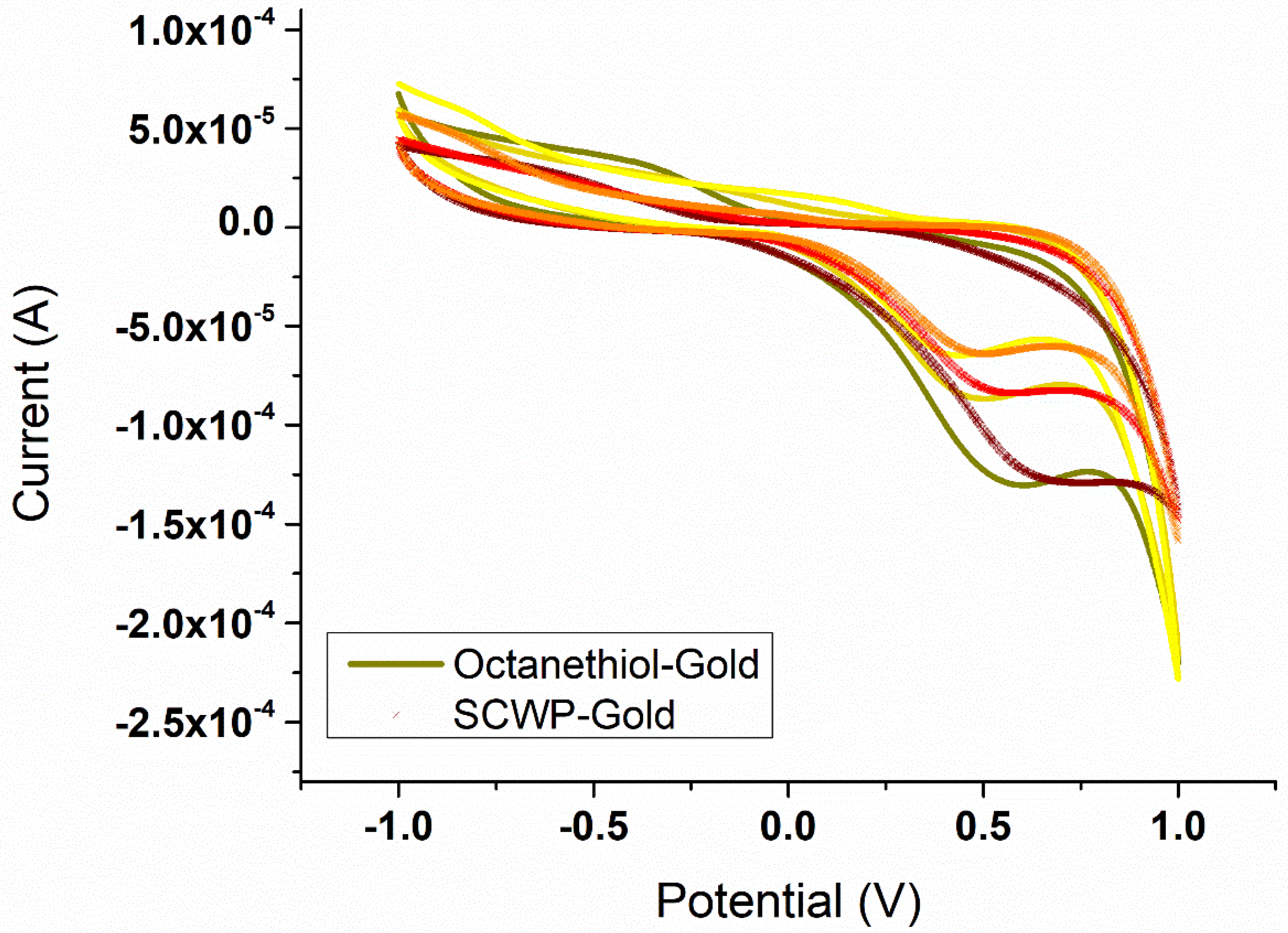
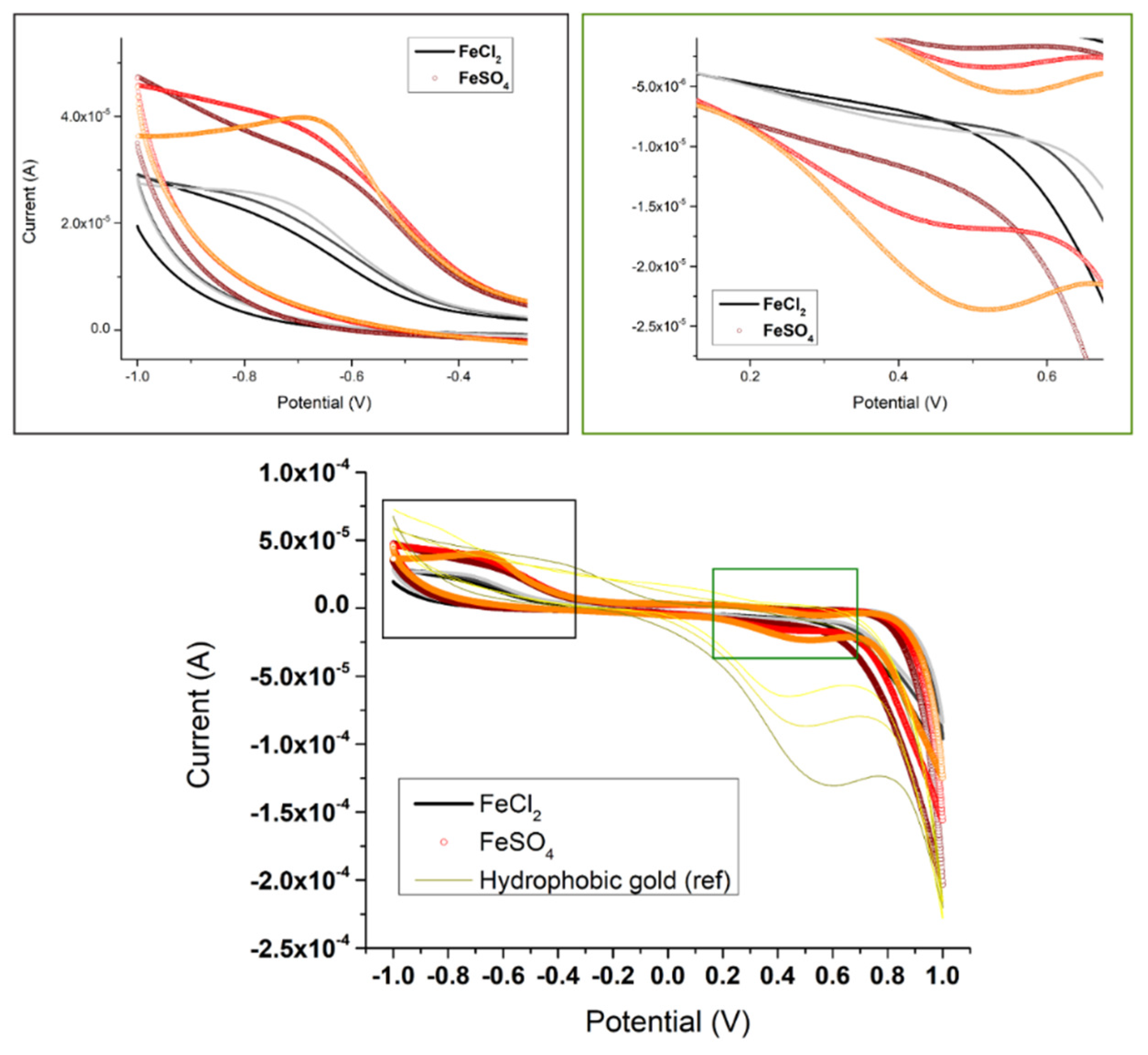
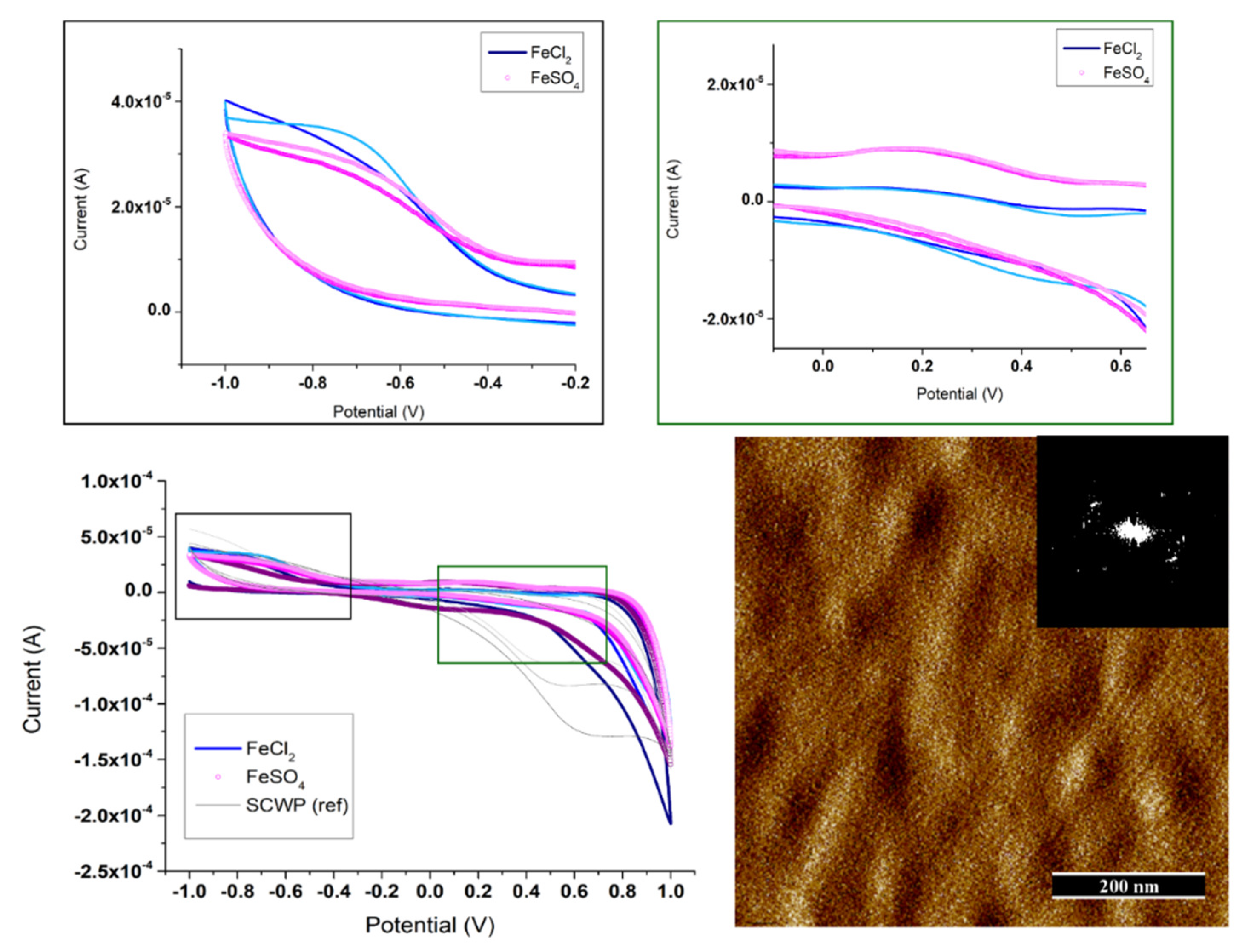
3.4. Determination of the S-Layer Ion Permeability by Cyclic Voltammetry
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A



References
- Sleytr, U.B.; Schuster, B.; Egelseer, E.M.; Pum, D. S-layers: Principles and applications. FEMS Microbiol. Rev. 2014, 38, 823–864. [Google Scholar] [CrossRef] [PubMed]
- Pavkov-Keller, T.; Howorka, S.; Keller, W. Chapter 3 – the structure of Bacterial S-layer proteins. Prog. Mol. Biol. Transl. Sci. 2011, 103, 73–130. [Google Scholar] [CrossRef] [PubMed]
- Albers, S.-V.; Meyer, B.H. The archaeal cell envelope. Nat. Rev. Microbiol. 2011, 9, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.M.; Strickland, A.; Mark, S.S.; Bergkvist, M.; Sierra-Sastre, Y.; Batt, C.A. Entropically driven self-assembly of lysinibacillus sphaericus S-layer proteins analysed under various environmental conditions. Macromol. Biosci. 2010, 10, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Eleta-Lopez, A.; Moreno-Flores, S.; Pum, D.; Sleytr, U.B.; Toca-Herrera, J.L. Surface dependence of protein nanocrystal formation. Small 2010, 6, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Iturri, J.; Moreno-Cencerrado, A.; Toca-Herrera, J.L. Polyelectrolyte brushes as supportive substrate for bacterial s-layer recrystallization: Polymer charge and chain extension factors. Colloids Surf. A 2017, 526, 56–63. [Google Scholar] [CrossRef]
- Breitwieser, A.; Iturri, J.; Toca-Herrera, J.L.; Sleytr, U.B.; Pum, D. In vitro characterization of the two-stage non-classical reassembly pathway of s-layers. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Baranova, E.; Fronzes, R.; Garcia-Pino, A.; Van Gerven, N.; Papapostolou, D.; Péhau-Arnaudet, G.; Pardon, E.; Steyaert, J.; Howorka, S.; Remaut, H. SbsB structure and lattice reconstruction unveil Ca2+ triggered S-layer assembly. Nature 2012, 487, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Rad, B.; Haxton, T.K.; Shon, A.; Shin, S.-H.; Whitelam, S.; Ajo-Franklin, C.M. Ion-Specific control of the self-assembly dynamics of a nanostructured protein lattice. ACS Nano 2015, 9, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Falke, S.; Drobot, B.; Oberthuer, D.; Kikhney, A.; Guenther, T.; Fahmy, K.; Svergun, D.; Betzel, C.; Raff, J. Analysis of self-assembly of s-layer protein slp-B53 from lysinibacillus sphaericus. Eur. Biophys. J. 2017, 46, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Nomellini, J.F.; Kupcu, S.; Sleytr, U.B.; Smit, J. Factors controlling in vitro recrystallization of the caulobacter crescentus paracrystalline S-layer. J. Bacteriol. 1997, 179, 6349–6354. [Google Scholar] [CrossRef] [PubMed]
- Kish, A.; Miot, J.; Lombard, C.; Guigner, J.-M.; Bernard, S.; Zirah, S.; Guyot, F. Preservation of archaeal surface layer structure during mineralization. Sci. Rep. 2016, 6, 26152. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, L.; Dussan, J. Biosorption and bioaccumulation of heavy metals on dead and living biomass of bacillus sphaericus. J. Hazard. Mater. 2009, 167, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Schneewind, O.; Missiakas, D.M. Protein secretion and surface display in gram-positive bacteria. Philos. Trans. R. Soc. B 2012, 367, 1123–1139. [Google Scholar] [CrossRef] [PubMed]
- Tarlovsky, Y.; Fabian, M.; Solomaha, E.; Honsa, E.; Olson, J.S.; Maresso, A.W. A bacillus anthracis S-layer homology protein that binds heme and mediates heme delivery to isdc. J. Bacteriol. 2010, 192, 3503–3511. [Google Scholar] [CrossRef] [PubMed]
- Ilbert, M.; Bonnefoy, V. Insight into the evolution of the iron oxidation pathways. Biochim. Biophys. Acta Biophys. 2013, 1827, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Ilk, N.; Kosma, P.; Puchberger, M.; Egelseer, E.M.; Mayer, H.F.; Sleytr, U.B.; Sára, M. Structural and functional analyses of the secondary cell wall polymer of bacillus sphaericus CCM 2177 that serves as an S-layer-specific anchor. J. Bacteriol. 1999, 181, 7643–7646. [Google Scholar] [PubMed]
- Norville, J.E.; Kelly, D.F.; knight, T.F., Jr.; Belcher, A.M.; Walz, T. 7 Å projection map of the S-layer protein sbpA obtained with trehalose-embedded monolayer crystals. J. Struct. Biol. 2007, 160, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Comolli, L.R.; Siegerist, C.E.; Shin, S.-H.; Bertozzi, C.; Regan, W.; Zettl, A.; De Yoreo, J. Conformational transitions at an s-layer growing boundary resolved by Cryo-TEM. Angew. Chem. 2013, 125, 4929–4932. [Google Scholar] [CrossRef]
- Chung, S.W.; Shin, S.-H.; Bertozzi, C.R.; De Yoreo, J.J. Self-catalyzed growth of S layers via an amorphous-to-crystalline transition limited by folding kinetics. Proc. Natl. Acad. Sci. USA 2010, 107, 16536–16541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.-H.; Chung, S.M.; Sanii, B.; Comolli, L.R.; Bertozzi, C.R.; De Yoreo, J.J. Direct observation of kinetic traps associated with structural transformations leading to multiple pathways of S-layer assembly. Proc. Natl. Acad. Sci. USA 2012, 109, 12968–12973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iturri, J.; Vianna, A.C.; Moreno-Cencerrado, A.; Pum, D.; Sleytr, U.B.; Toca-Herrera, J.L. Impact of surface wettability on S-layer recrystallization: A real-time characterization by QCM-D. Beilstein J. Nanotechnol. 2017, 8, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Handrea, M.; Sahre, M.; Neubauer, A.; Sleytr, U.B.; Kautek, W. Electrochemistry of nano-scale bacterial surface protein layers on gold. Bioelectrochemistry 2003, 61, 1–8. [Google Scholar] [CrossRef]
- Bhalla, N.; Lee, D.; Sathish, S.; Shen, A.Q. Dual-mode refractive index and charge sensing to investigate complex surface chemistry on nanostructures. Nanoscale 2017, 9, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Ono, T.; Kanai, Y.; Ikuta, T.; Shimatani, M.; Ogawa, S.; Maehashi, K.; Inoue, K.; Matsumoto, K. Graphene surface acoustic wave sensor for simultaneous detection of charge and mass. ACS Sens. 2018, 3, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.-J.; Jaschke, M. Calculation of thermal noise in atomic force microscopy. Nanotechnology 1995, 6, 1. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Tadolini, B. Iron autoxidation in mops and hepes buffers. Free Radic. Res.Commun. 1987, 4, 149–160. [Google Scholar] [CrossRef]
- Hofmeister, F. Zur lehre von der wirkung der salze. Arch. Exp. Pathol. Pharmakol. 1888, 24, 247–260. [Google Scholar] [CrossRef]
- Okur, H.I.; Hladilkova, J.; Rembert, K.B.; Cho, Y.; Heyda, J.; Dzubiella, J.; Cremer, P.S.; Jungwirth, P. Beyond the hofmeister series: Ion-specific effects on proteins and their biological functions. J. Phys. Chem. B 2017, 121, 1997–2014. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.L.; Lorenzo, A.; Marmisolle, W.A.; von Bilderling, C.; Maza, E.; Pietrasanta, L.; Battaglini, F.; Ceolin, M.; Azzaroni, O. Highly-organized stacked multilayers via layer-by-layer assembly of lipid-like surfactants and polyelectrolytes. Stratified supramolecular structures for (bio)electrochemical nanoarchitectonics. Soft Matter 2018, 14, 1939–1952. [Google Scholar] [CrossRef] [PubMed]
- Fenoy, G.E.; Giussi, J.M.; von Bilderling, C.; Maza, E.M.; Pietrasanta, L.I.; Knoll, W.; Marmisolle, W.A.; Azzaroni, O. Reversible modulation of the redox activity in conducting polymer nanofilms induced by hydrophobic collapse of a surface-grafted polyelectrolyte. J. Colloid Interface Sci. 2018, 518, 92–101. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iturri, J.; Breitwieser, A.; Pum, D.; Sleytr, U.B.; Toca-Herrera, J.L. Electrochemical-QCMD Control over S-Layer (SbpA) Recrystallization with Fe2+ as Specific Ion for Self-Assembly Induction. Appl. Sci. 2018, 8, 1460. https://doi.org/10.3390/app8091460
Iturri J, Breitwieser A, Pum D, Sleytr UB, Toca-Herrera JL. Electrochemical-QCMD Control over S-Layer (SbpA) Recrystallization with Fe2+ as Specific Ion for Self-Assembly Induction. Applied Sciences. 2018; 8(9):1460. https://doi.org/10.3390/app8091460
Chicago/Turabian StyleIturri, Jagoba, Andreas Breitwieser, Dietmar Pum, Uwe B. Sleytr, and José Luis Toca-Herrera. 2018. "Electrochemical-QCMD Control over S-Layer (SbpA) Recrystallization with Fe2+ as Specific Ion for Self-Assembly Induction" Applied Sciences 8, no. 9: 1460. https://doi.org/10.3390/app8091460