Effect of Heat Treatment on Water Absorption of Chinese fir Using TD-NMR
Abstract
:1. Introduction
2. Materials and Methods
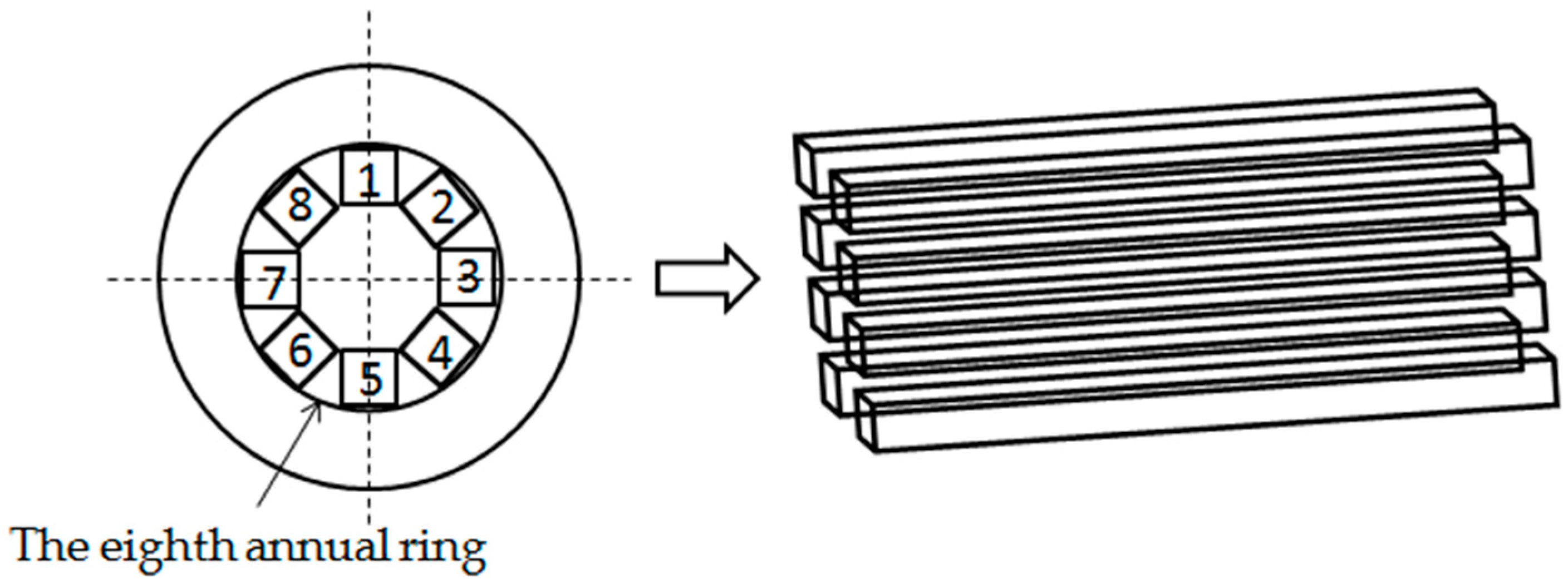
2.1. Sample Preparation and Heat Treatment Process
2.2. TD-NMR Experiments
3. Results and Discussion
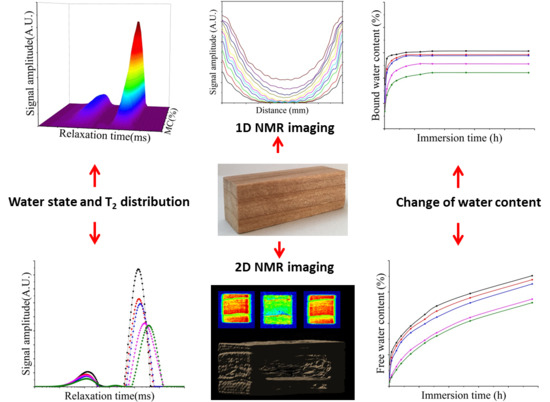
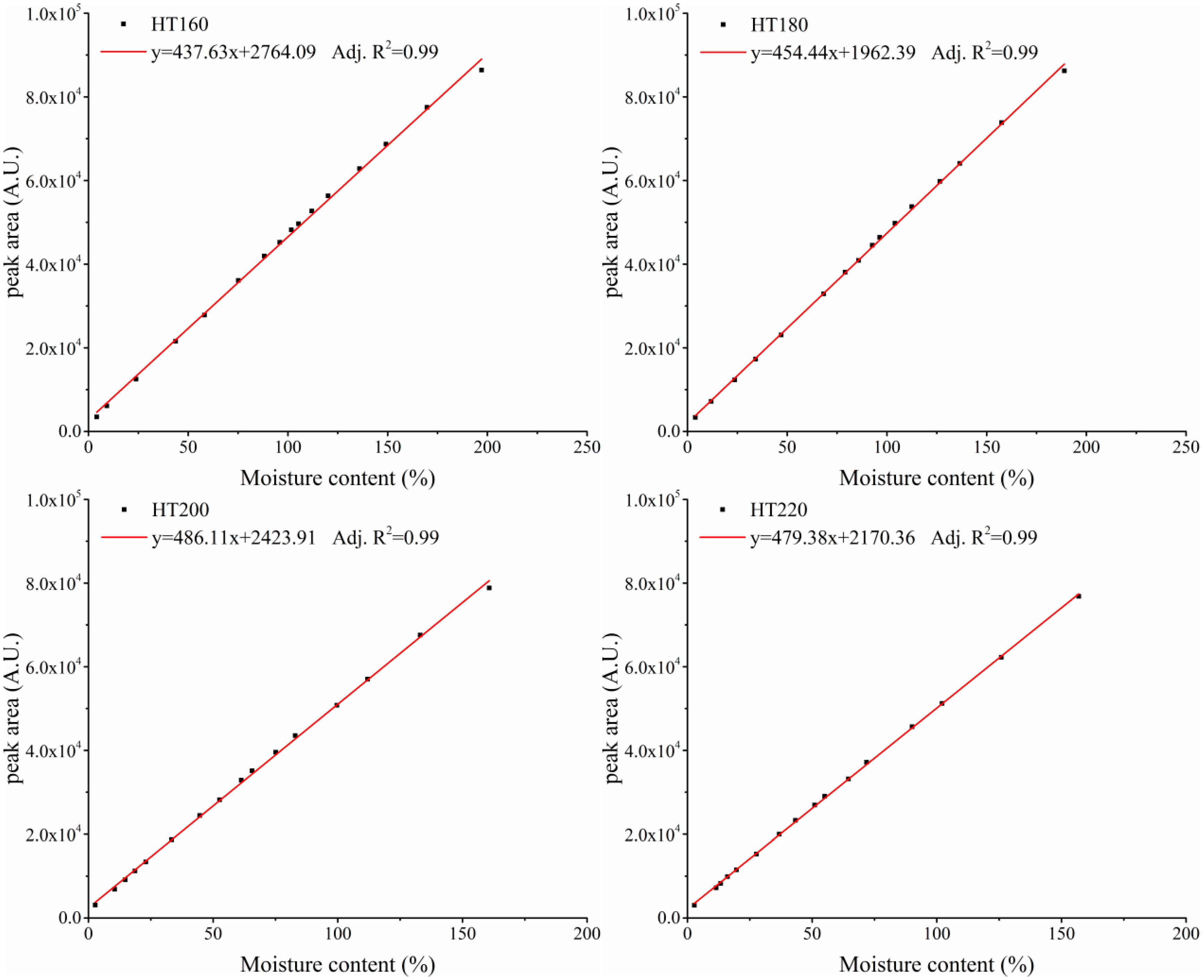
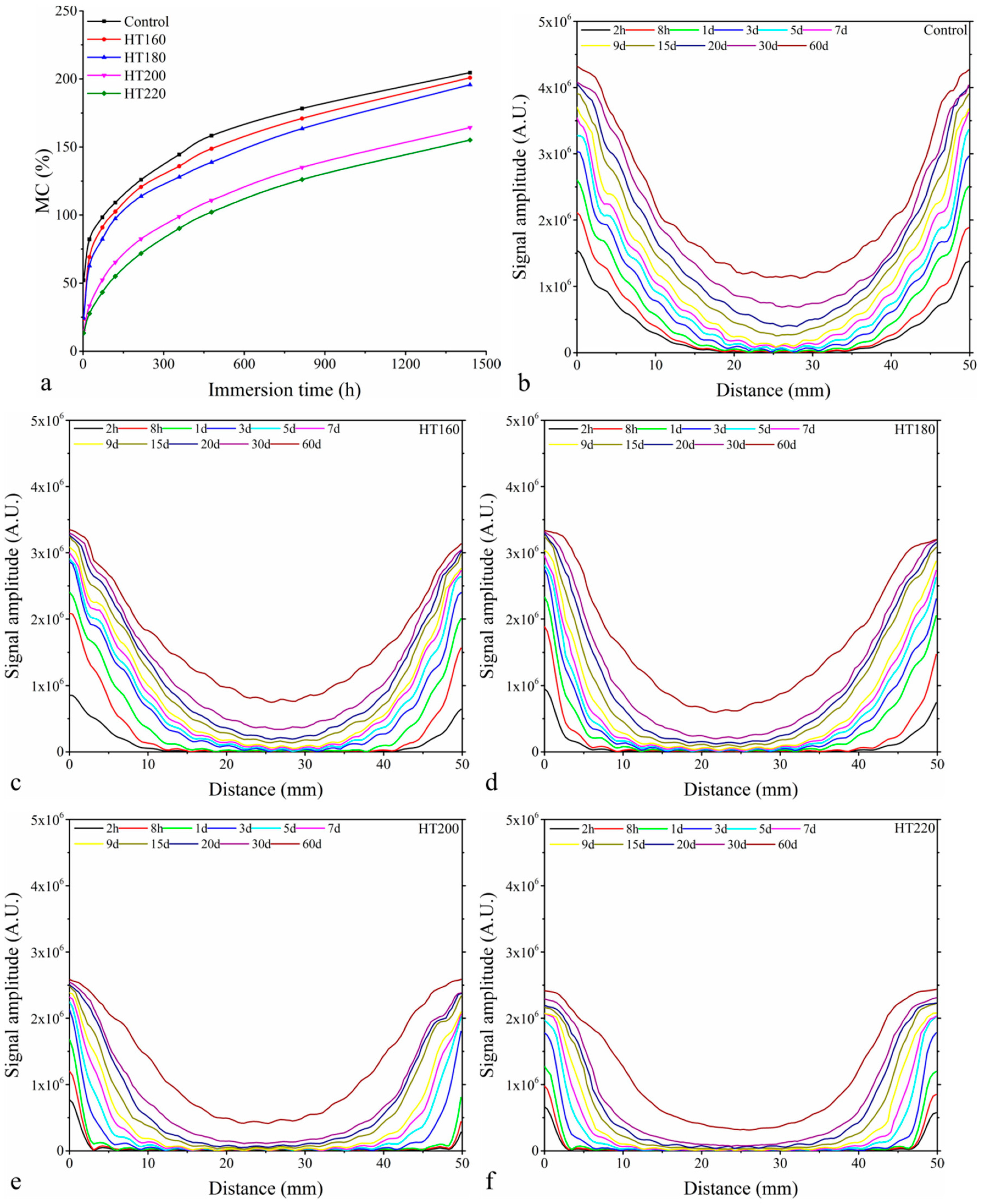
3.1. Relationship between MC and NMR Signal
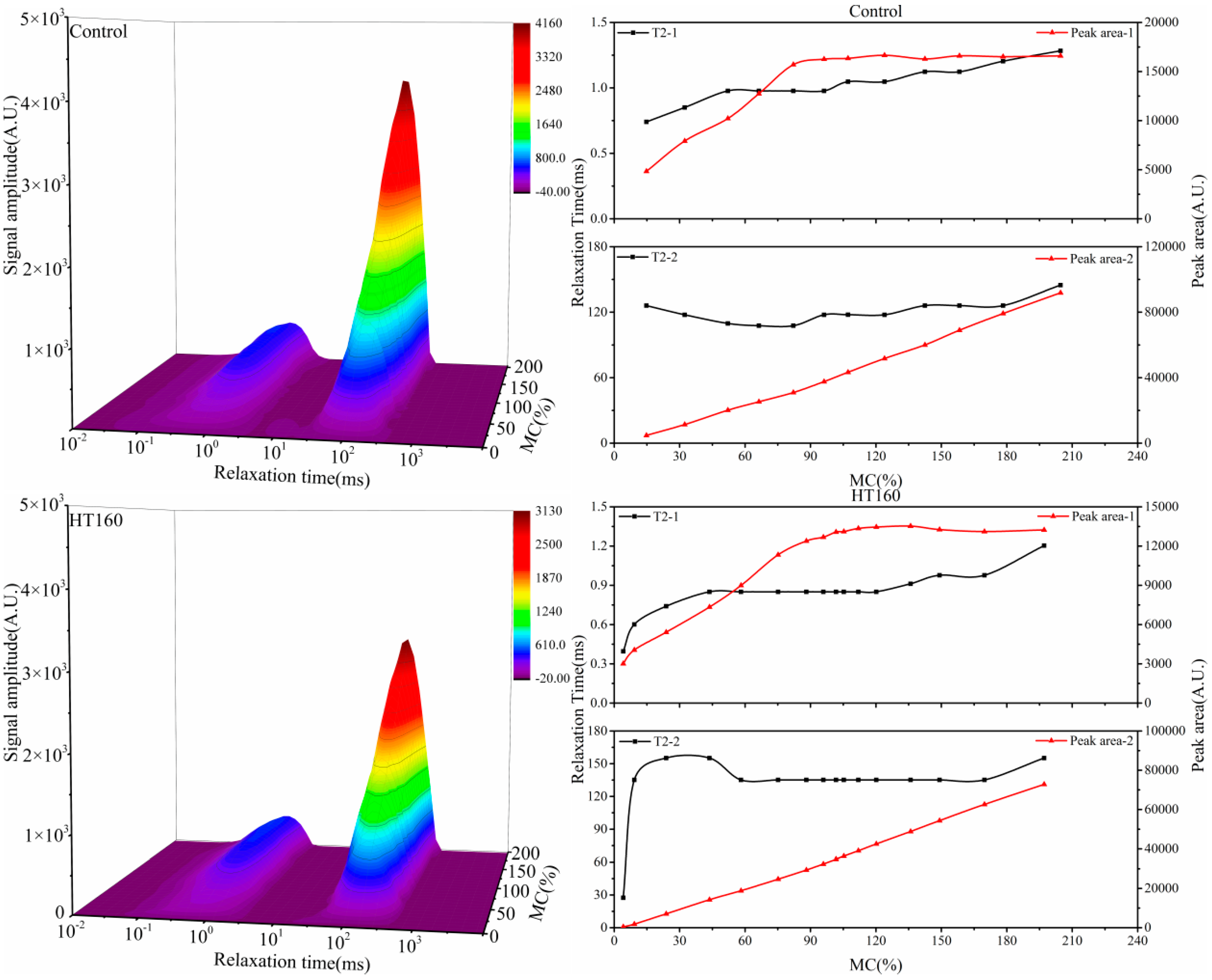
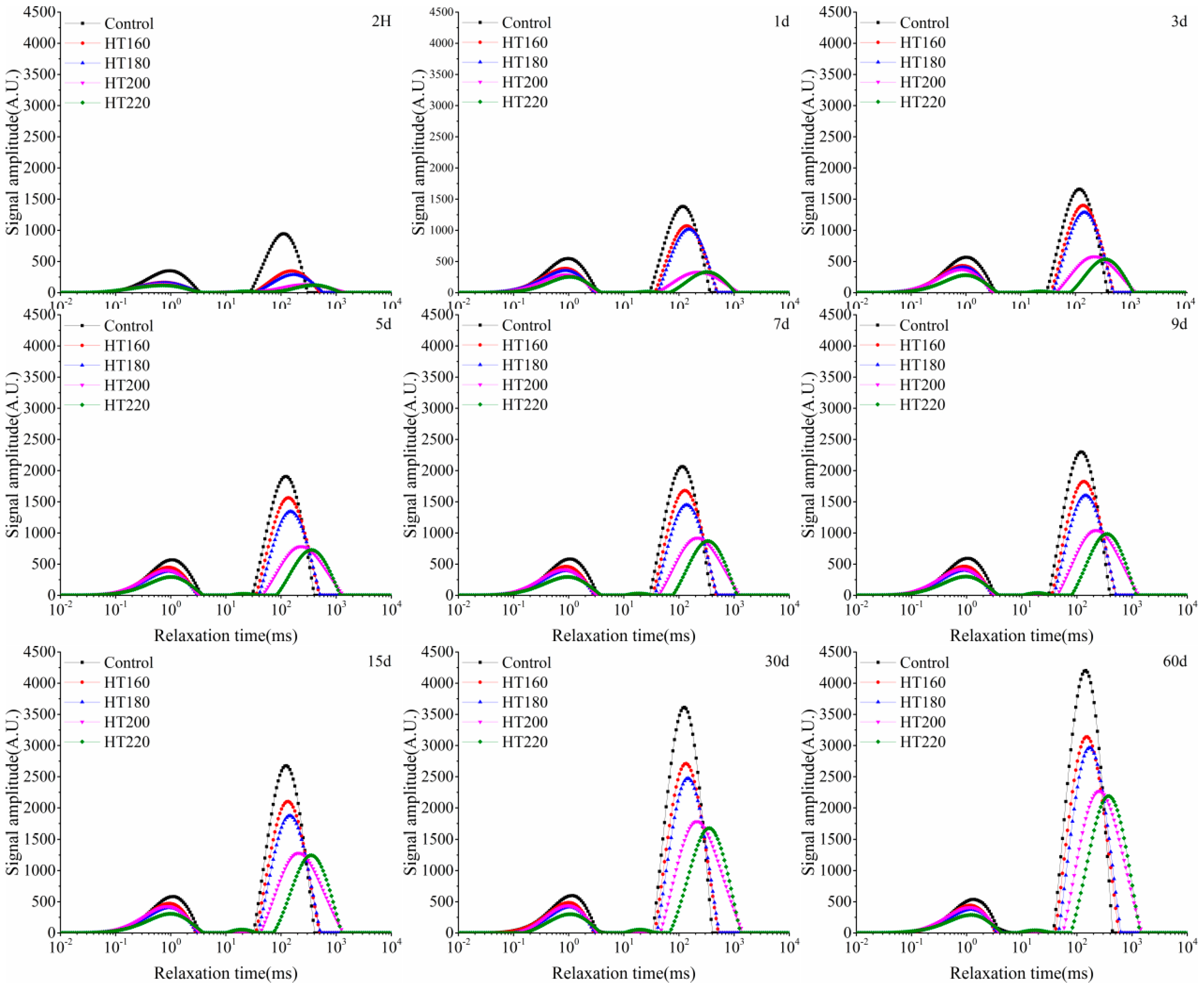
3.2. Water States and Migration
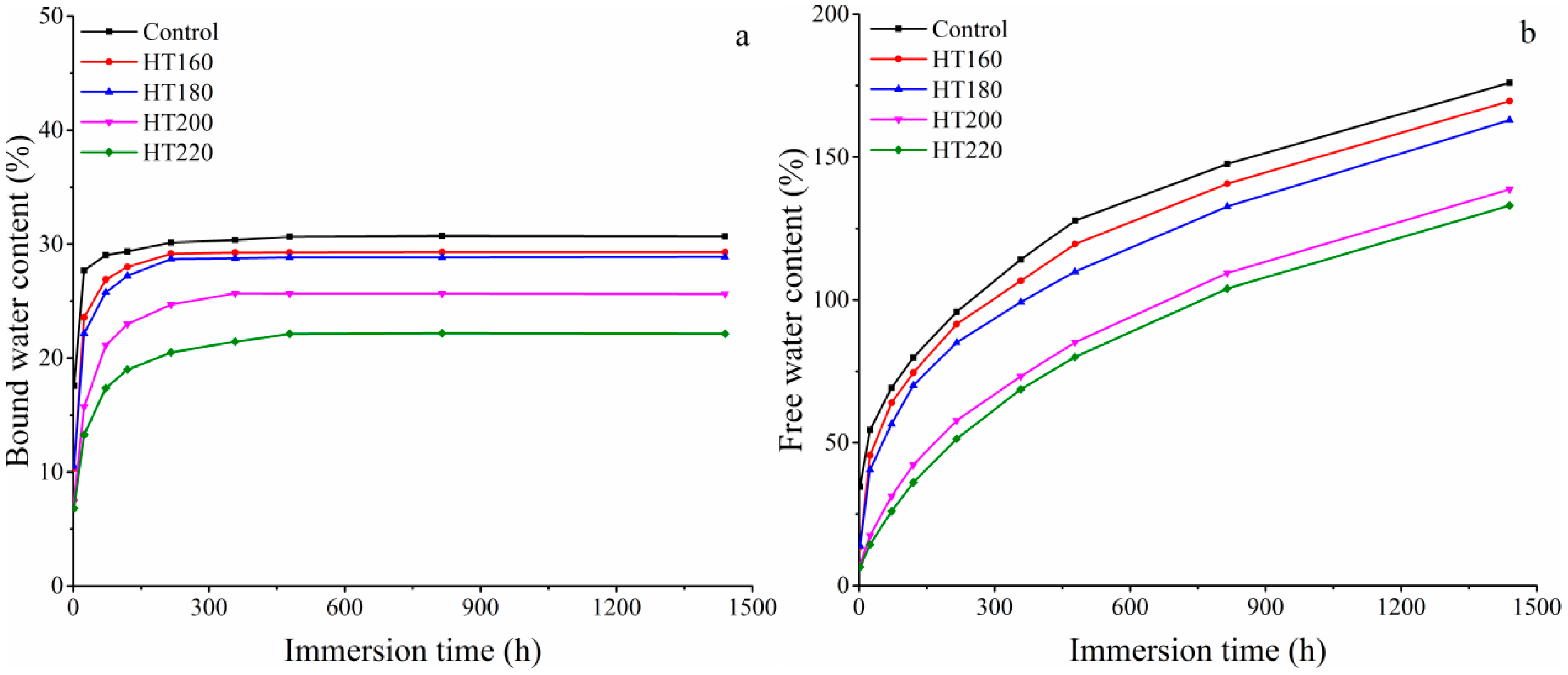
3.3. Content of Free Water and Bound Water
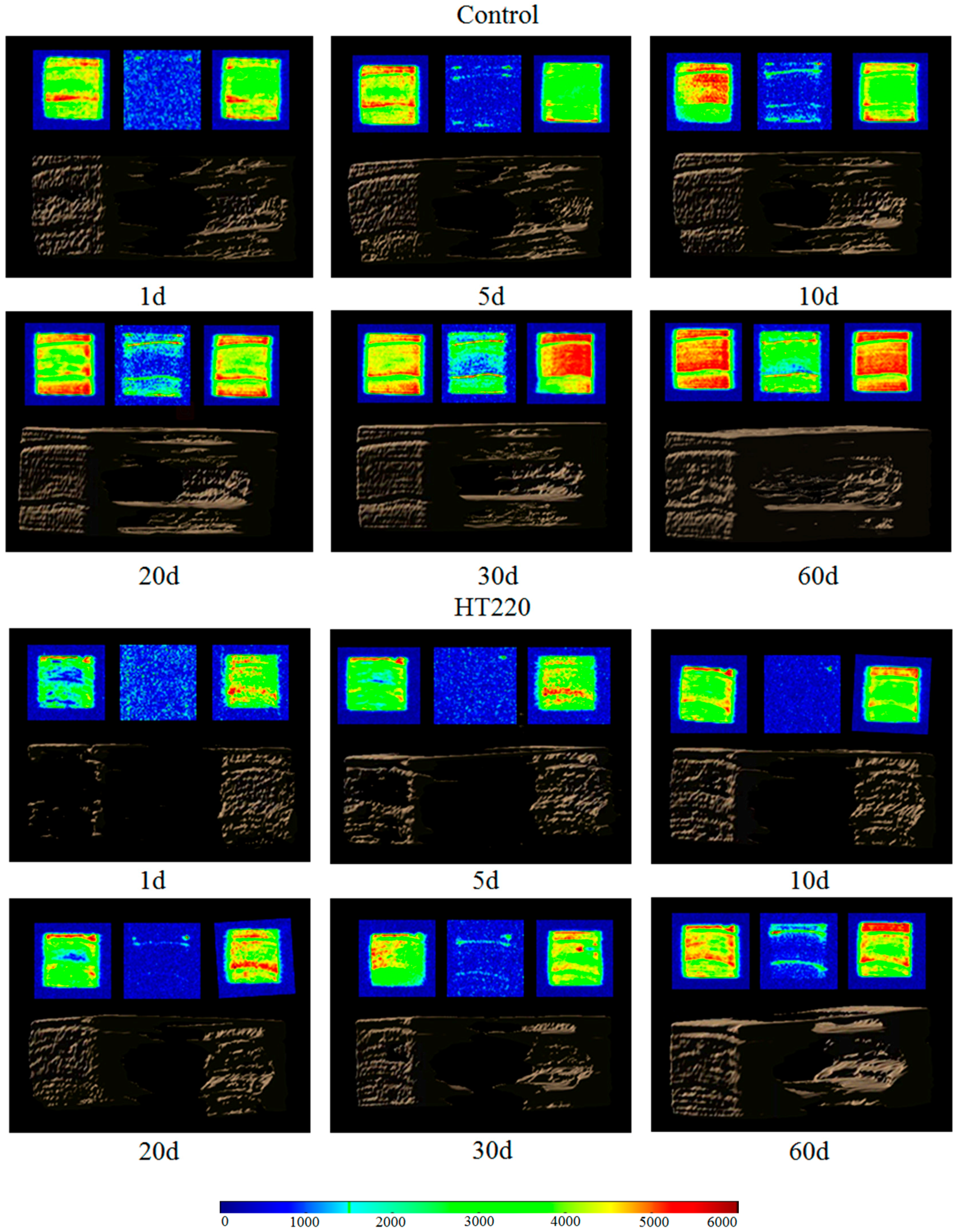
3.4. MC Profiles and NMR Imaging of Absorption of Free Water
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bal, B.C. Wood-water relationships and biological durability of heat-treated Taurus Fir wood. Pro Ligno 2015, 11, 3–10. [Google Scholar]
- Sun, Q.F.; Yu, H.P.; Liu, Y.X.; Jian, L.; Yun, L.; Hunt, J.F. Improvement of water resistance and dimensional stability of wood through titanium dioxide coating. Holzforschung 2010, 64, 757–761. [Google Scholar] [CrossRef]
- Zhan, T.; Jiang, J.; Lu, J.; Zhang, Y.; Chang, J. Influence of hygrothermal condition on dynamic viscoelasticity of Chinese fir (Cunninghamia lanceolata). Part 1: Moisture adsorption. Holzforschung 2018. [Google Scholar] [CrossRef]
- Zhan, T.; Jiang, J.; Lu, J.; Zhang, Y.; Chang, J. Influence of hygrothermal condition on dynamic viscoelasticity of Chinese fir (Cunninghamia lanceolata). Part 2: Moisture desorption. Holzforschung 2018. [Google Scholar] [CrossRef]
- Källbom, S.; Rautkari, L.; Wålinder, M.; Johansson, L.S.; Campbell, J.M.; Segerholm, K.; Jones, D.; Laine, K. Water vapour sorption characteristics and surface chemical composition of thermally modified spruce (Picea abies karst). Int. Wood Prod. J. 2016, 7, 116–123. [Google Scholar] [CrossRef]
- Tjeerdsma, B.F.; Militz, H. Chemical changes in hydrothermal treated wood: FTIR analysis of combined hydrothermal and dry heat-treated wood. Holz Als Roh-Und Werkst. 2005, 63, 102–111. [Google Scholar] [CrossRef]
- Altgen, M.; Hofmann, T.; Militz, H. Wood moisture content during the thermal modification process affects the improvement in hygroscopicity of Scots pine sapwood. Wood Sci. Technol. 2016, 50, 1–15. [Google Scholar] [CrossRef]
- Nuopponen, M.; Vuorinen, T.; Jämsä, S.; Viitaniemi, P. Thermal Modifications in Softwood Studied by FT-IR and UV Resonance Raman Spectroscopies. J. Wood Chem. Technol. 2005, 24, 13–26. [Google Scholar] [CrossRef]
- Tjeerdsma, B.F.; Boonstra, M.; Pizzi, A.; Tekely, P.; Militz, H. Characterisation of thermally modified wood: Molecular reasons for wood performance improvement. Holz Als Roh-Und Werkst. 1998, 56, 149–153. [Google Scholar] [CrossRef]
- Windeisen, E.; Strobel, C.; Wegener, G. Chemical changes during the production of thermo-treated beech wood. Wood Sci. Technol. 2007, 41, 523–536. [Google Scholar] [CrossRef]
- Akgül, M.; Gümüşkaya, E.; Korkut, S. Crystalline structure of heat-treated Scots pine [Pinus sylvestris L.] and Uludağ fir [Abies nordmanniana (Stev.) subsp. bornmuelleriana (Mattf.)] wood. Wood Sci. Technol. 2007, 41, 281. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Gazo, R. Water states in yellow poplar during drying studied by time-domain nuclear magnetic resonance. Wood Fiber Sci. 2013, 45, 423–428. [Google Scholar]
- Nanassy, A.J. True dry mass and moisture content of wood by NMR. Wood Sci. 1976, 9, 104–109. [Google Scholar]
- Sharp, A.R.; Riggin, M.T.; Kaiser, R.; Schneider, M.H. Determination of moisture content of wood by pulsed nuclear magnetic resonance. Wood Fiber Sci. 1978, 10, 74–81. [Google Scholar]
- Hartley, I.D.; Kamke, F.A.; Peemoeller, H. Absolute Moisture Content Determination of Aspen Wood Below the Fiber Saturation Point using Pulsed NMR. Holzforschung 1994, 48, 474–479. [Google Scholar] [CrossRef]
- Merela, M.; Oven, P.; Serša, I.; Mikac, U. A single point NMR method for an instantaneous determination of the moisture content of wood. Holzforschung 2009, 63, 348–351. [Google Scholar] [CrossRef]
- Hartley, I.D.; Avramidis, S.; Mackay, A.L. H-NMR studies of water interactions in sitka spruce and western hemlock: Moisture content determination and second moments. Wood Sci. Technol. 1996, 30, 141–148. [Google Scholar] [CrossRef]
- Dvinskikh, S.V.; Furó, I. Moisture content profiles and uptake kinetics in wood cladding materials evaluated by a portable nuclear magnetic resonance spectrometer. Wood Mater. Sci. Eng. 2011, 6, 119–127. [Google Scholar] [CrossRef]
- Labbé, N.; Jéso, B.D.; Lartigue, J.-C.; Daudé, G.; Pétraud, M.; Ratier, M. Time-domain 1H NMR characterization of the liquid phase in greenwood. Holzforschung 2006, 60, 265–270. [Google Scholar] [CrossRef]
- Xu, K.; Lv, J.; Gao, Y.; Wu, Y.; Li, X. Determination of Moisture Content and Moisture Content Profiles in Wood during Drying by Low Field Nuclear Magnetic Resonance. Dry. Technol. 2017, 35, 1909–1918. [Google Scholar] [CrossRef]
- Almeida, G. A NMR study of water distribution in hardwoods at several equilibrium moisture contents. Wood Sci. Technol. 2007, 41, 293–307. [Google Scholar] [CrossRef]
- Menon, R.S.; Mackay, A.L.; Hailey, J.R.T.; Bloom, M.; Burgess, A.E.; Swanson, J.S. An NMR determination of the physiological water distribution in wood during drying. J. Appl. Polym. Sci. 1987, 33, 1141–1155. [Google Scholar] [CrossRef]
- Riggin, M.T.; Sharp, A.R.; Kaiser, R.; Schneider, M.H. Transverse NMR relaxation of water in wood. J. Appl. Polym. Sci. 1979, 23, 3147–3154. [Google Scholar] [CrossRef]
- Araujo, C.D.; Mackay, A.L.; Hailey, J.R.T.; Whittall, K.P.; Le, H. Proton magnetic resonance techniques for characterization of water in wood: Application to white spruce. Wood Sci. Technol. 1992, 26, 101–113. [Google Scholar] [CrossRef]
- Labbé, N.; Jéso, B.D.; Lartigue, J.-C.; Daudé, G.; Pétraud, M.; Ratier, M.; Labbé, N.; Jéso, B.D.; Lartigue, J.C.; Daudé, G. Moisture Content and Extractive Materials in Maritime Pine Wood by Low Field 1H NMR. Holzforschung 2002, 56, 25–31. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Elder, T. Moisture in untreated, acetylated, and furfurylated Norway spruce studied during drying using time domain NMR. Wood Fiber Sci. 2008, 40, 309–320. [Google Scholar]
- Telkki, V.V.; Yliniemi, M.; Jokisaari, J. Moisture in softwoods: Fiber saturation point, hydroxyl site content, and the amount of micropores as determined from NMR relaxation time distributions. Holzforschung 2013, 67, 291–300. [Google Scholar] [CrossRef]
- Kekkonen, P.M.; Ylisassi, A.; Telkki, V.V. Absorption of Water in Thermally Modified Pine Wood As Studied by Nuclear Magnetic Resonance. J. Phys. Chem. C 2014, 118, 2146–2153. [Google Scholar] [CrossRef]
- Dvinskikh, S.V.; Henriksson, M.; Berglund, L.A.; Furo, I. A multinuclear magnetic resonance imaging (MRI) study of wood with adsorbed water: Estimating bound water concentration and local wood density. Holzforschung 2011, 65, 103–107. [Google Scholar] [CrossRef]
- Mazela, B.; Kowalczuk, J.; Ratajczak, I.; Szentner, K. Moisture content (MC) and multinuclear magnetic resonance imaging (MRI) study of water absorption effect on wood treated with aminofunctional silane. Eur. J. Wood Wood Prod. 2014, 72, 243–248. [Google Scholar] [CrossRef]
- Javed, M.A.; Kekkonen, P.M.; Ahola, S. Magnetic resonance imaging study of water absorption in thermally modified pine wood. Holzforschung 2014, 69, 899–907. [Google Scholar] [CrossRef]
- Kymäläinen, M.; Havimo, M.; Louhelainen, J. Sorption properties of torrefied wood and charcoal. Wood Mater. Sci. Eng. 2014, 9, 170–178. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Morén, T. Moisture properties of heat-treated Scots pine and Norway spruce sapwood impregnated with wood preservatives. Ann. Des Sci. For. 2012, 46, 85–93. [Google Scholar]
- Kaygin, B.; Gunduz, G.; Aydemir, D. Some Physical Properties of Heat-Treated Paulownia (Paulownia elongata) Wood. Dry. Technol. 2009, 27, 89–93. [Google Scholar] [CrossRef]
- Unsal, O.; Kartal, S.N.; Candan, Z.; Arango, R.A.; Clausen, C.A.; Iii, F.G. Decay and termite resistance, water absorption and swelling of thermally compressed wood panels. Int. Biodeterior. Biodegrad. 2009, 63, 548–552. [Google Scholar] [CrossRef]
- Scheiding, W.; Direske, M.; Zauer, M. Water absorption of untreated and thermally modified sapwood and heartwood of Pinus sylvestris L. Eur. J. Wood Wood Prod. 2016, 74, 585–589. [Google Scholar] [CrossRef]
- Cox, J.; Mcdonald, P.J.; Gardiner, B.A. A study of water exchange in wood by means of 2D NMR relaxation correlation and exchange. Holzforschung 2010, 64, 259–266. [Google Scholar] [CrossRef]
- Borrega, M.; Karenlämpi, P.P. Cell wall porosity in Norway spruce wood as affected by high-temperature drying. Wood Fiber Sci. J. Soc. Wood Sci. Technol. 2011, 43, 206–214. [Google Scholar]
- Olek, W.; Bonarski, J.T. Effects of thermal modification on wood ultrastructure analyzed with crystallographic texture. Holzforschung 2014, 68, 721–726. [Google Scholar] [CrossRef]
- Junghans, K.; Niemz, P.; Bächle, F. Investigations into the influence of thermal treatment on the porosity of spruce. Eur. J. Wood Prod. 2005, 63, 243–344. [Google Scholar] [CrossRef]
- Gezici-Koç, Ö.; Erich, S.J.F.; Huinink, H.P.; van der Ven, L.G.; Adan, O.C.G. Bound and free water distribution in wood during water uptake and drying as measured by 1D magnetic resonance imaging. Cellulose 2017, 24, 535–553. [Google Scholar] [CrossRef]
- Kocaefe, D.; Poncsak, S.; Doré, G.; Younsi, R. Effect of heat treatment on the wettability of white ash and soft maple by water. Holz Als Roh Werkst. 2008, 66, 355–361. [Google Scholar] [CrossRef]
- Esteves, B.; Graça, J.; Pereira, H. Extractive composition and summative chemical analysis of thermally treated eucalypt wood. Holzforschung 2008, 62, 344–351. [Google Scholar] [CrossRef]
- Mitsui, K.; Inagaki, T.; Tsuchikawa, S. Monitoring of hydroxyl groups in wood during heat treatment using NIR spectroscopy. Biomacromolecules 2008, 9, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Dubey, M.K.; Pang, S.; Walker, J. Changes in chemistry, color, dimensional stability and fungal resistance of Pinus radiata D. Don wood with oil heat-treatment. Holzforschung 2012, 66, 49–57. [Google Scholar] [CrossRef]
- Cademartori, P.H.G.; Santos, P.S.B.D.; Serrano, L.; Labidi, J.; Gatto, D.A. Effect of thermal treatment on physicochemical properties of Gympie messmate wood. Ind. Crop. Prod. 2013, 45, 360–366. [Google Scholar] [CrossRef]
- Sandak, A.; Sandak, J.; Allegretti, O. Quality control of vacuum thermally modified wood with near infrared spectroscopy. Vacuum 2015, 114, 44–48. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification: Chemical, Thermal and other Processes; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 99–127. [Google Scholar]
- Telkki, V.V.; Saunavaara, J.; Jokisaari, J. Time-of-flight remote detection MRI of thermally modified wood. J. Magn. Reson. 2010, 202, 78–84. [Google Scholar] [CrossRef]
- Hakkou, M.; Pétrissans, M.; Zoulalian, A.; Gérardin, P. Investigation of wood wettability changes during heat treatment on the basis of chemical analysis. Polym. Degrad. Stab. 2005, 89, 1–5. [Google Scholar] [CrossRef]
- Kekkonen, P.M.; Telkki, V.V.; Jokisaari, J. Determining the highly anisotropic cell structures of Pinus sylvestris in three orthogonal directions by PGSTE NMR of absorbed water and methane. J. Phys. Chem. B 2009, 113, 1080–1084. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Xu, K.; Peng, H.; Jiang, J.; Zhao, R.; Lu, J. Effect of Heat Treatment on Water Absorption of Chinese fir Using TD-NMR. Appl. Sci. 2019, 9, 78. https://doi.org/10.3390/app9010078
Gao Y, Xu K, Peng H, Jiang J, Zhao R, Lu J. Effect of Heat Treatment on Water Absorption of Chinese fir Using TD-NMR. Applied Sciences. 2019; 9(1):78. https://doi.org/10.3390/app9010078
Chicago/Turabian StyleGao, Yulei, Kang Xu, Hui Peng, Jiali Jiang, Rongjun Zhao, and Jianxiong Lu. 2019. "Effect of Heat Treatment on Water Absorption of Chinese fir Using TD-NMR" Applied Sciences 9, no. 1: 78. https://doi.org/10.3390/app9010078