Characterization of Slag Cement Mortar Containing Nonthermally Treated Dried Red Mud
Abstract
:1. Introduction
2. Nonthermally Treated Dried Red Mud (NTRM) Manufacturing
2.1. Raw Materials
2.2. Experimental Plan to Manufacture NTRM
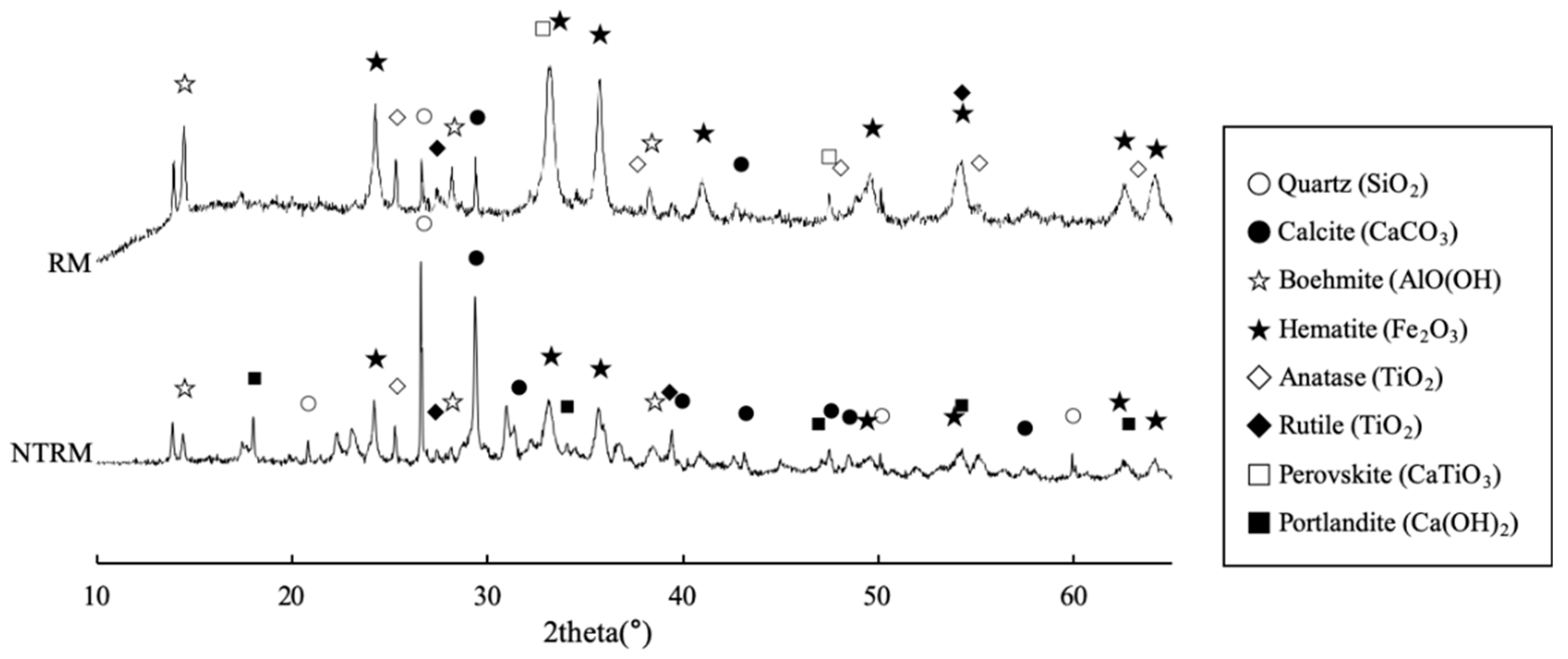
2.3. Characteristics of NTRM
2.3.1. Water Content
2.3.2. Chemical properties
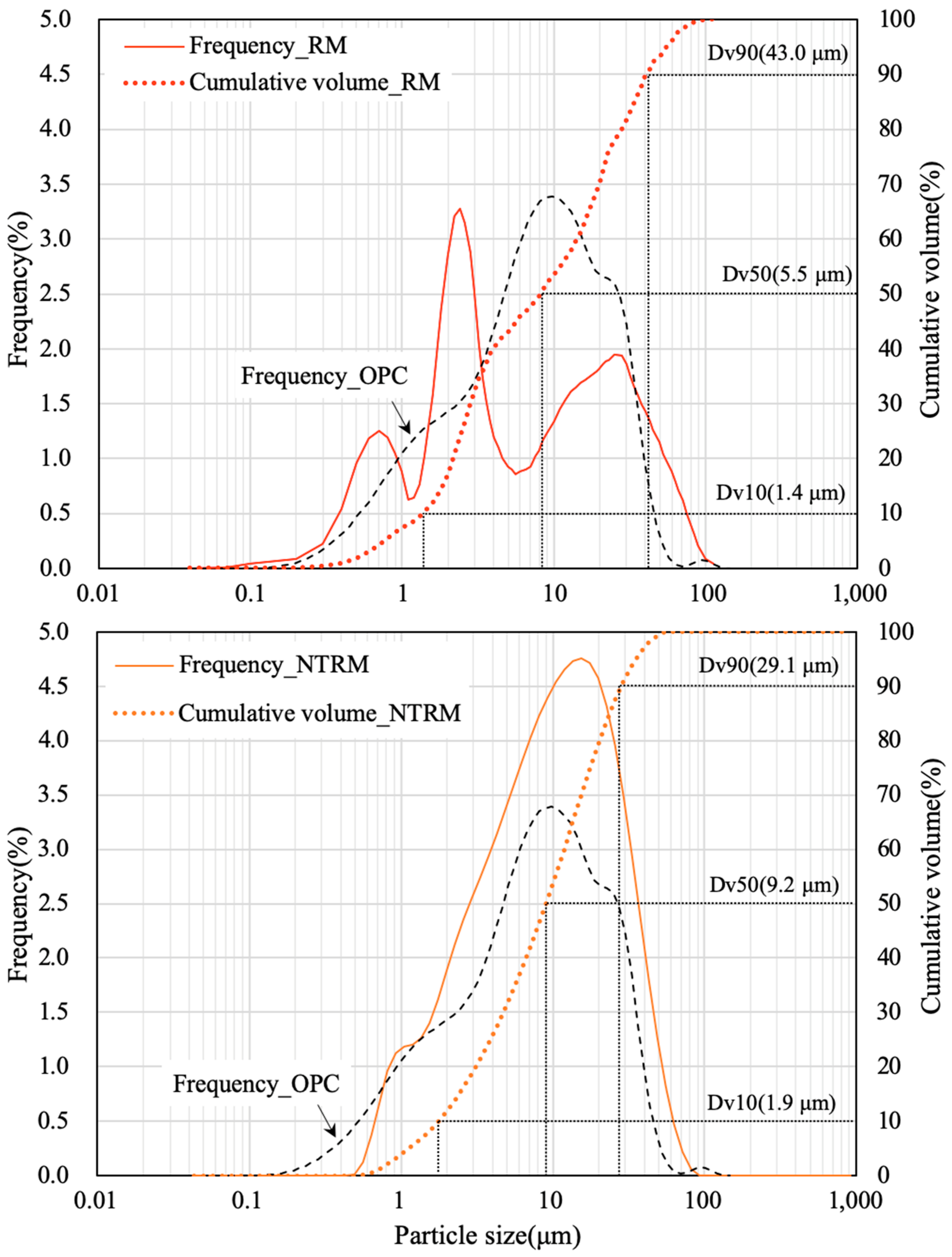
2.3.3. Particle Size
3. Characterization of Slag Cement Mortar Containing RM and NTRM
3.1. Materials
3.2. Experimental Outline
3.3. Experimental Method
3.3.1. Compressive Strength
3.3.2. Porosity
3.3.3. Water Absorption Coefficient
3.4. Experimental Results and Discussion
3.4.1. Compressive strength
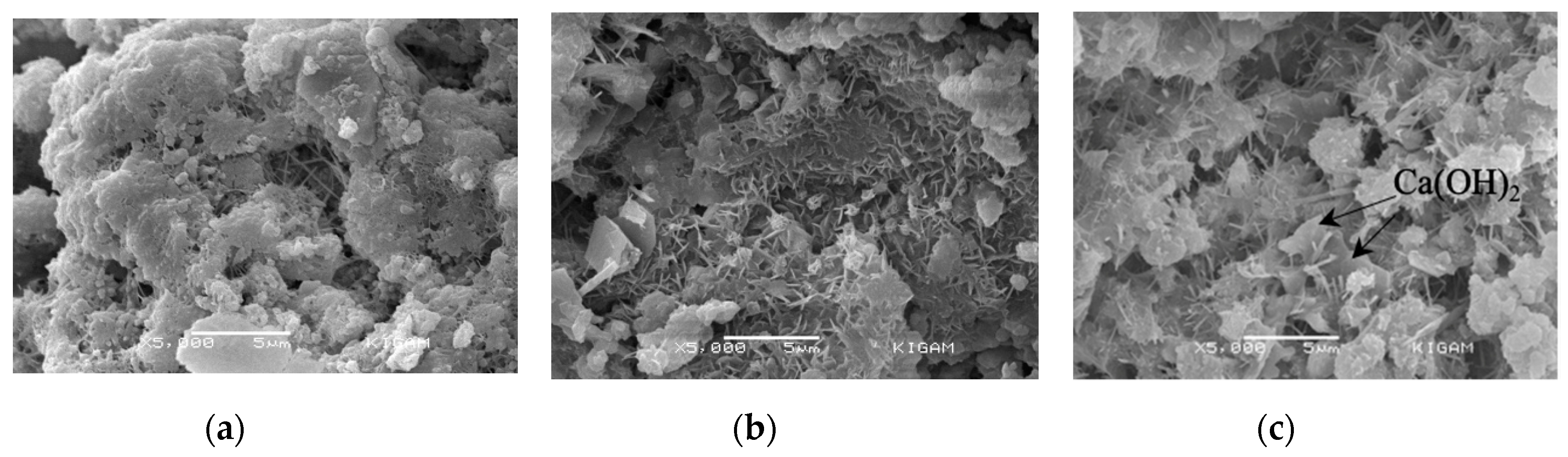
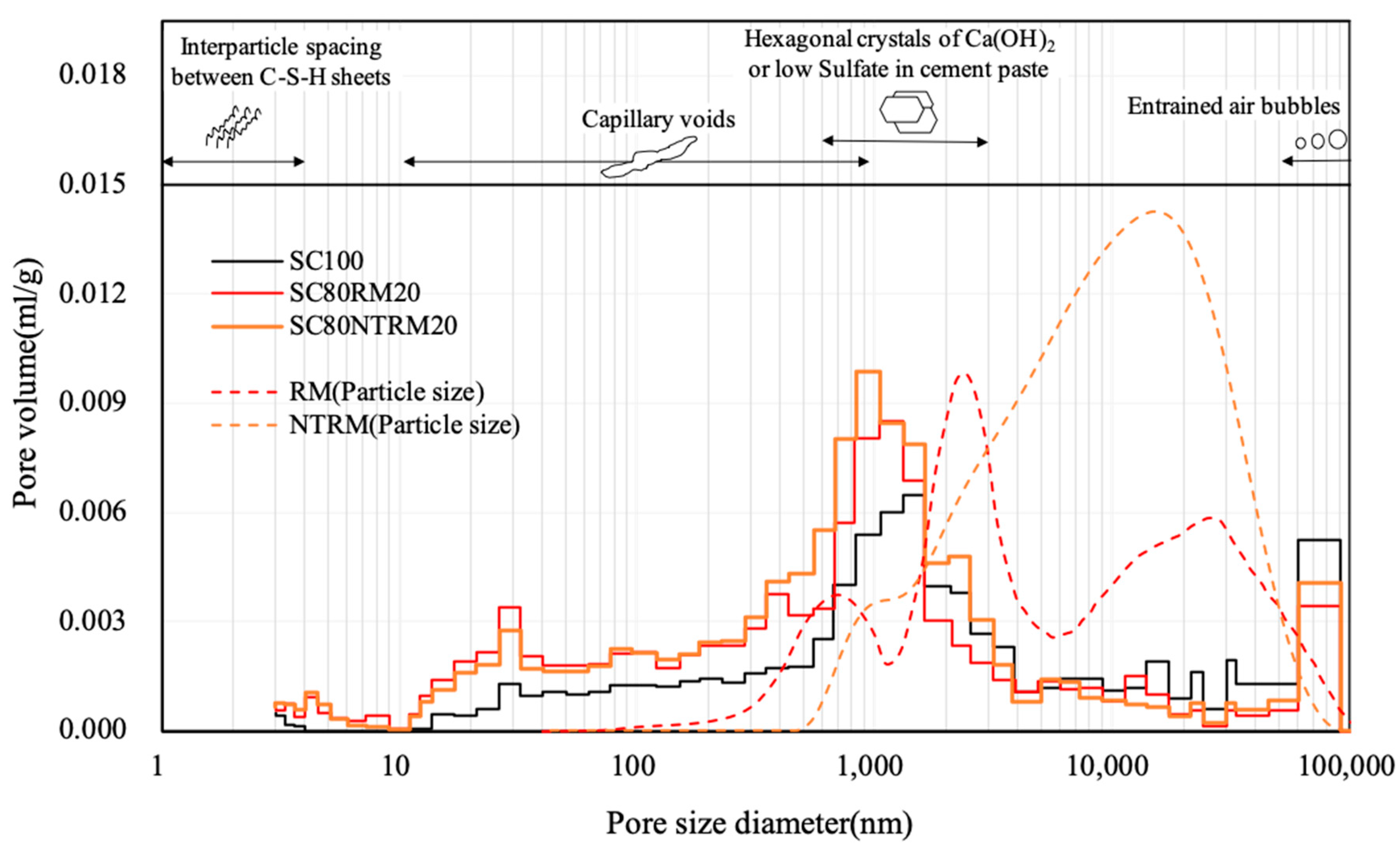
3.4.2. Porosity
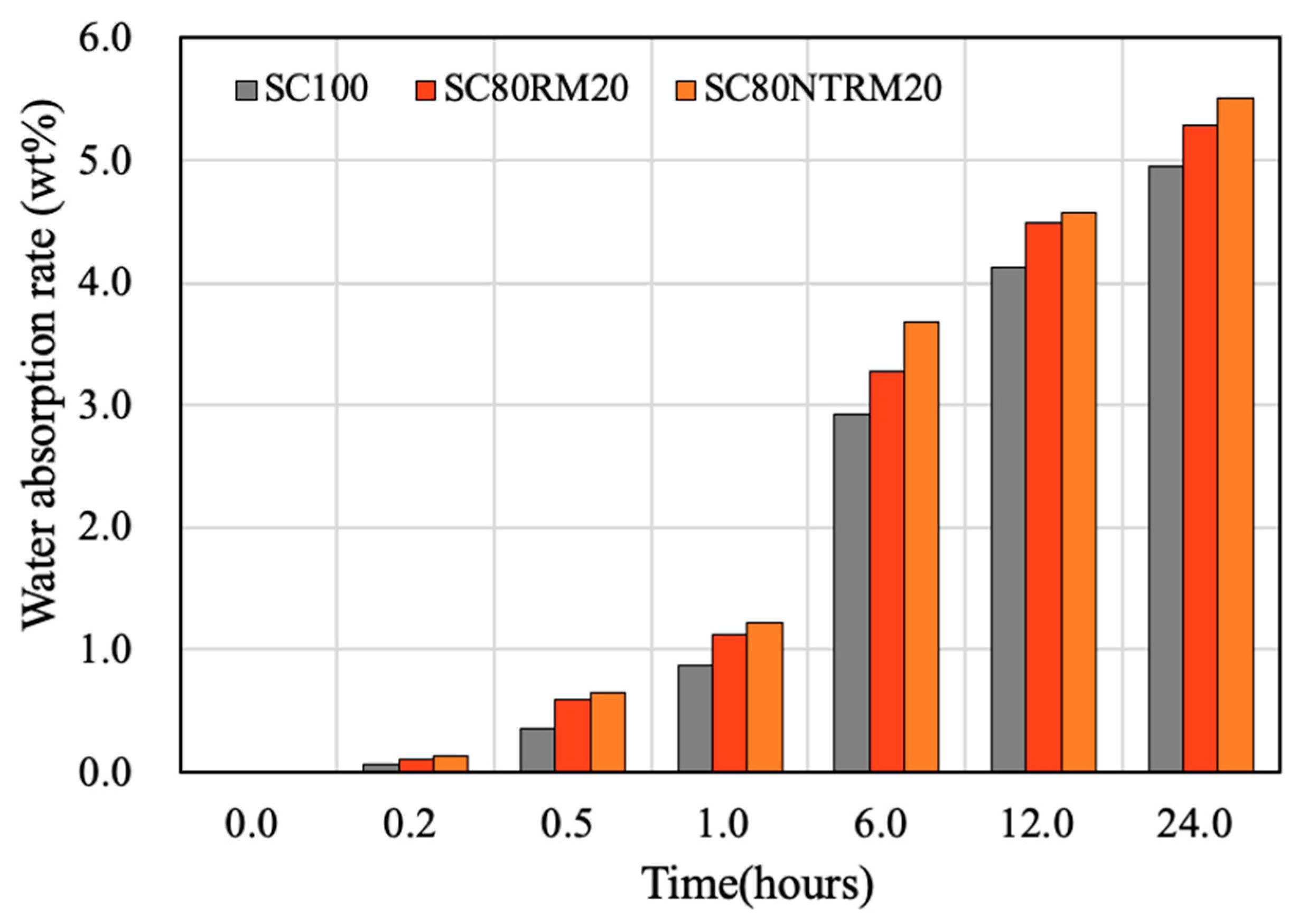
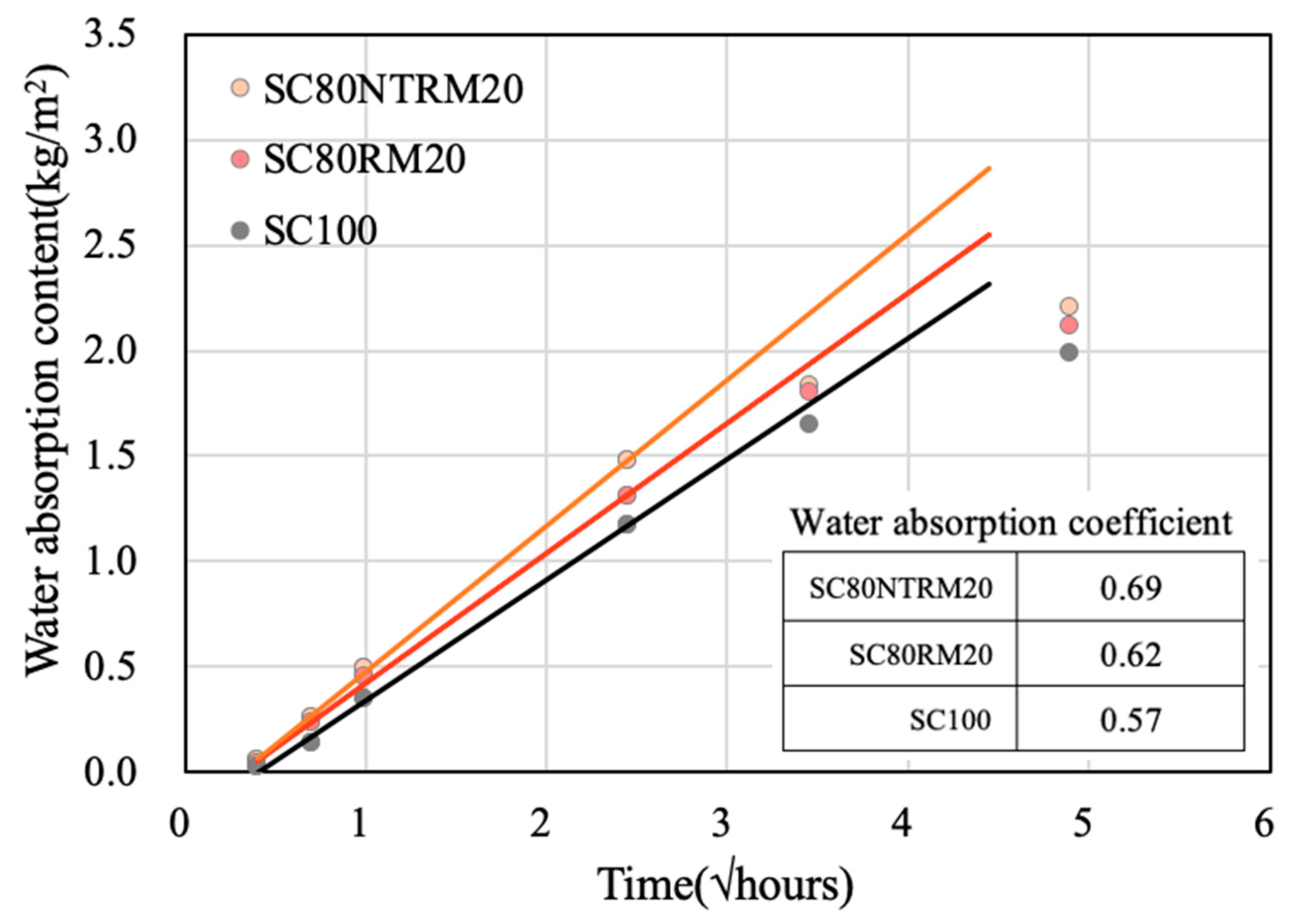
3.4.3. Moisture Absorption Coefficient
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alam, S.; Das, S.K.; Rao, B.H. Characterization of Coarse Fraction of Red Mud as a Civil Engineering Construction Material. J. Clean. Prod. 2017, 168, 679–691. [Google Scholar] [CrossRef]
- Mymrin, V.; Guidolin, M.A.; Klitzke, W.; Alekseev, K.; Guidolin, R.H.; Avanci, M.A.; Pawlowsky, U.; Winter, E., Jr.; Catai, R.E. Environmentally Clean Ceramics from Printed Circuit Board Sludge, Red Mud of Bauxite Treatment and Steel Slag. J. Clean. Prod. 2017, 164, 831–839. [Google Scholar] [CrossRef]
- Li, J.; Xu, L.; Sun, P.; Zhai, P.; Chen, X.; Zhang, H.; Zhang, Z.; Zhu, W. Novel Application of Red Mud: Facile Hydrothermal-Thermal Conversion Synthesis of Hierarchical Porous AlOOH and Al2O3 Microspheres as Adsorbents for Dye Removal. Chem. Eng. J. 2017, 321, 622–634. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jun, Y.; Jeon, D.; Oh, J.E. Synthesis of Structural Binder for Red Brick Production Based on Red Mud and Fly Ash Activated Using Ca(OH)2 and Na2CO3. Constr. Build. Mater. 2017, 147, 101–116. [Google Scholar] [CrossRef]
- Mayes, W.M.; Burke, I.T.; Gomes, H.I.; Anton, Á.D.; Molnár, M.; Feigl, V.; Ujaczki, É. Advances in Understanding Environmental Risks of Red Mud after the Ajka Spill, Hungary. J. Sustain. Metall. 2016, 2, 332–343. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, N. Utilization of Red Mud in Cement Production: A Review. Waste Manag. Res. 2011, 29, 1053–1063. [Google Scholar] [CrossRef]
- Senff, L.; Hotza, D.; Labrincha, J.A. Effect of Red Mud Addition on the Rheological Behaviour and on Hardened State Characteristics of Cement Mortars. Constr. Build. Mater. 2011, 25, 163–170. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Agatzini-Leonardou, S.; Oustadakis, P. Red Mud Addition in the Raw Meal for the Production of Portland Cement Clinker. J. Hazard. Mater. 2004, 116, 103–110. [Google Scholar] [CrossRef]
- Villarejo, L.P.; Corpas-Iglesias, F.A.; Martínez-Martínez, S.; Artiaga, R.; Pascual-Cosp, J. Manufacturing New Ceramic Materials from Clay and Red Mud Derived from the Aluminium Industry. Constr. Build. Mater. 2012, 35, 656–665. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Labrincha, J.A.; Morelli, M.R. Use of Red Mud as Addition for Portland Cement Mortars. J. Mater. Sci. Eng. 2010, 4, 1–9. [Google Scholar]
- Liu, R.-X.; Poon, C.S. Utilization of Red Mud Derived from Bauxite in Self-Compacting Concrete. J. Clean. Prod. 2016, 112, 384–391. [Google Scholar] [CrossRef]
- Geng, J.; Zhou, M.; Li, Y.; Chen, Y.; Han, Y.; Wan, S.; Zhou, X.; Hou, H. Comparison of Red Mud and Coal Gangue Blended Geopolymers Synthesized through Thermal Activation and Mechanical Grinding Preactivation. Constr. Build. Mater. 2017, 153, 185–192. [Google Scholar] [CrossRef]
- Jobbágy, V.; Somlai, J.; Kovács, J.; Szeiler, G.; Kovacs, T. Dependence of Radon Emanation of Red Mud Bauxite Processing Wastes on Heat Treatment. J. Hazard. Mater. 2009, 172, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xiao, B. Development of Unsintered Construction Materials from Red Mud Wastes Produced in the Sintering Alumina Process. Constr. Build. Mater. 2008, 22, 2299–2307. [Google Scholar] [CrossRef]
- KS L 5405 Flyash; Korean Agency for Technology and Standards (KATS): Seoul, Korea, 2009.
- Kang, S.K. Hydration Characteristics of Coal-Fly Ash Containing High CaO Compound. J. Korean Ceram. Soc. 2012, 49, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Pascual, J.; Corpas, F.A.; López-Beceiro, J.; Benítez-Guerrero, M.; Artiaga, R. Thermal Characterization of a Spanish Red Mud. J. Therm. Anal. Calorim. 2009, 96, 407–412. [Google Scholar] [CrossRef]
- Yalçin, N.; Sevinç, V. Utilization of Bauxite Waste in Ceramic Glazes. Ceram. Int. 2000, 26, 485–493. [Google Scholar] [CrossRef]
- Kang, S.; Kwon, S.-J. Effects of Red Mud and Alkali-Activated Slag Cement on Efflorescence in Cement Mortar. Constr. Build. Mater. 2017, 133, 459–467. [Google Scholar] [CrossRef]
- Krivenko, P.; Kovalchuk, O.; Pasko, A.; Croymans, T.; Hult, M.; Lutter, G.; Vandevenne, N.; Schreurs, S.; Schroeyers, W. Development of Alkali Activated Cements and Concrete Mixture Design with High Volumes of Red Mud. Constr. Build. Mater. 2017, 151, 819–826. [Google Scholar] [CrossRef]
- Kim, H.; Kaxng, S.; Choe, G. Effect of Red Mud Content on Strength and Efflorescence in Pavement Using Alkali-Activated Slag Cement. Int. J. Concr. Struct. Mater. 2018, 12, 18. [Google Scholar] [CrossRef]
- ISO 679: 2009: Cement—Test methods—Determination of Strength; International Organisation for Standardisation: Geneva, Switzerland, 2009.
- ASTM C 349 Standard Test Method for Compressive Strength of Hydraulic-Cement Mortars (Using Portions of Prisms Broken in Flexure); Annual Book of ASTM: Pennsylvania, PA, USA, 2005.
- KS F 2609 Determination of the Water Absorption Coefficient of Building Materials; Korean Agency for Technology and Standards (KATS): Seoul, Korea, 2008.
- Fujii, A.L.; Torres, D.D.R.; Romano, R.C.D.O.; Cincotto, M.A.; Pileggi, R.G. Impact of Superplasticizer on the Hardening of Slag Portland Cement Blended with Red Mud. Constr. Build. Mater. 2015, 101, 432–439. [Google Scholar] [CrossRef]
- Senff, L.; Modolo, R.C.E.; Silva, A.S.; Ferreira, V.M.; Hotza, D.; Labrincha, J.A. Influence of Red Mud Addition on Rheological Behavior and Hardened Properties of Mortars. Constr. Build. Mater. 2014, 65, 84–91. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Huang, H.; Meng, K.; Hu, K.; Hu, P.; Wang, X.; Zhang, Z.; Meng, X. Novel Glass Ceramic Foams Materials Based on Red Mud. Ceram. Int. 2014, 40, 6677–6683. [Google Scholar] [CrossRef]
- Singh, M.; Upadhayay, S.N.; Prasad, P.M. Preparation of Special Cements from Red Mud. Waste Manag. 1996, 16, 665–670. [Google Scholar] [CrossRef]
- Lee, S.S.; Song, H.Y.; Lee, Y.S.; Lee, K.P. A Study on the Strength and Flowing Properties of the Non-Cement Inorganic Composite by Using Blast Furnace Slag and Red Mud. Adv. Mater. Res. 2011, 261–263, 491–495. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Y.; Liu, X.; Jiang, S.; Feng, C.; Rafanan, E. Characterization on a Cementitious Material Composed of Red Mud and Coal Industry Byproducts. Constr. Build. Mater. 2013, 47, 496–501. [Google Scholar] [CrossRef]
- Yang, C.C.; Cho, S.W.; Wang, L.C. The Relationship between Pore Structure and Chloride Diffusivity from Ponding Test in Cement-Based Materials. Mater. Chem. Phys. 2006, 100, 203–210. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill: New York, NY, USA, 2006; ISBN 0071462899. [Google Scholar]
- Zhang, S.P.; Zong, L. Evaluation of Relationship between Water Absorption and Durability of Concrete Materials. Adv. Mater. Sci. Eng. 2014, 2014, 650373. [Google Scholar] [CrossRef]




| Element Oxide | Content (mass%) | ||
|---|---|---|---|
| Red Mud (RM) Sludge | Paper Sludge Ash (PSA) | High-Calcium Fly Ash (HCFA) | |
| SiO2 | 38.80 | 17.00 | 26.70 |
| Al2O3 | 16.10 | 9.83 | 13.10 |
| Fe2O3 | 22.80 | 6.77 | 8.71 |
| MgO | 0.20 | 4.05 | 4.40 |
| Na2O | 10.00 | - | - |
| CaO | 3.40 | 59.70 | 41.20 |
| TiO2 | 2.40 | 0.39 | - |
| SO3 | 0.24 | 1.80 | 5.28 |
 |  |  |
| Materials | Proportion (wt%) | Mixing Method | Measured Parameters |
|---|---|---|---|
| RM sludge PSA HCFA | 50 35 15 | Mixer type: Asphalt mixer Mixing time: 4 min | Water content Particle size XRF spectra XRD patterns |
| Type 1 | Specific Surface Area (cm2/g) | Density (g/cm3) | Lg. Loss | Chemical Composition (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | ||||
| OPC | 3.144 | 3.15 | 2.44 | 21.7 | 5.7 | 3.2 | 63.1 | 2.8 | 2.2 |
| GGBS | 4196 | 2.90 | 1.32 | 32.75 | 15.61 | 0.5 | 43.51 | 4.41 | - |
| Specimen ID | W/B 1 | B:S 2 | Binder (wt%) | Measured Parameters | ||
|---|---|---|---|---|---|---|
| SC | RM | NTRM | ||||
| SC100 | 0.6 | 1:3 | 100 | - | - | Compressive strength (MPa) |
| SC80RM20 | 80 | 20 | - | Porosity | ||
| SC80NTRM20 | 80 | - | 20 | Water absorption coefficient | ||
| Specimen ID | Age (d) | Compressive Strength (MPa) | ||||
|---|---|---|---|---|---|---|
| no.1 | no.2 | no.3 | Average | CV 1 | ||
| SC100 | 1 | 6.24 | 6.34 | 6.19 | 6.26 | 0.07 |
| 3 | 13.36 | 13.99 | 14.49 | 13.95 | 0.56 | |
| 7 | 23.55 | 18.63 | 21.96 | 21.38 | 2.50 | |
| 28 | 30.08 | 30.53 | 29.81 | 30.14 | 0.35 | |
| SC80RM20 | 1 | 5.18 | 4.87 | 5.01 | 5.02 | 0.15 |
| 3 | 11.81 | 11.83 | 11.87 | 11.84 | 0.03 | |
| 7 | 20.18 | 17.62 | 18.71 | 18.83 | 1.28 | |
| 28 | 24.74 | 27.67 | 25.33 | 25.91 | 1.55 | |
| SC80NTRM20 | 1 | 4.19 | 3.82 | 3.84 | 3.95 | 0.21 |
| 3 | 9.31 | 10.28 | 10.26 | 9.95 | 0.55 | |
| 7 | 15.92 | 13.24 | 15.70 | 14.95 | 1.49 | |
| 28 | 20.84 | 18.78 | 20.33 | 19.98 | 1.07 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, G.; Kang, S.; Kang, H. Characterization of Slag Cement Mortar Containing Nonthermally Treated Dried Red Mud. Appl. Sci. 2019, 9, 2510. https://doi.org/10.3390/app9122510
Choe G, Kang S, Kang H. Characterization of Slag Cement Mortar Containing Nonthermally Treated Dried Red Mud. Applied Sciences. 2019; 9(12):2510. https://doi.org/10.3390/app9122510
Chicago/Turabian StyleChoe, Gyeongcheol, Sukpyo Kang, and Hyeju Kang. 2019. "Characterization of Slag Cement Mortar Containing Nonthermally Treated Dried Red Mud" Applied Sciences 9, no. 12: 2510. https://doi.org/10.3390/app9122510





