Optimizing a Simple Natural Dye Production Method for Dye-Sensitized Solar Cells: Examples for Betalain (Bougainvillea and Beetroot Extracts) and Anthocyanin Dyes
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Dyes Preparation and Characterization
3.1.1. Flavonoids
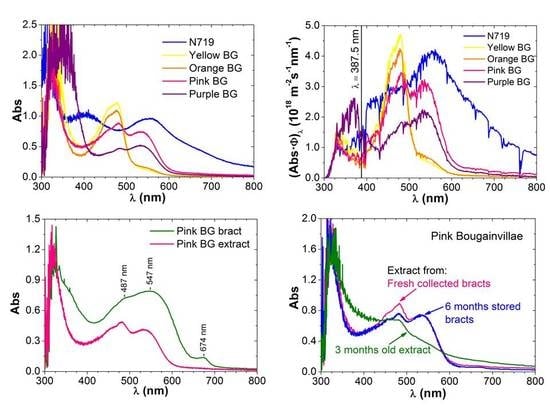
3.1.2. Betalains from Bougainvillea Bracts
Comparison of Dye Extracts and Dry Bracts Absorption Spectra
3.1.3. Betalains from Beetroots
3.2. Exam Dyes Behavior
3.2.1. How the Dye Solution Adsorbs onto Semiconductor DSSC Film
3.2.2. Mixing Different Dyes: Co-sensitization
3.2.3. How Stable the Dye Extract is
3.2.4. Role of Other Parameters Like pH or Pigment Concentration
3.3. NDSSC Tests
4. Conclusions
- The best extraction solution and method: for betalains, Solution 1 (acetone/water, moderately acid), for anthocyanins, Solutions 2 and 3 (very acidic solutions).
- The relative concentration of different pigment molecules for the different extracts.
- Some candidate vegetable material can be a priori selected or ruled out by inspection of the extract spectra. Besides, comparison with the corresponding spectra of dry raw material performed with a spectroradiometer, gives reliable information about the dye molecules which can be potentially extracted. We show the example of bougainvillea-colored bracts spectra.
- The dye stability or ageing: Anthocyanins are more stable and betalain molecules degrade, but conservation of the dry precursor (collected bracts) for at least 6 months is possible.
- How the dye adsorbs onto photoelectrode film, and the effect of mixing dye extracts. In this paper, we focus on mixing dyes from the betalain family to complement results in the bibliography.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohd, Y.; Mohd, S.; Faqeer, M. Natural Colorants: Historical, Processing and Sustainable Prospects. Nat. Prod. Bioprospect. 2017, 7, 123–145. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.; Owens, S.J.; Rørslett, B. Plants and colour: Flowers and pollination. Opt. Laser Technol. 2011, 43, 282–294. [Google Scholar] [CrossRef]
- Narayan, M.R. Review: Dye sensitized solar cells based on natural photosensitizers. Renew. Sust. Energ. Rev. 2012, 16, 208–215. [Google Scholar] [CrossRef]
- Sugathan, V.; John, E.; Sudhakar, K. Recent improvements in dye sensitized solar cells: A review. Renew. Sust. Energ. Rev. 2015, 52, 54–64. [Google Scholar] [CrossRef]
- Sharma, S.; Siwach, B.; Ghoshal, S.K.; Mohan, D. Dye sensitized solar cells: From genesis to recent drifts. Renew. Sust. Energ. Rev. 2017, 70, 529–537. [Google Scholar] [CrossRef]
- Mohiuddin, O.; Obaidullah, M.; Sabah, C. Improvement in dye sensitized solar cells from past to present. Opt. Quant. Electron. 2018, 50, 377. [Google Scholar] [CrossRef]
- Hug, H.; Bader, M.; Mair, P.; Glatzel, T. Biophotovoltaics: Natural pigments in dye-sensitized solar cells. Appl. Energ. 2014, 115, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Ludin, N.A.; Mahmoud, A.M.A.A.; Mohamad, A.B.; Amir, H.K.A.; Sopian, K.; Karim, N.S.A. Review on the development of natural dye photosensitizer for dye-sensitized solar cells. Renew. Sust. Energ. Rev. 2014, 31, 386–396. [Google Scholar] [CrossRef]
- Shalini, S.; Balasundara Prabhu, R.; Prasanna, S.; Mallick, T.K.; Senthilarasu, S. Review on natural dye sensitized solar cells: Operation, materials and methods. Renew. Sust. Energ. Rev. 2015, 51, 1306–1325. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sust. Energ. Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent advances in dye-sensitized solar cells: From photoanodes, sensitizers and electrolytes to counter electrodes. Mater. Today 2015, 18, 155–162. [Google Scholar] [CrossRef]
- Mozaffari, S.; Nateghi, M.R.; Zarandi, M.B. An overview of the challenges in the commercialization of dye sensitized solar cells. Renew. Sust. Energ. Rev. 2017, 71, 675–686. [Google Scholar] [CrossRef]
- Chawla, P.; Tripathi, M. Novel improvements in the sensitizers of dye-sensitized solar cells for enhancement in efficiency—A review. Int. J. Energy Res. 2015, 39, 1579–1596. [Google Scholar] [CrossRef]
- Shalini, S.; Balasundaraprabhu, R.; Satish-Kumar, T.; Prabavathy, N.; Senthilarasu, S.; Prasanna, S. Status and outlook of sensitizers/dyes used in dye sensitized solar cells (DSSC): A review. Int. J. Energy Res. 2016, 40, 1303–1320. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, L.; Gao, Y.; Ma, T. Dye-sensitized solar cells using 20 natural dyes as sensitizers. J. Photoch. Photobio. A 2011, 219, 188–194. [Google Scholar] [CrossRef]
- Kumara, N.T.R.N.; Lim, A.; Lim, S.M.; Petra, M.I.; Ekanayake, P. Recent progress and utilization of natural pigments in dye sensitized solar cells: A review. Renew. Sust. Energ. Rev. 2017, 78, 301–317. [Google Scholar] [CrossRef]
- Richhariya, G.; Kumar, A.; Tekasakul, P.; Gupta, B. Natural dyes for dye sensitized solar cell: A review. Renew. Sust. Energ. Rev. 2017, 69, 705–718. [Google Scholar] [CrossRef]
- Gu, P.; Yang, D.; Zhu, X.; Sun, H.; Li, J. Fabrication and characterization of dye-sensitized solar cells based on natural plants. Chem. Phys. Lett. 2018, 693, 16–22. [Google Scholar] [CrossRef]
- Mahmoud, A.M.A.A.; Ludin, N.A.; Mohamad, A.B.; Kadhum, A.A.H.; Mukhlus, A. Application of dyes extracted from Alternanthera dentata leaves and Musa acuminata bracts as natural sensitizers for dye-sensitized solar cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 192, 487–498. [Google Scholar] [CrossRef]
- Maurya, I.C.; Singh, S.; Neetu; NeetuGupta, A.K.; Srivastava, P.; Bahadur, L. Dye-sensitized solar cells employing extracts from four cassia flowers as natural sensitizers: Studies on dye ingredient effect on photovoltaic performance. J. Electron. Mater. 2018, 47, 225–232. [Google Scholar] [CrossRef]
- Adedokun, O.; Sanusi, Y.K.; Awodugba, A.O. Solvent dependent natural dye extraction and its sensitization effect for dye sensitized solar cells. Optik 2018, 174, 497–507. [Google Scholar] [CrossRef]
- Calogero, G.; Barichello, J.; Citro, I.; Mariani, P.; Vesce, L.; Bartolotta, A.; Di Carlo, A.; Di Marco, G. Photoelectrochemical and spectrophotometric studies on dye-sensitized solar cells (DSCs) and stable modules (DSCMs) based on natural apocarotenoids pigments. Dyes Pigments 2018, 155, 75–83. [Google Scholar] [CrossRef]
- Sanjay, P.; Deepa, K.; Madhavan, J.; Senthil, S. Optical, spectral and photovoltaic characterization of natural dyes extracted from leaves of Peltophorum pterocarpum and Acalypha amentacea used as sensitizers for ZnO based dye sensitized solar cells. Opt. Mater. 2018, 83, 192–199. [Google Scholar] [CrossRef]
- Manaa, M.B.; Bouaziz, N.; Schmaltz, B.; Van, F.T.; Lamine, A.B. Study of the effect of variation in temperature and pH on the adsorption process of natural Gardenia yellow dye into TiO2 mesoporous for dye sensitized solar cells using the statistical physics formalism: Physicochemical and thermodynamic investigation. Micropor. Mesopor. Mat. 2018, 270, 82–92. [Google Scholar] [CrossRef]
- Gu, P.; Yang, D.; Zhu, X.; Sun, H.; Li, J. Performance of dye-sensitized solar cells based on natural dyes. Opt. Quant. Electron. 2018, 50, 223. [Google Scholar] [CrossRef]
- Tahir, D.; Satriani, W.; Gareso, P.L.; Abdullah, B. Dye sensitized solar cell (DSSC) with natural dyes extracted from Jatropha leaves and purple Chrysanthemum flowers as sensitizer. IOP Conf. Ser. J. Phys. Conf. Ser. 2018, 979, 012056. [Google Scholar] [CrossRef]
- Rajkumar, S.; Nirmal Kumar, M.; Suguna, K.; Muthulakshmi, S.; Ashok Kumar, R. Enhanced performance of dye-sensitized solar cells using natural cocktail dye as sensitizer. Optik 2019, 178, 224–230. [Google Scholar] [CrossRef]
- Hosseinnezhad, M.; Rouhani, S.; Gharanjig, K. Extraction and application of natural pigments for fabrication of green dye-sensitized solar cells. Opto. Electron. Rev. 2018, 26, 165–171. [Google Scholar] [CrossRef]
- Yildiz, Z.K.; Atilgan, A.; Atli, A.; Özel, K.; Altinkaya, C.; Yildiz, A. Enhancement of efficiency of natural and organic dye sensitized solar cells using thin film TiO2 photoanodes fabricated by spin-coating. J. Photochem. Photobiol. A 2019, 368, 23–29. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, N.; Liu, D.; Liu, J.; Li, Y. Structure and photoelectrical properties of natural photoactive dyes for solar cells. Appl. Sci. 2018, 8, 1697. [Google Scholar] [CrossRef]
- Cassone, G.; Calogero, G.; Sponer, J.; Saija, F. Mobilities of iodide anions in aqueous solutions for applications in natural dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2018, 20, 13038–13046. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.K.; Arof, A.K. Performance of natural dyes as sensitizer in dye-sensitized solar cells employing LiBOB-based liquid electrolyte. Ionics 2018, 24, 915–922. [Google Scholar] [CrossRef]
- Swathi, K.E.; Jinchu, I.; Sreelatha, K.S.; Sreekala, C.O.; Menon, S.K. Effect of microwave exposure on the photo anode of DSSC sensitized with natural dye. IOP Conf. Ser. Mater. Sci. Eng. 2018, 310, 012141. [Google Scholar] [CrossRef]
- Tractz, G.T.; Viomar, A.; Dias, B.V.; de Lima, C.A.; Banczek, E.P.; da Cunha, M.T.; Antunes, S.R.M.; Rodrigues, P.R.P. Recombination Study of Dye Sensitized Solar Cells with Natural Extracts. J. Braz. Chem. Soc. 2019, 30, 371–378. [Google Scholar] [CrossRef]
- Lucioli, S.; Di Bari, C.; Forni, C.; Di Carlo, A.; Barrajón-Catalán, E.; Micol, V.; Nota, P.; Teoli, F.; Matteocci, F.; Frattarelli, A.; et al. Anthocyanic pigments from elicited in vitro grown shoot cultures of Vaccinium corymbosum L., cv. Brigitta Blue, as photosensitizer in natural dye-sensitized solar cells (NDSSC). J. Photochem. Photobiol. B 2018, 188, 69–76. [Google Scholar] [CrossRef]
- Prabavathy, N.; Shalini, S.; Balasundaraprabhu, R.; Velauthapillai, D.; Prasanna, S.; Balaji, G.; Muthukumarasamy, N. Algal buffer layers for enhancing the efficiency of anthocyanins extracted from rose petals for natural dye-sensitized solar cell (DSSC). Int. J. Energy Res. 2018, 42, 790–801. [Google Scholar] [CrossRef]
- Galera-Moreno, S.; González-Galindo, E.; Ariza, M.J.; García-Salinas, M.J. Influence of natural dyes in low-cost dye-sensitized solar cell’s efficiency. In Materials and Technologies for Energy Efficiency; Méndez-Vilas, A., Ed.; BrownWalker Press: Boca Ratón, FL, USA, 2015; pp. 48–52. [Google Scholar]
- Maldonado-Valdivia, A.I.; Galindo, E.G.; Ariza, M.J.; Garcia-Salinas, M.J. Surfactant influence in the performance of titanium dioxide photoelectrodes for dye-sensitized solar cells. Sol. Energy 2013, 91, 263–272. [Google Scholar] [CrossRef]
- Ito, S.; Kitamura, T.; Wada, Y.; Yanagida, S. Facile fabrication of mesoporous TiO2 electrodes for dye solar cells: Chemical modification and repetitive coating. Sol. Energy Mater. Sol. Cells 2003, 76, 3–13. [Google Scholar] [CrossRef]
- Ito, S.; Murakami, T.N.; Comte, P.; Liska, P.; Grätzel, C.; Nazeeruddin, M.K.; Grätzel, M. Fabrication of thin film dye sensitized solar cells with solar to electric power conversion efficiency over 10%. Thin Solid Films 2008, 516, 4613–4619. [Google Scholar] [CrossRef]
- Galindo, E.G.; Ariza, M.J.; De las Nieves, F.J.; García-Salinas, M.J. Effects of multilayer coating and calcination procedures on the morphology of dye-sensitized solar cell semiconductor photoelectrodes. Thin Solid Films 2015, 590, 230–240. [Google Scholar] [CrossRef]
- Syafinar, R.; Gomesh, N.; Irwanto, M.; Fareq, M.; Irwan, Y.M. Potential of Purple Cabbage, Coffee, Blueberry and Turmeric as Nature Based Dyes for Dye Sensitized Solar Cell (DSSC). Energy Procedia 2015, 79, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Belay, A.; Ture, K.; Redi, M.; Asfaw, A. Measurement of caffeine in coffee beans with UV/vis spectrometer. Food Chem. 2008, 108, 310–315. [Google Scholar] [CrossRef]
- Setiawan, T.; Subekti, W.Y.; Nur’Adya, S.S.; Ilmiah, K.; Ulfa, S.M. Performance of natural dye and counter electrode from robusta coffee beans peel waste for fabrication of dye-sensitized solar cell (DSSC). IOP Conf. Ser.: Mat. Sci. Eng. 2018, 299, 012073. [Google Scholar] [CrossRef]
- Kawase, M.; Takahashi, M. Chemical composition of sporopollenin in magnolia grandiflora (Magnoliaceae) and hibiscus syriacus (Malvaceae). Grana 1995, 34, 242–245. [Google Scholar] [CrossRef]
- Di Paola-Naranjo, R.D.; Sánchez-Sánchez, J.; González-Paramás, A.M.; Rivas-Gonzalo, J.C. Liquid chromatographic–mass spectrometric analysis of anthocyanin composition of dark blue bee pollen from Echium plantagineum. J. Chromatogr. A 2004, 1054, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Urbanczyk, J.; Fernandez-Casado, M.A.; Díaz, T.E.; Heras, P.; Infante, M.; Borrego, A.G. Spectral fluorescence variation of pollen and spores from recent peat-forming plants. Int. J. Coal Geol. 2014, 131, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Mularczyk-Oliwa, M.; Bombalska, A.; Kaliszewski, M.; Włodarski, M.; Trafny, A.; Kopczyński, K.; Kwaśny, M.; Szpakowska, M.; Zbieta, E. Comparison of fluorescence spectroscopy and FTIR in differentiation of plant pollens. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Calogero, G.; Di Marco, G.; Caramori, S.; Cazzanti, S.; Argazzi, R.; Bignozzi, C.A. Natural dye sensitizers for photoelectrochemical cells. Energy Environ. Sci. 2009, 2, 1162–1172. [Google Scholar] [CrossRef]
- Shanmugam, V.; Manoharan, S.; Anandan, S.; Murugan, R. Performance of dye-sensitized solar cells fabricated with extracts from fruits of ivy gourd and flowers of red frangipani as sensitizers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 104, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Gholamrezaei, S.; Salavati-Niasari, M. Natural sensitizer for low cost dye sensitized solar cell based on Strontium Titanate nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 27, 2467–2472. [Google Scholar] [CrossRef]
- Sashank, T.V.S.S.P.; Manikanta, B.; Pasula, A. Fabrication and experimental investigation on dye sensitized solar cells using titanium dioxide nano particles. Mater. Today Proc. 2017, 4, 3918–3925. [Google Scholar] [CrossRef]
- Liao, S.K.; Chang, Y.H.; Wu, C.T.; Lai, Y.R.; Chen, W.Y. Fabrication of anthocyanin-sensitized nanocrystalline titanium dioxide solar cells using supercritical carbon dioxide. J. CO2 Util. 2017, 21, 513–520. [Google Scholar] [CrossRef]
- Teoli, F.; Lucioli, S.; Nota, P.; Frattarelli, A.; Matteocci, F.; Di Carlo, A.; Caboni, E.; Forni, C. Role of pH and pigment concentration for natural dye-sensitized solar cells treated with anthocyanin extracts of common fruits. J. Photoch. Photobio. A 2016, 316, 24–30. [Google Scholar] [CrossRef]
- Mahmoud, A.M.A.A.; Mohamad, A.B.; Kadhum, A.A.H.; Ludin, N.A. Effect of solvents on the extraction of natural pigments and adsorption onto TiO2 for dye-sensitized solar cell applications. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 130–137. [Google Scholar] [CrossRef]
- Maabong, K.; Muiva, C.M.; Monowe, P.; Sathiaraj, T.S.; Hopkins, M.; Nguyen, L.; Malungwa, K.; Thobega, M. Natural pigments as photosensitizers for dye-sensitized solar cells with TiO2 thin films. Int. J. Renew. Sustain. Energy Res. 2015, 5, 54–60. [Google Scholar]
- Isah, K.U.; Ahmadu, U.; Idris, A.; Kimpa, M.I.; Uno, U.E.; Ndamitso, M.M.; Alu, N. Betalain pigments as natural photosensitizers for dye-sensitized solar cells: The effect of dye pH on the photoelectric parameters. Mater. Renew. Sustain. Energy 2015, 4, 39. [Google Scholar] [CrossRef]
- Kumar, S.N.A.; Ritesh, S.K.; Sharmila, G.; Muthukumaran, C. Extraction optimization and characterization of water soluble red purple pigment from floral bracts of Bougainvillea glabra. Arab. J. Chem. 2017, 10, 2145–2150. [Google Scholar] [CrossRef]
- Damit, D.N.F.P.; Galappaththi, K.; Lim, A.; Petra, M.I.; Ekanayake, P. Formulation of water to ethanol ratio as extraction solvents of Ixora coccinea and Bougainvillea glabra and their effect on dye aggregation in relation to DSSC performance. Ionics 2017, 23, 485–495. [Google Scholar] [CrossRef]
- Hernandez-Martinez, A.R.; Estevez, M.; Vargas, S.; Quintanilla, F.; Rodriguez, R. New dye-sensitized solar cells obtained from extracted bracts of bougainvillea Glabra and Spectabilis Betalain Pigments by Different Purification Processes. Int. J. Mol. Sci. 2011, 12, 5565–5576. [Google Scholar] [CrossRef]
- Oprea, C.I.; Dumbravă, A.; Enache, I.; Georgescu, A.; Gîrţu, M.A. A combined experimental and theoretical study of natural betalain pigments used in dye-sensitized solar cells. J. Photochem. Photobio. A 2012, 240, 5–13. [Google Scholar] [CrossRef]
- Shanmugam, V.; Manoharan, S.; Sharafali, A.; Anandan, S.; Murugan, R. Green grasses as light harvesters in dye sensitized solar cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 135, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Upadhyay, R.; Pandey, A. Novel dye based photoelectrode for improvementof solar cell conversion efficiency. Appl. Sol. Energy 2013, 49, 54–57. [Google Scholar] [CrossRef]
- Sengupta, D.; Mondal, B.; Mukherjee, K. Visible light absorption and photo-sensitizing properties of spinach leaves and beetroot extracted natural dyes. Spectrochim. Acta A 2015, 148, 85–92. [Google Scholar] [CrossRef]
- Hemmatzadeh, R.; Mohammadi, A. Improving optical absorptivity of natural dyes for fabrication of efficient dye-sentitized solar cells. J. Theor. Appl. Phys. 2013, 7, 57. [Google Scholar] [CrossRef]
- Senthil, T.S.; Muthukumarasamy, N.; Velauthapillai, D.; Agilan, S.; Thambidurai, M.; Balasundaraprabhu, R. Natural dye (cyaniding 3-O-glucoside) sensitized nanocrystalline TiO2 solar cell fabricated using liquid electrolyte/quasi-solid-state polymer electrolyte. Renew. Energy 2011, 36, 2484–2488. [Google Scholar] [CrossRef]
- Calogero, G.; Yum, J.H.; Sinopoli, A.; Di Marco, G.; Grätzel, M.; Nazeeruddin, M.K. Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Sol. Energy 2012, 86, 1563–1575. [Google Scholar] [CrossRef]
- Hemalatha, K.V.; Karthick, S.N.; Justin-Raj, C.; Hong, N.Y.; Kim, S.K.; Kim, H.-J. Performance of Kerria japonica and Rosa chinensis flower dyes as sensitizers for dye-sensitized solar cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 96, 305–309. [Google Scholar] [CrossRef]
- Torchani, A.; Gharbi, R.; Fathallah, M. Study of natural dyes for sensitized solar cells applications. Sens. Transducers 2014, 27, 185–189. [Google Scholar]
- Suyitno, S.; Saputra, T.J.; Supriyanto, A.; Arifin, Z. Stability and efficiency of dye-sensitized solar cells based on papaya-leaf dye. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 148, 99–104. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, T.Y.; Park, J.Y.; Jin, E.M.; Yim, S.H.; Choi, D.Y.; Lee, J.W. Adsorption characteristics of gardenia yellow as natural photosensitizer for dye-sensitized solar cells. Dyes Pigments 2013, 96, 595–601. [Google Scholar] [CrossRef]
- Chang, H.; Kao, M.J.; Chen, T.L.; Fuo, H.G.; Choand, K.C.; Lin, X.P. Natural sensitizer for dye-sensitized solar cells using three layers of photoelectrode thin films with a Schottky barrier. Am. J. Eng. Appl. Sci. 2011, 4, 214–222. [Google Scholar] [CrossRef]
- Kimpa, M.I.; Momoh, M.; Isah, K.U.; Yahya, H.N.; Ndamitso, M.M. Photoelectric characterization of dye sensitized solar cells using natural dye from pawpaw leaf and flame tree flower as sensitizers. Mater. Sci. Appl. 2012, 3, 281–286. [Google Scholar] [CrossRef]
- Kumar, K.A.; Subalakshmi, K.; Senthilselvan, J. Effect of mixed valence state of titanium on reduced recombination for natural dye-sensitized solar cell applications. J. Solid State Electrochem. 2016, 20, 1921–1932. [Google Scholar] [CrossRef]
- Chang, H.; Lo, Y.J. Pomegranate leaves and mulberry fruit as natural sensitizers for dye-sensitized solar cells. Sol. Energy 2010, 84, 1833–1837. [Google Scholar] [CrossRef]
- Wongcharee, K.; Meeyoo, V.; Chavadej, S. Dye-sensitized solar cell using natural dyes extracted from rosella and blue pea flowers. Sol. Energy Mater. Sol. Cell 2007, 91, 566–571. [Google Scholar] [CrossRef]
- Torchani, A.; Saadaoui, S.; Gharbi, R.; Fathallah, M. Sensitized solar cells based on natural dyes. Curr. Appl. Phys. 2015, 15, 307–312. [Google Scholar] [CrossRef]
- Hernandez-Martinez, A.R.; Estevez, M.; Vargas, S.; Rodriguez, R. Stabilized conversion efficiency and dye-sensitized solar cells from beta vulgaris pigment. Int. J. Mol. Sci. 2013, 14, 4081–4093. [Google Scholar] [CrossRef]
- Galindo, E.G. Estudio de la Dinámica de Liquidos Ionicos Confinados en Medios Nanoporosos Mediante Experimentos y Simulaciones. Aplicación a las Celulas Solares de Colorante. Ph.D. Thesis, University of Almeria, Almeria, Spain, 2015. [Google Scholar]
- Askar, K.A.; Alsawad, Z.H.; Khalaf, M.N. Evaluation of the pH and thermal stabilities of rosella anthocyanin extracts under solar light. Beni Suef Univ. J. Basic Appl. Sci. 2015, 4, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Norman, M.; Bartczak, P.; Zdarta, J.; Ehrlich, H.; Jesionowski, T. Anthocyanin dye conjugated with Hippospongia communis marine demosponge skeleton and its antiradical activity. Dyes Pigments 2016, 134, 541–552. [Google Scholar] [CrossRef]
- Calogero, G.; Di Marco, G. Red Sicilian orange and purple eggplant fruits as natural sensitizers for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cell 2008, 92, 1341–1346. [Google Scholar] [CrossRef]
- Vankar, P.S.; Shukla, D. Natural Dyeing with Anthocyanins from Hibiscus rosa sinensis Flowers. J. Appl. Polym. Sci. 2011, 122, 3361–3368. [Google Scholar] [CrossRef]
- Zhang, D.; Lanier, S.M.; Downing, J.A.; Avent, J.L.; Lum, J.; McHale, J.L. Betalain pigments for dye-sensitized solar cells. J. Photochem. Photobiol. A 2008, 195, 72–80. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, sources, applications and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Chien, C.Y.; Hsu, B.D. Optimization of the dye-sensitized solar cell with anthocyanin as photosensitizer. Sol. Energy 2013, 98, 203–211. [Google Scholar] [CrossRef]









| Extraction Method | Acetone (% vol.) | Ethanol (% vol.) | HCl (M) | H2O (% vol.) | pH Range |
|---|---|---|---|---|---|
| Solution 1 | 32 | --- | --- | 68 | 5.0–5.6 |
| Solution 2 | --- | Solvent | 0.1 | --- | 1.6–1.7 |
| Solution 3 | --- | --- | 0.1 | Solvent | 1.3–1.4 |
| Solution 4 | --- | 80 | --- | 20 | 6.5–7.0 |
| Spectrum (Figure) | Peak Type | λ (nm) | Abs |
|---|---|---|---|
| N719 (3) | Peak 1 | 405 | 1.03 |
| Peak 2 | 553 | 0.96 | |
| Yellow BG (3) | Shoulder 1 | 455 | 1.10 |
| Peak 1 | 478 | 1.21 | |
| Shoulder 2 | 543 | 0.12 | |
| Orange BG (3) | Shoulder 1 | 455 | 1.01 |
| Peak 1 | 478 | 1.08 | |
| Shoulder 2 | 543 | 0.16 | |
| Pink BG (3) | Shoulder 1 | 451 | 0.74 |
| Peak 1 | 482 | 0.88 | |
| Peak 2 | 535 | 0.74 | |
| Purple BG (3) | Shoulder 1 | 454 | 0.40 |
| Peak 1 | 484 | 0.47 | |
| Peak 2 | 535 | 0.52 | |
| Pink BG bract (4) | Shoulder 1 | 487 | 0.72 |
| Peak 2 | 547 | 0.79 | |
| Peak 3 | 674 | 0.13 | |
| Orange BG bract (4) | Shoulder 1 | 451 | 0.76 |
| Peak 1 | 484 | 0.81 | |
| Shoulder 2 | 553 | 0.54 | |
| Peak 3 | 674 | 0.19 | |
| Beetroot 1 (5) | Peak 1 | 526 | 0.62 |
| Beetroot 2 (5) | Peak 1 | 519 | 0.24 |
| Beetroot 3 (5) | Peak 1 | 516 | 0.56 |
| Peak 1 | [Betaxanthins] | Peak 2 | [Betanin] | Nϕ | |||
|---|---|---|---|---|---|---|---|
| Abs | μM | (%) | Abs | μM | (%) | (%) | |
| Purple BG | 0.47 | 7.2 | 47.3 | 0.52 | 8.0 | 52.7 | 34.7 |
| Pink BG | 0.88 | 15.2 | 57.3 | 0.74 | 11.4 | 42.7 | 53.2 |
| Orange BG | 1.08 | 24.5 | 90.9 | 0.16 | 2.5 | 9.1 | 35.0 |
| Yellow BG | 1.21 | 27.9 | 93.8 | 0.12 | 1.8 | 6.2 | 38.7 |
| DSSC Dye | ηav (%) | ηmax (%) | FFav | FFmax |
|---|---|---|---|---|
| N-719 | 2.3 ± 0.5 | 3.235 | 0.44 ± 0.08 | 0.553 |
| Eggplant 2 [37] | 0.090 ± 0.006 | 0.101 | 0.51 ± 0.06 | 0.623 |
| Eggplant 3 [37] | 0.069 ± 0.011 | 0.083 | 0.54 ± 0.02 | 0.568 |
| Pink bougainvillea | 0.19 ± 0.07 | 0.250 | 0.51 ± 0.08 | 0.595 |
| Orange Bougainvillea | 0.19 ± 0.02 | 0.215 | 0.427 ± 0.013 | 0.448 |
| Concentrated orange BG | 0.188 ± 0.012 | 0.202 | 0.521 ± 0.016 | 0.542 |
| Yellow Bougainvillea | 0.162 ± 0.017 | 0.185 | 0.39 ± 0.04 | 0.451 |
| Beetroot 1 | 0.32 ± 0.12 | 0.467 | 0.41 ± 0.08 | 0.469 |
| Beetroot 2 | 0.08 ± 0.04 | 0.112 | 0.35 ± 0.06 | 0.414 |
| Beetroot 3 | 0.17 ± 0.06 | 0.229 | 0.35 ± 0.03 | 0.392 |
| 1:1 Orange BG + Beetroot 1 | 0.269 ± 0.004 | 0.274 | 0.55 ± 0.02 | 0.563 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Salinas, M.J.; Ariza, M.J. Optimizing a Simple Natural Dye Production Method for Dye-Sensitized Solar Cells: Examples for Betalain (Bougainvillea and Beetroot Extracts) and Anthocyanin Dyes. Appl. Sci. 2019, 9, 2515. https://doi.org/10.3390/app9122515
García-Salinas MJ, Ariza MJ. Optimizing a Simple Natural Dye Production Method for Dye-Sensitized Solar Cells: Examples for Betalain (Bougainvillea and Beetroot Extracts) and Anthocyanin Dyes. Applied Sciences. 2019; 9(12):2515. https://doi.org/10.3390/app9122515
Chicago/Turabian StyleGarcía-Salinas, María José, and María Jesús Ariza. 2019. "Optimizing a Simple Natural Dye Production Method for Dye-Sensitized Solar Cells: Examples for Betalain (Bougainvillea and Beetroot Extracts) and Anthocyanin Dyes" Applied Sciences 9, no. 12: 2515. https://doi.org/10.3390/app9122515