Impact of Aeration on the Removal of Organic Matter and Nitrogen Compounds in Constructed Wetlands Treating the Liquid Fraction of Piggery Manure
Abstract
:1. Introduction
2. Materials and Methods
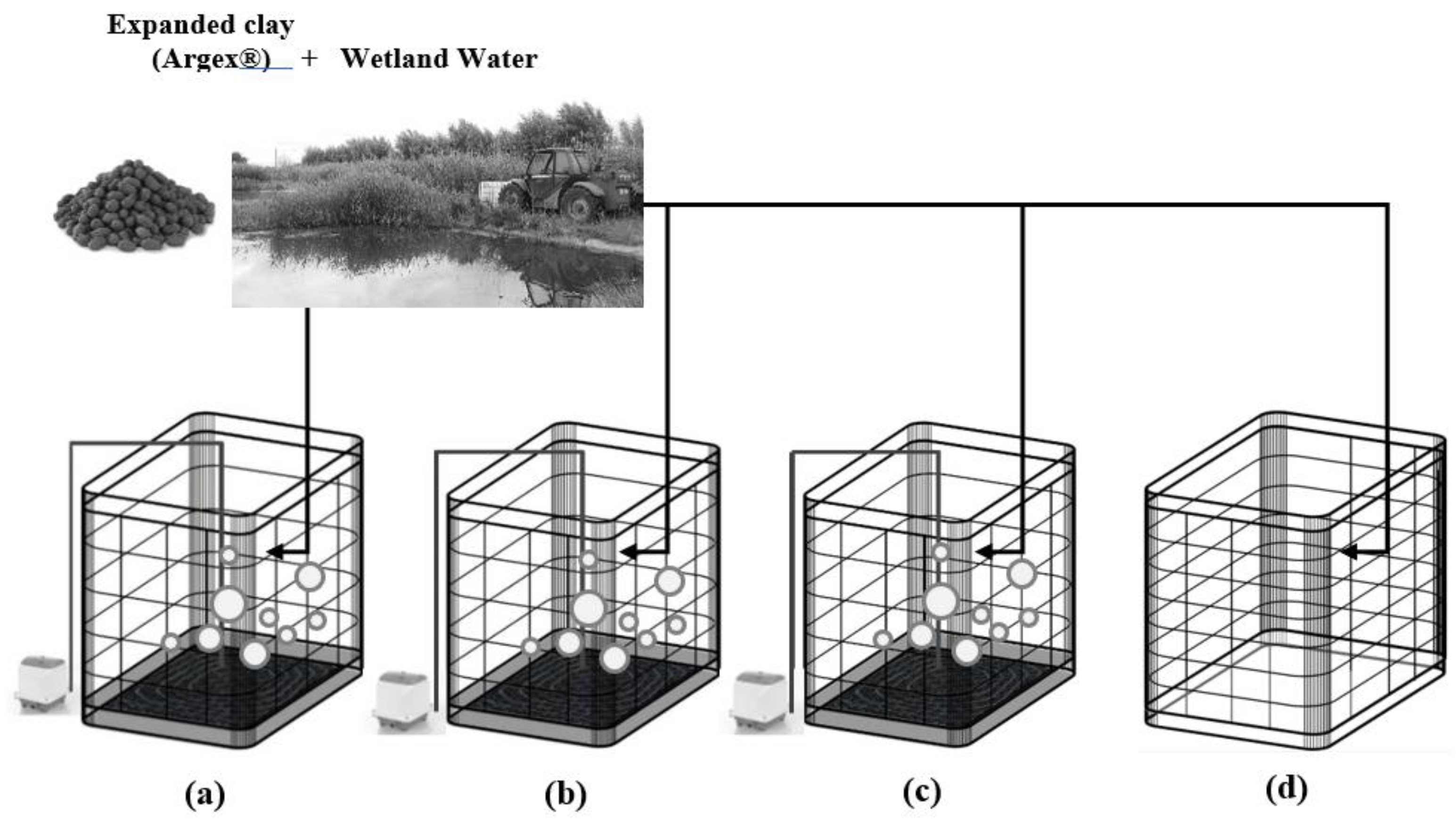
2.1. Experimental Setup
2.2. Physicochemical Analyses
2.3. Hypotheses Testing Through a Mixed Model Procedure
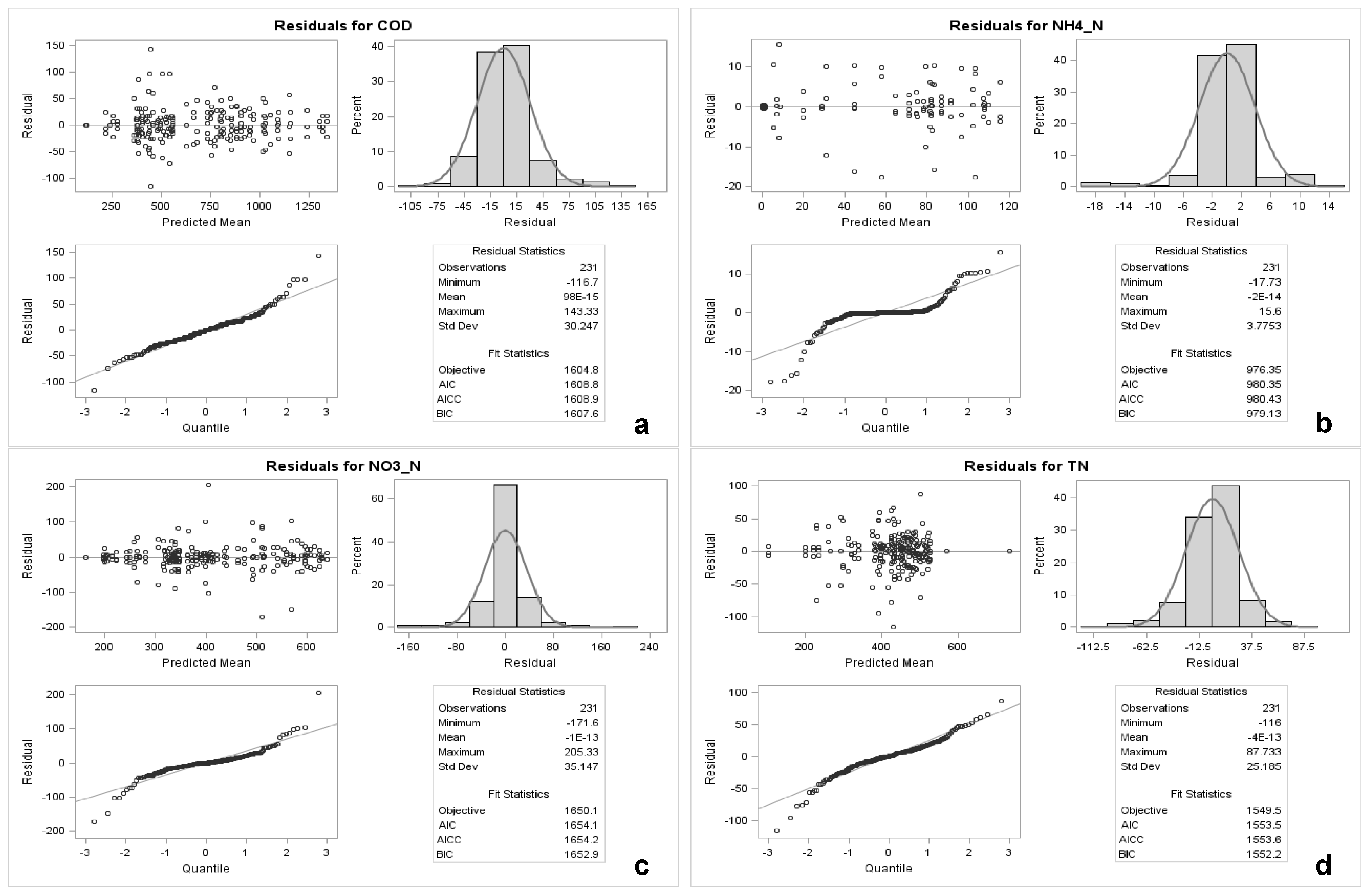
3. Results
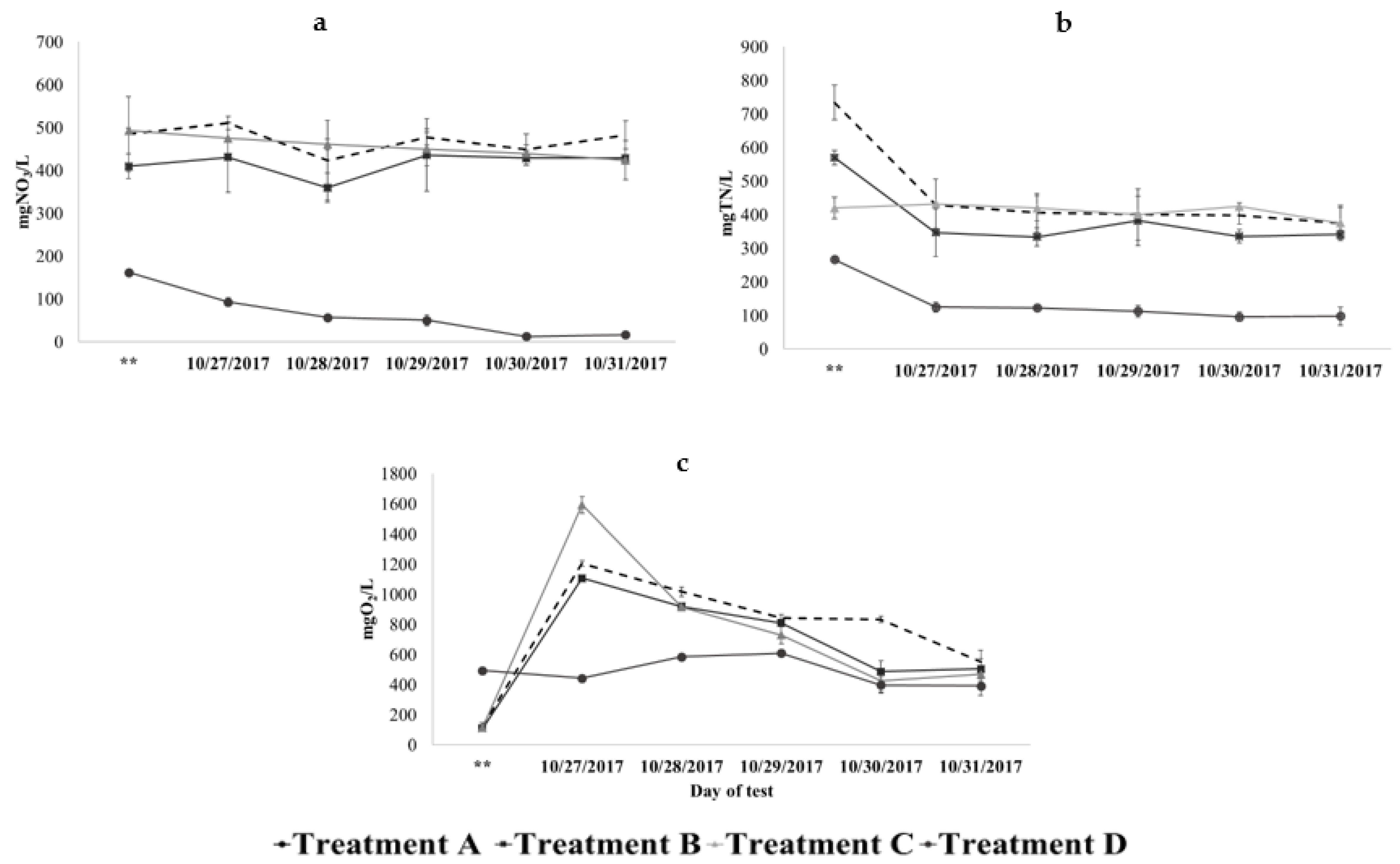
3.1. First Stage: Testing Ammonium Volatilisation
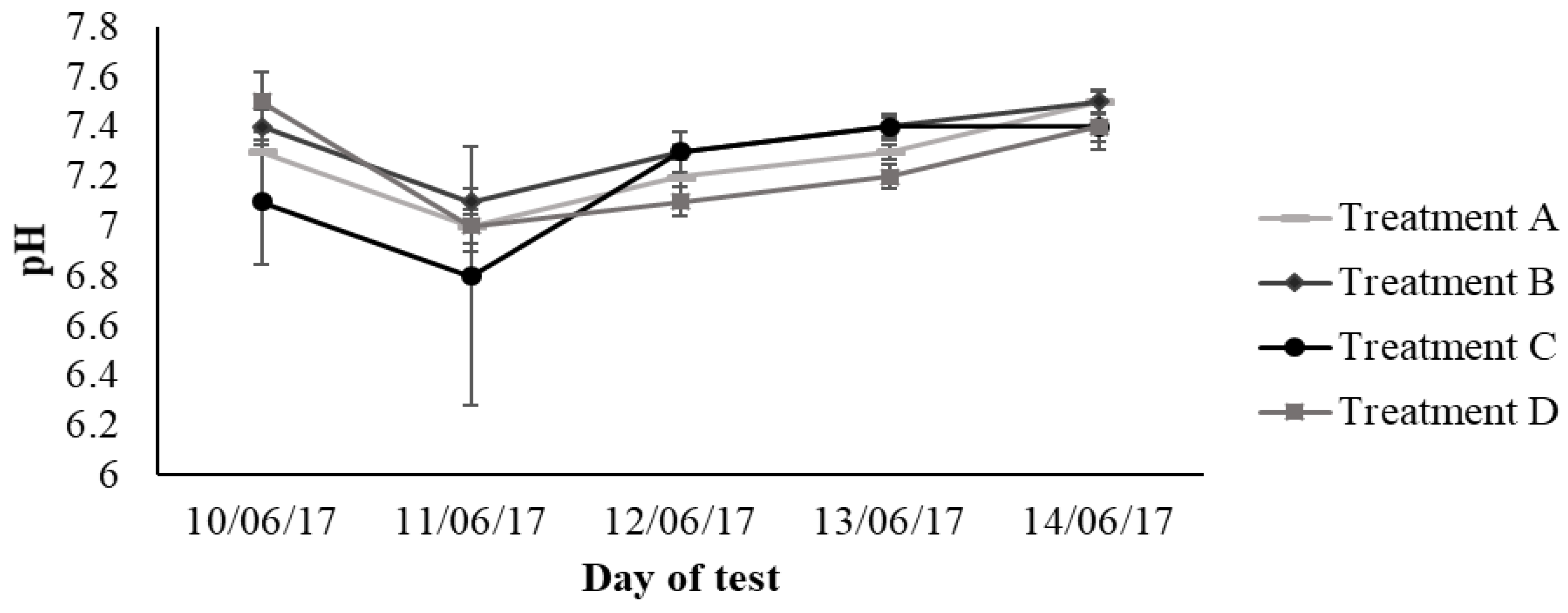
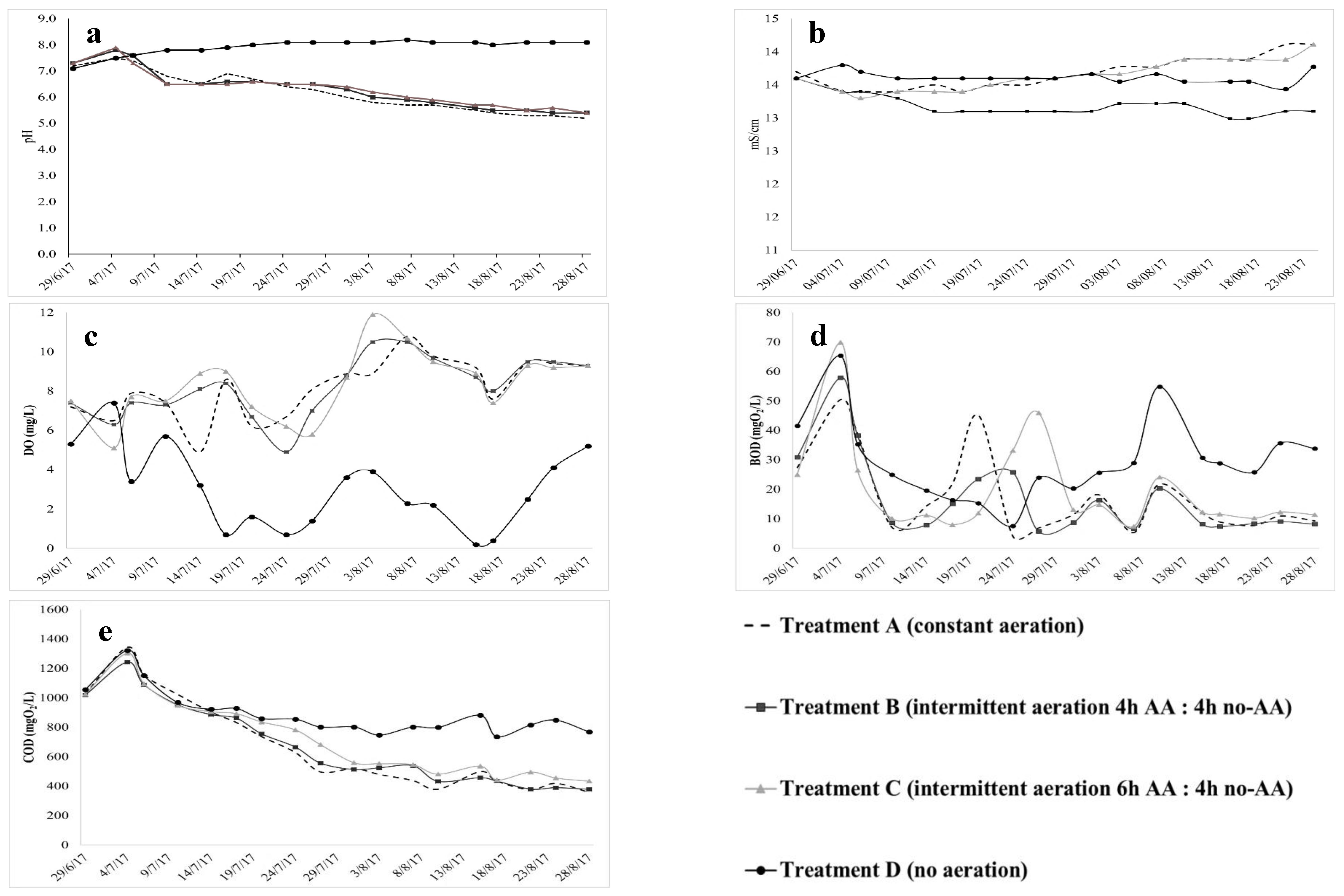
3.2. Second Stage: Evolution of Physicochemical Parameters, Organic and Nitrogen Compound Concentrations under Different Aeration Regimes
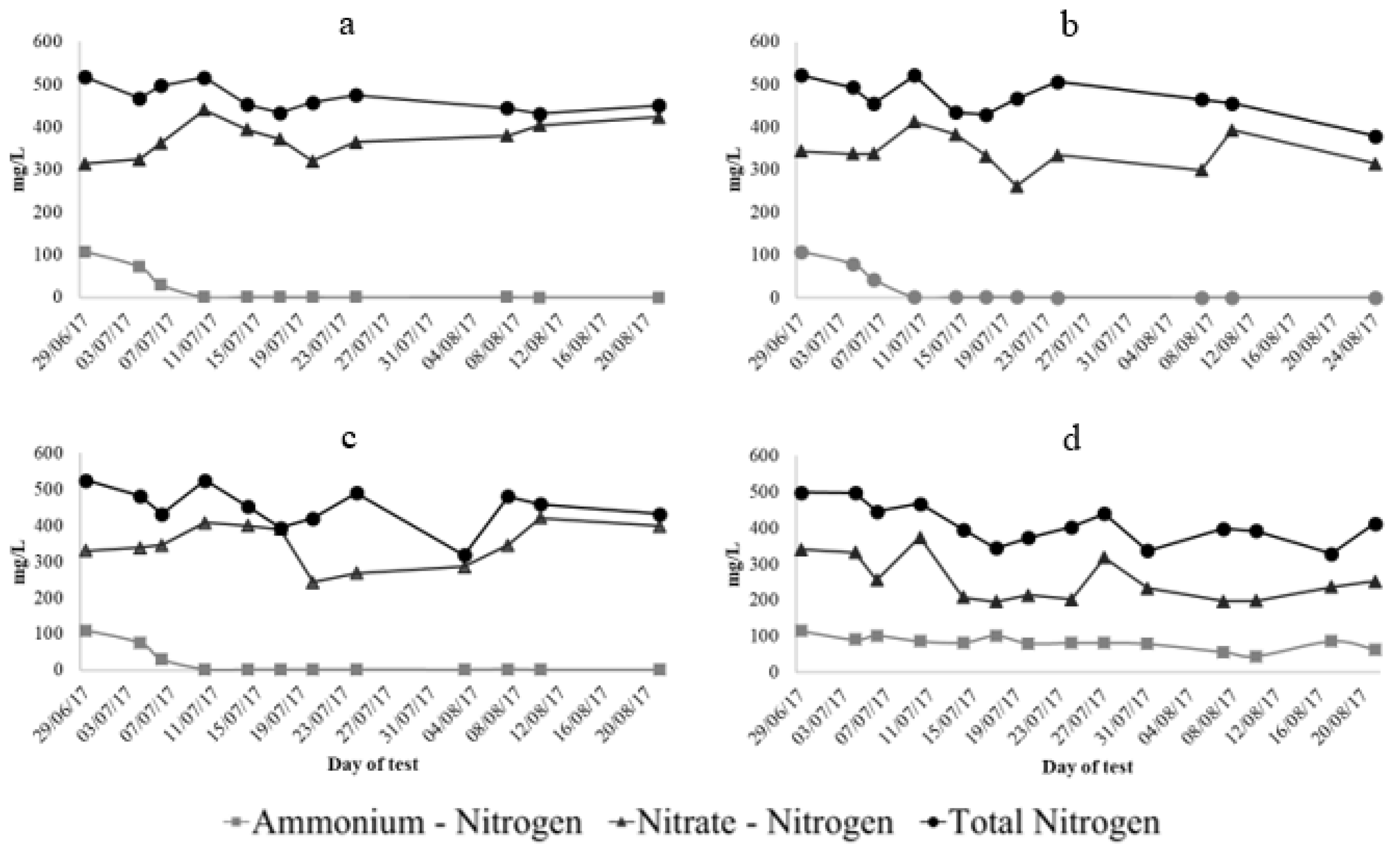
 ) measurements showed great variability. In the case of NO3−-N, the variation amongst the measurements could be attributed to the different analytical methods used. The test kits were used to determine TN, whereas, NO3−-N and NH4+-N were determined by ion chromatography. The high dilution factors were needed to analyse the samples by ion chromatography due to high sensitivity and low detection limits of the equipment. Considering that this fact could have increased the error or variation in the analysis, the results were corroborated and some samples re-analysed using quick test kits. The inconsistent NO3−-N values evidenced through the comparison with the TN concentration, and after testing similar methodologies, were considered as outliers and removed from the dataset. The overall tendency indicates that NO3−-N concentrations decreased only in treatment D from 341 mg/L to 223 mg/L, indicating a removal of 35%. The estimated effects of this treatment supported that nitrate concentrations significantly decreased (p < 0.05) in the non-aerated container. The ammonium concentrations started to decrease significantly after a one-week period of the second stage of the experiment (Figure 5
) measurements showed great variability. In the case of NO3−-N, the variation amongst the measurements could be attributed to the different analytical methods used. The test kits were used to determine TN, whereas, NO3−-N and NH4+-N were determined by ion chromatography. The high dilution factors were needed to analyse the samples by ion chromatography due to high sensitivity and low detection limits of the equipment. Considering that this fact could have increased the error or variation in the analysis, the results were corroborated and some samples re-analysed using quick test kits. The inconsistent NO3−-N values evidenced through the comparison with the TN concentration, and after testing similar methodologies, were considered as outliers and removed from the dataset. The overall tendency indicates that NO3−-N concentrations decreased only in treatment D from 341 mg/L to 223 mg/L, indicating a removal of 35%. The estimated effects of this treatment supported that nitrate concentrations significantly decreased (p < 0.05) in the non-aerated container. The ammonium concentrations started to decrease significantly after a one-week period of the second stage of the experiment (Figure 5  ). A full NH4+-N removal was obtained in treatment A, B and C, whereas, a lower removal efficiency of 35% was achieved in treatment D. Looking at Figure 5
). A full NH4+-N removal was obtained in treatment A, B and C, whereas, a lower removal efficiency of 35% was achieved in treatment D. Looking at Figure 5  , it can be stated that there is no clear distinction among the effects of aeration on TN concentrations between treatments. However, the 55% of TN removal achieved by treatment D was just slightly above the 50% removal at treatment A. By assessing the whole testing period through the model procedure estimate effects, the results corroborated that statistically significant effects on the reduction of TN concentrations were mostly recorded in the non-aerated container, and a few other occasions when intermittent aeration, as in treatment C, was applied.
, it can be stated that there is no clear distinction among the effects of aeration on TN concentrations between treatments. However, the 55% of TN removal achieved by treatment D was just slightly above the 50% removal at treatment A. By assessing the whole testing period through the model procedure estimate effects, the results corroborated that statistically significant effects on the reduction of TN concentrations were mostly recorded in the non-aerated container, and a few other occasions when intermittent aeration, as in treatment C, was applied.3.3. Third Stage: Intensification of Nitrification and Denitrification Processes by the Addition of a Supplementary Carbon Source
4. Discussion
4.1. Changes in Physicochemical Profile under Different Aeration Regimes
4.2. Hypotheses Testing Through a Mixed Model Procedure
4.3. Addition of Carbon Source to Enhance The Denitrification Process
4.4. Moving Towards Sustainable Concepts
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| pH | EC | DO | NH4+-N | NO3−-N | TN | COD | BOD | |
|---|---|---|---|---|---|---|---|---|
| mS/cm | mg/L | mg/L | mg/L | mg/L | mgO2/L | mgO2/L | ||
| Treatment A | ||||||||
| Initial conc. | 7.2 | 7.2 | 14 | 107 | 314 | 518 | 1027 | 27 |
| Std Dev | ±0.06 | ±0.06 | - | ±2.3 | ±40 | ±8.9 | ±15 | ±3.6 |
| Final conc. | 5.2 | 9.3 | 14 | 0.3 | 476 | 257 | 358 | 9.4 |
| Std Dev | ±0.06 | ±0.21 | ±0.06 | 0.1 | ±20 | ±47 | ±44 | ±1.2 |
| % Removal | 99 | -52 | 50 | 65 | 66 | |||
| Treatment B | ||||||||
| Initial conc. | 7.3 | 7.4 | 14 | 108 | 344 | 522 | 1019 | 31 |
| Std Dev | - | ±0.06 | ±0.06 | ±0.8 | ±30 | ±25 | ±50 | ±6.1 |
| Final conc. | 5.4 | 9.3 | 13 | 0.3 | 435 | 298 | 379 | 8.3 |
| Std Dev | ±0.26 | ±0.20 | ±0.06 | ±0.2 | ±17 | ±41 | ±76 | ±2.6 |
| % Removal | 99 | -27 | 43 | 63 | 73 | |||
| Treatment C | ||||||||
| Initial conc. | 7.3 | 7.5 | 14 | 110 | 331 | 525 | 1026 | 25 |
| Std Dev | - | ±0.12 | ±0.10 | ±3.7 | ±43 | ±15 | ±45 | ±9.5 |
| Final conc. | 5.4 | 9.3 | 14 | 0.3 | 463 | 291 | 434 | 12 |
| Std Dev | ±0.06 | ±0.20 | - | ±0.1 | ±22 | ±53 | ±57 | ±0.8 |
| % Removal | 99 | -49 | 45 | 58 | 54 | |||
| Treatment D | ||||||||
| Initial conc. | 7.1 | 5.3 | 14 | 116 | 341 | 499 | 1057 | 42 |
| Std Dev | ±0.06 | ±0.06 | ±0.12 | ±5.4 | ±16 | ±11 | ±32 | ±2.0 |
| Final conc. | 8.1 | 5.2 | 14 | 75 | 223 | 222 | 768 | 34 |
| Std Dev | ±0.06 | ±1.0 | ±0.06 | ±2.3 | ±13 | ±65 | ±61 | ±8.5 |
| % Removal | 35 | 35 | 55 | 27 | 19 |
Appendix B
| Treatment | Sampling Day | Temp | pH | DO | EC | NH4+-N | NO3−-N | TN | COD | BOD |
|---|---|---|---|---|---|---|---|---|---|---|
| °C | mgO2/L | mS/cm | mg/L | mg/L | mg/L | mg/L | mg/L | |||
| Treatment A | 29/6/17 | 20 | 7.2 | 7.2 | 14 | 107 | 314 | 518 | 1027 | 27 |
| 4/7/17 | 20 | 7.5 | 6.5 | 13 | 72 | 324 | 468 | 1343 | 51 | |
| 6/7/17 | 20 | 7.4 | 7.9 | 13 | 30 | 362 | 497 | 1157 | 39 | |
| 10/7/17 | 19 | 6.8 | 7.4 | 13 | 1.3 | 440 | 517 | 1023 | 7.1 | |
| 14/7/17 | 22 | 6.5 | 4.9 | 14 | 1.3 | 394 | 452 | 903 | 15 | |
| 17/7/17 | 23 | 6.9 | 8.6 | 13 | 1.0 | 371 | 433 | 832 | 22 | |
| 20/7/17 | 21 | 6.7 | 6.2 | 14 | 0.7 | 320 | 457 | 737 | 45 | |
| 24/7/17 | 21 | 6.4 | 6.7 | 14 | 0.5 | 364 | 475 | 629 | 4.1 | |
| 27/7/17 | 20 | 6.3 | 8.1 | 14 | 0.4 | 589 | 484 | 497 | 7.0 | |
| 31/7/17 | 19 | 6.0 | 8.9 | 14 | 0.6 | 539 | 509 | 520 | 12 | |
| 3/8/17 | 20 | 5.8 | 8.9 | 14 | 0.3 | 639 | 481 | 480 | 18 | |
| 7/8/17 | 20 | 5.7 | 11 | 14 | 0.7 | 379 | 444 | 438 | 5.4 | |
| 10/8/17 | 19 | 5.7 | 9.8 | 14 | 0.2 | 404 | 431 | 379 | 22 | |
| 15/8/17 | 21 | 5.5 | 9.2 | 14 | 0.2 | 580 | 429 | 499 | 12 | |
| 17/8/17 | 23 | 5.4 | 7.6 | 14 | 0.3 | 605 | 454 | 434 | 9.0 | |
| 21/8/17 | 21 | 5.3 | 9.5 | 14 | 0.3 | 558 | 414 | 377 | 7.8 | |
| 24/8/17 | 20 | 5.3 | 9.4 | 14 | 0.3 | 493 | 422 | 419 | 11 | |
| 28/8/17 | 22 | 5.2 | 9.3 | 14 | 0.3 | 476 | 257 | 358 | 9.4 | |
| Treatment B | 29/6/17 | 19 | 7.3 | 7.4 | 14 | 108 | 344 | 522 | 1019 | 31 |
| 4/7/17 | 21 | 7.8 | 6.3 | 13 | 78 | 337 | 493 | 1243 | 58 | |
| 6/7/17 | 19 | 7.6 | 7.4 | 13 | 43 | 338 | 455 | 1090 | 38 | |
| 10/7/17 | 18 | 6.5 | 7.3 | 13 | 1.4 | 413 | 522 | 953 | 8.7 | |
| 14/7/17 | 23 | 6.5 | 8.1 | 13 | 0.8 | 383 | 434 | 886 | 8.0 | |
| 17/7/17 | 23 | 6.6 | 8.4 | 13 | 1.0 | 332 | 430 | 863 | 15 | |
| 20/7/17 | 21 | 6.6 | 6.7 | 13 | 0.9 | 261 | 467 | 756 | 24 | |
| 24/7/17 | 19 | 6.5 | 4.9 | 13 | 0.6 | 334 | 507 | 666 | 26 | |
| 27/7/17 | 19 | 6.5 | 7.0 | 13 | 0.6 | 572 | 489 | 557 | 5.8 | |
| 31/7/17 | 19 | 6.3 | 8.8 | 13 | 0.2 | 495 | 467 | 513 | 8.8 | |
| 3/8/17 | 20 | 6.0 | 11 | 13 | 0.3 | 596 | 471 | 525 | 16 | |
| 7/8/17 | 19 | 5.9 | 11 | 13 | 0.4 | 299 | 466 | 539 | 6.7 | |
| 10/8/17 | 19 | 5.8 | 9.7 | 13 | 0.2 | 393 | 456 | 433 | 20 | |
| 15/8/17 | 20 | 5.6 | 8.7 | 13 | 0.3 | 557 | 381 | 457 | 8.2 | |
| 17/8/17 | 23 | 5.5 | 8.0 | 13 | 0.3 | 532 | 412 | 434 | 7.5 | |
| 21/8/17 | 21 | 5.5 | 9.5 | 13 | 0.3 | 555 | 399 | 380 | 8.5 | |
| 24/8/17 | 20 | 5.4 | 9.5 | 13 | 0.3 | 514 | 444 | 389 | 9.2 | |
| 28/8/17 | 21 | 5.4 | 9.3 | 13 | 0.3 | 435 | 298 | 379 | 8.3 | |
| Treatment C | 29/6/17 | 20 | 7.3 | 7.5 | 14 | 110 | 331 | 525 | 1026 | 25 |
| 4/7/17 | 21 | 7.9 | 5.1 | 13 | 75 | 339 | 482 | 1303 | 70 | |
| 6/7/17 | 20 | 7.3 | 7.7 | 13 | 29 | 346 | 432 | 1097 | 26 | |
| 10/7/17 | 19 | 6.5 | 7.5 | 13 | 1.6 | 408 | 525 | 950 | 10 | |
| 14/7/17 | 23 | 6.5 | 8.9 | 13 | 0.8 | 399 | 452 | 906 | 11 | |
| 17/7/17 | 23 | 6.5 | 9.0 | 13 | 1.0 | 390 | 386 | 893 | 8.1 | |
| 20/7/17 | 21 | 6.6 | 7.2 | 14 | 1.0 | 244 | 420 | 837 | 12 | |
| 24/7/17 | 21 | 6.5 | 6.2 | 14 | 0.9 | 269 | 490 | 783 | 33 | |
| 27/7/17 | 20 | 6.5 | 5.8 | 14 | 0.7 | 513 | 514 | 683 | 46 | |
| 31/7/17 | 19 | 6.4 | 8.7 | 14 | 1.1 | 614 | 487 | 560 | 13 | |
| 3/8/17 | 20 | 6.2 | 12 | 14 | 0.4 | 625 | 473 | 553 | 15 | |
| 7/8/17 | 19 | 6.0 | 11 | 14 | 1.6 | 347 | 481 | 547 | 7.5 | |
| 10/8/17 | 19 | 5.9 | 9.5 | 14 | 0.2 | 421 | 460 | 482 | 24 | |
| 15/8/17 | 20 | 5.7 | 8.9 | 14 | 0.3 | 599 | 423 | 537 | 12 | |
| 17/8/17 | 23 | 5.7 | 7.4 | 14 | 0.3 | 340 | 451 | 443 | 12 | |
| 21/8/17 | 21 | 5.5 | 9.3 | 14 | 0.4 | 625 | 424 | 496 | 10 | |
| 24/8/17 | 20 | 5.6 | 9.2 | 14 | 0.3 | 510 | 497 | 456 | 12 | |
| 28/8/17 | 21 | 5.4 | 9.3 | 14 | 0.3 | 463 | 291 | 434 | 12 | |
| Treatment D | 29/6/17 | 20 | 7.1 | 5.3 | 14 | 116 | 341 | 499 | 1056 | 42 |
| 4/7/17 | 22 | 7.5 | 7.4 | 14 | 91 | 332 | 498 | 1323 | 65 | |
| 6/7/17 | 20 | 7.6 | 3.4 | 14 | 102 | 256 | 446 | 1153 | 35 | |
| 10/7/17 | 19 | 7.8 | 5.7 | 14 | 87 | 375 | 468 | 970 | 25 | |
| 14/7/17 | 22 | 7.8 | 3.2 | 14 | 83 | 328 | 387 | 923 | 20 | |
| 17/7/17 | 23 | 7.9 | 0.7 | 14 | 102 | 346 | 385 | 929 | 17 | |
| 20/7/17 | 21 | 8.0 | 1.6 | 14 | 81 | 214 | 373 | 860 | 15 | |
| 24/7/17 | 19 | 8.1 | 0.7 | 14 | 82 | 203 | 403 | 856 | 7.7 | |
| 27/7/17 | 20 | 8.1 | 1.4 | 14 | 82 | 318 | 440 | 803 | 24 | |
| 31/7/17 | 20 | 8.1 | 3.6 | 14 | 79 | 567 | 404 | 803 | 20 | |
| 3/8/17 | 20 | 8.1 | 3.9 | 14 | 71 | 335 | 397 | 748 | 26 | |
| 7/8/17 | 19 | 8.2 | 2.3 | 14 | 57 | 198 | 399 | 803 | 29 | |
| 10/8/17 | 19 | 8.1 | 2.2 | 14 | 45 | 199 | 392 | 800 | 55 | |
| 15/8/17 | 20 | 8.1 | 0.2 | 14 | 96 | 281 | 341 | 883 | 31 | |
| 17/8/17 | 24 | 8.0 | 0.4 | 14 | 87 | 381 | 311 | 736 | 29 | |
| 21/8/17 | 21 | 8.1 | 2.5 | 13 | 64 | 259 | 322 | 817 | 26 | |
| 24/8/17 | 20 | 8.1 | 4.1 | 14 | 82 | 248 | 328 | 849 | 36 | |
| 28/8/17 | 22 | 8.1 | 5.2 | 14 | 75 | 223 | 222 | 768 | 34 |
References
- Donoso, N.; Gobeyn, S.; Boets, P.; Goethals, P.L.M.; Wilde, D.D.E.; Meers, E. Assessing the Integration of Wetlands along Small European Waterways to Address Diffuse Nitrate Pollution. Water 2017, 9, 369. [Google Scholar] [CrossRef]
- Meers, E.; Tack, F.M.G.; Tolpe, I.; Michels, E. Application of a Full-scale Constructed Wetland for Tertiary Treatment of Piggery Manure: Monitoring Results. Water Air Soil Pollut. 2008, 193, 15–24. [Google Scholar] [CrossRef]
- Borin, M.; Politeo, M.; De Stefani, G. Performance of a hybrid constructed wetland treating piggery wastewater. Ecol. Eng. 2013, 51, 229–236. [Google Scholar] [CrossRef]
- Wu, H.; Fan, J.; Zhang, J.; Ngo, H.H.; Guo, W.; Liang, S.; Lv, J.; Lu, S.; Wu, W.; Wu, S. Intensified organics and nitrogen removal in the intermittent-aerated constructed wetland using a novel sludge-ceramsite as substrate. Bioresour. Technol. 2016, 210, 101–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donoso, N.; Boets, P.; Michels, E.; Goethals, P.L.M.; Meers, E. Environmental Impact Assessment (EIA) of Effluents from Constructed Wetlands on Water Quality of Receiving Watercourses. Water Air Soil Pollut. 2015, 226, 205. [Google Scholar] [CrossRef]
- Donoso, N.; Gobeyn, S.; Villa-Cox, G.; Boets, P.; Meers, E.; Goethals, P. Assessing the Ecological Relevance of Organic Discharge Limits for Constructed Wetlands by Means of a Model-Based Analysis. Water 2018, 10, 63. [Google Scholar] [CrossRef]
- Meers, E.; Rousseau, D.P.L.; Lesage, E.; Demeersseman, E.; Tack, F.M.G. Physico-chemical P removal from the liquid fraction of pig manure as an intermediary step in manure processing. Water Air Soil Pollut. 2006, 169, 317–330. [Google Scholar] [CrossRef]
- Donoso, N. Environmental Assessment of Constructed Wetlands for Agricultural Wastewater Treatment. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2018. [Google Scholar]
- Orhon, D.; Çokgör, E.U. COD Fractionation in Wastewater Characterization—The State of the Art. J. Chem. Technol. Biotechnol. 1997, 68, 283–293. [Google Scholar] [CrossRef]
- Meers, E.; Rousseau, D.P.L.; Blomme, N.; Lesage, E.; Du Laing, G.; Tack, F.M.G.; Verloo, M.G. Tertiary treatment of the liquid fraction of pig manure with Phragmites australis. Water Air Soil Pollut. 2005, 160, 15–26. [Google Scholar] [CrossRef]
- Dotro, G.; Langergraber, G.; Molle, P.; Nivala, J.; Puigagut, J.; Stein, O.; von Sperling, M. Treatment Wetlands; Nova, T., Ed.; Biological Wastewater Treatment Series; IWA Publishing: Chennai, India, 2017; Volume 7, p. 21. [Google Scholar]
- Kadlec, R.H. The inadequacy of first-order treatment wetland models. Ecol. Eng. 2000, 15, 105–119. [Google Scholar] [CrossRef]
- Nguyen, L.M. Organic matter composition, microbial biomass and microbial activity in gravel-bed constructed wetlands treating farm dairy wastewaters. Ecol. Eng. 2000, 16, 119–221. [Google Scholar] [CrossRef]
- Auvinen, H.; Laing, G.D.; Meers, E.; Rousseau, D.; Vymazal, J. Constructed Wetlands Treating Municipal and Agricultural Wastewater: An Overview for Flanders, Belgium. In Natural and Constructed Wetlands: Nutrients, Heavy Metals and Energy Cycling, and Flow; Vymazal, J., Ed.; Springer: Cham, Switzerland, 2016; pp. 179–207. [Google Scholar]
- Rousseau, D.P.; Vanrolleghem, P.A.; De Pauw, N. Constructed wetlands in Flanders: A performance analysis. Ecol. Eng. 2004, 23, 151–163. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, B.; Zhang, J.; Ngo, H.H.; Guo, W.; Liu, F.; Guo, Y.; Wu, H. Intermittent aeration strategy to enhance organics and nitrogen removal in subsurface flow constructed wetlands. Bioresour. Technol. 2013, 141, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Ouellet-Plamondon, C.; Chazarenc, F.; Comeau, Y.; Brisson, J. Artificial aeration to increase pollutant removal efficiency of constructed wetlands in cold climate. Ecol. Eng. 2006, 27, 258–264. [Google Scholar] [CrossRef]
- Wu, H.; Fan, J.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Lv, J. Optimization of organics and nitrogen removal in intermittently aerated vertical flow constructed wetlands: Effects of aeration time and aeration rate. Int. Biodeterior. Biodegrad. 2016, 113, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Lei, M.; Lu, Q.; Guo, L.; Dong, R. Treatment of pig manure liquid digestate in horizontal flow constructed wetlands: Effect of aeration. Eng. Life Sci. 2016, 16, 263–271. [Google Scholar] [CrossRef]
- Gupta, P.; Ann, T.; Lee, S.-M. Use of biochar to enhance constructed wetland performance in wastewater reclamation. Environ. Eng. Res. 2016, 21, 36–44. [Google Scholar] [CrossRef] [Green Version]
- U.S. Environmental Protection Agency. Recommendation for Sampling and Preservation of Samples, Arranged According to Measurements. In Methods for Chemical Analysis of Water and Wastes; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1983; p. 5. [Google Scholar]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. 5210 B. 5-Day Biochemical Oxygen Demand (BOD) Test. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1998; pp. 1–11. [Google Scholar]
- MACHEREY-NAGEL GmbH & Co. Kg Nanocolor Cod 160 Chemical Oxygen Demand. Available online: ftp://ftp.mn-net.com/english/Instruction_leaflets/NANOCOLOR/985026en.pdf (accessed on 18 May 2017).
- Merck Millipore. Nitrate Test Method: Photometric 0.2–20.0 mg/L NO₃-N. Available online: http://www.merckmillipore.com/BE/fr/product/Nitrate-Test,MDA_CHEM-114773?ReferrerURL=https%3A%2F%2Fwww.google.be%2F#anchor_TI (accessed on 10 September 2018).
- Littell, R.C.; Stroup, W.W.; Freund, R.J. SAS for Linear Models, 4th ed.; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- SAS Institute Inc. SAS/STAT(R) 9.22 User’s Guide. Available online: https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_mixed_sect031.htm (accessed on 5 March 2018).
- Mantovi, P.; Bonazzi, G.; Maestri, E.; Marmiroli, N. Accumulation of copper and zinc from liquid manure in agricultural soils and crop plants. Plant Soil 2003, 250, 249–257. [Google Scholar] [CrossRef]
- Metcalf, E.; Eddy, H. Wastewater Engineering, Treatment, Disposal, and Reuse, 3rd ed.; McGraw-Hill: Singapore, 1991. [Google Scholar]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid-liquid separation of animal slurry in theory and practice. A review. Agron. Sustain. Dev. 2010, 30, 153–180. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; Taylor & Francis Group/CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- van Dyck, M.; van Deun, R. Constructed wetlands for on-site wastewater treatment, experiences in Flanders. Lucr. Științifice 2010, 12, 107–111. [Google Scholar]
- Meng, P.; Pei, H.; Hu, W.; Shao, Y.; Li, Z. Bioresource Technology How to increase microbial degradation in constructed wetlands: Influencing factors and improvement measures. Bioresour. Technol. 2014, 157, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wang, W.; Zhang, B.; Guo, Y.; Ngo, H.H.; Guo, W.; Zhang, J.; Wu, H. Nitrogen removal in intermittently aerated vertical flow constructed wetlands: Impact of influent COD/N ratios. Bioresour. Technol. 2013, 143, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, H.; Gebhardt, W.; Linnemann, V.; Du Laing, G.; Rousseau, D.P.L. Laboratory- and full-scale studies on the removal of pharmaceuticals in an aerated constructed wetland: Effects of aeration and hydraulic retention time on the removal efficiency and assessment of the aquatic risk. Water Sci. Technol. 2017, 76, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; He, K.; Wu, S.; Sun, H.; Wang, Y.; Huang, X.; Dong, R. Optimization of high-rate TN removal in a novel constructed wetland integrated with microelectrolysis system treating high-strength digestate supernatant. J. Environ. Manag. 2016, 178, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.; Silverstein, J. Denitrification kinetics of high nitrate concentration water: pH effect on inhibition and nitrite accumulation. Water Res. 1998, 32, 831–839. [Google Scholar] [CrossRef]
- He, Y.; Tao, W.; Wang, Z.; Shayya, W. Effects of pH and seasonal temperature variation on simultaneous partial nitrification and anammox in free-water surface wetlands. J. Environ. Manag. 2012, 110, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, R.H.; Knight, R.L. Treatment Wetlands; Lewis-CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Wu, S.; Wallace, S.; Brix, H.; Kuschk, P.; Kirui, W.K.; Masi, F.; Dong, R. Treatment of industrial effluents in constructed wetlands: Challenges, operational strategies and overall performance. Environ. Pollut. 2015, 201. [Google Scholar] [CrossRef]
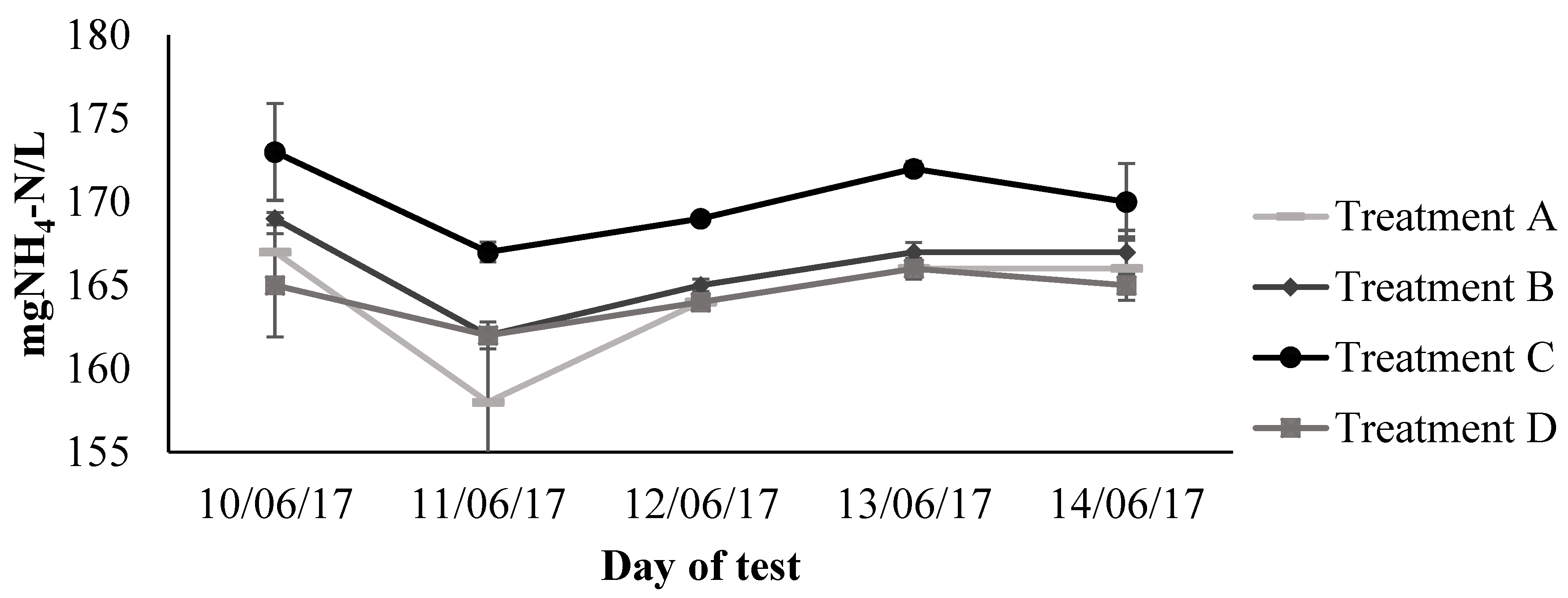
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donoso, N.; van Oirschot, D.; Kumar Biswas, J.; Michels, E.; Meers, E. Impact of Aeration on the Removal of Organic Matter and Nitrogen Compounds in Constructed Wetlands Treating the Liquid Fraction of Piggery Manure. Appl. Sci. 2019, 9, 4310. https://doi.org/10.3390/app9204310
Donoso N, van Oirschot D, Kumar Biswas J, Michels E, Meers E. Impact of Aeration on the Removal of Organic Matter and Nitrogen Compounds in Constructed Wetlands Treating the Liquid Fraction of Piggery Manure. Applied Sciences. 2019; 9(20):4310. https://doi.org/10.3390/app9204310
Chicago/Turabian StyleDonoso, Natalia, Dion van Oirschot, Jayanta Kumar Biswas, Evi Michels, and Erik Meers. 2019. "Impact of Aeration on the Removal of Organic Matter and Nitrogen Compounds in Constructed Wetlands Treating the Liquid Fraction of Piggery Manure" Applied Sciences 9, no. 20: 4310. https://doi.org/10.3390/app9204310




