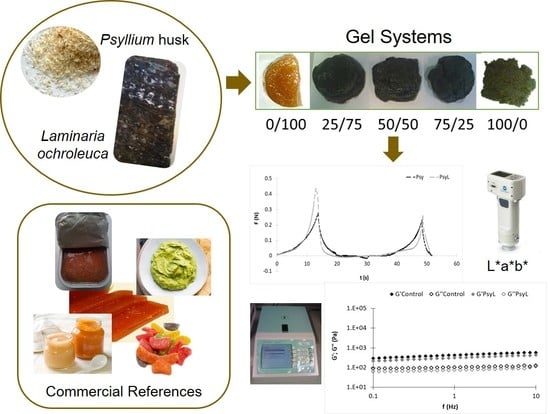
Psyllium and Laminaria Partnership—An Overview of Possible Food Gel Applications
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Formulations Development
2.2.1. Laminaria ochroleuca Sample Production
2.2.2. Preparation of the Laminaria-Psyllium Gels
2.3. Physicochemical Measurements
2.4. Color Measurements
2.5. Dynamic Viscoelasticity
2.6. Texture Profile Analysis (TPA)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of Samples
3.2. Color Evaluation of Samples
3.3. Effect of Laminaria-Psyllium Ratio on the Dynamic Viscoelasticity
3.4. Texture Properties of the Laminaria-Psyllium Gel Systems
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, S.-K.; Bhatnagar, I. Physical, Chemical, and Biological Properties of Wonder Kelp—Laminaria. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 85–96. [Google Scholar] [CrossRef]
- Adebisi, A.O.; Laity, P.R.; Conway, B.R. Formulation and evaluation of floating mucoadhesive alginate beads for targeting Helicobacter pylori. J. Pharm. Pharmacol. 2015, 67, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; Fradinho, P.; Mata, P.; Moreira-Leite, B.; Raymundo, A. Exploring innovation in a traditional sweet pastry: Pastel de Nata. Int. J. Gastron. Food Sci. 2019, 17, 100160. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Fernandes, F.; Barbosa, M.; Oliveira, A.P.; Azevedo, I.C.; Sousa-Pinto, I.; Valentão, P.; Andrade, P.B. The pigments of kelps (Ochrophyta) as part of the flexible response to highly variable marine environments. J. Appl. Phycol. 2016, 28, 3689–3696. [Google Scholar] [CrossRef]
- Parada, J.; Pérez-Correa, J.R.; Pérez-Jiménez, J. Design of low glycemic response foods using polyphenols from seaweed. J. Funct. Foods. 2019, 56, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef]
- Roohinejad, S.; Koubaa, M.; Barba, F.J.; Saljoughian, S.; Amid, M.; Greiner, M. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Food Res. Int. 2017, 99, 1066–1083. [Google Scholar] [CrossRef]
- FDA. CFR–code of federal regulations title 21. U.S. Food and Drug Administration. 2012. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=101.81 (accessed on 26 July 2019).
- Raymundo, A.; Fradinho, P.; Nunes, M.C. Effect of Psyllium fibre content on the textural and rheological characteristics of biscuit and biscuit dough. Bioact. Carbohydr. Dietary Fibre. 2014, 3, 96–105. [Google Scholar] [CrossRef]
- Fischer, M.H.; Yu, N.; Gray, G.R.; Ralph, J.; Anderson, L.; Marlett, J.A. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk). Carbohyd. Res. 2004, 339, 2009–2017. [Google Scholar] [CrossRef]
- Askari, F.; Sadeghi, E.; Mohammadi, R.; Rouhi, M.; Taghizadeh, M.; Shirgardoun, M.H.; Kariminejad, M. The physicochemical and structural properties of psyllium gum/modified starch composite edible film. J. Food Process Preserv. 2018, 42, 13715. [Google Scholar] [CrossRef]
- Singh, B. Psyllium as therapeutic and drug delivery agent. Int. J. Pharm. 2007, 334, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Fradinho, P.; Mata, P.; Raymundo, A. Assessing gelling properties of chia (Salvia hispanica L.) flour through rheological characterization. J. Sci. Food Agric. 2016, 97, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Cambier, S.; Serchi, T.; Tsevdou, M.; Gaiani, C.; Ferrer, P.; Taoukis, P.S.; Hoffmann, L. Rheological and structural characterisation of whey protein acid gels co-structured with chia (Salvia hispanica L.) or flax seed (Linum usitatissimum L.) mucilage. Food Hydrocolloid. 2019, 89, 542–553. [Google Scholar] [CrossRef]
- Soukoulis, C.; Gaiani, C.; Hoffmann, L. Plant seed mucilage as emerging biopolymer in food industry applications. Curr. Opin. Food Sci. 2018, 22, 28–42. [Google Scholar] [CrossRef]
- Fradinho, P.; Flórez-Fernández, N.; Sousa, I.; Raymundo, A.; Domínguez, H.; Torres Pérez, M.D. Environmentally friendly processing of Laminaria ochroleuca for soft food applications with bioactive properties. J. Appl. Phycol. 2019. accepted for publication. [Google Scholar]
- Fradinho, P.; Sousa, I.; Raymundo, A. The role of Psyllium gels on the structuring of gluten-free fresh pasta –a rheological approach. In Book of Abstracts of AERC 2019. Proceedings of the Oral Communication FP11, Portoröz, Slovenia, 8–11 April 2019; The European Society of Rheology: Zurich, Switzerland, 2019; p. 29. [Google Scholar]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary fibre and physicochemical properties of several edible seaweeds from the northwestern Spanish coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Kiosseoglou, V.; Alevisopoulos, S.; Kasapis, S. Small deformation properties of model salad dressings prepared with reduced cholesterol egg yolk. J. Texture Stud. 1997, 28, 221–237. [Google Scholar] [CrossRef]
- Figueroa, L.E.; Genovese, D.B. Fruit jellies enriched with dietary fibre: Development and characterization of a novel functional food product. LWT Food Sci. Technol. 2019, 111, 423–428. [Google Scholar] [CrossRef]
- Flórez-Fernández, N.; Torres, M.D.; González-Muñoz, M.J.; Domínguez, H. Recovery of bioactive and gelling extracts from edible brown seaweed Laminaria ochroleuca by non-isothermal autohydrolysis. Food Chem. 2019, 277, 353–361. [Google Scholar] [CrossRef]
- Margier, M.; Georgé, S.; Hafnaoui, N.; Remond, D.; Nowicki, M.; Du Chaffaut, L.; Amiot, M.-J.; Reboul, E. Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method. Nutrients 2018, 10, 1668. [Google Scholar] [CrossRef]
- Jimoh, M.A.; MacNaughtan, W.; Williams, H.E.L.; Greetham, D.; Linforth, R.L.; Fisk, I.D. Sodium ion interaction with psyllium husk (Plantago sp.). Food Funct. 2016, 7, 4041. [Google Scholar] [CrossRef] [PubMed]
- Castellar, M.R.; Obón, J.M.; Fernández-López, J.A. The isolation and properties of a concentrated red-purple betacyanin food colorant from Opuntia stricta fruits. J. Sci. Food Agric. 2006, 86, 122–128. [Google Scholar] [CrossRef]
- Copetti, G.; Grassi, M.; Lapasin, R.; Pricl, S. Synergistic gelation of xanthan gum with locust bean gum: A rheological investigation. Glycoconj. J. 1997, 14, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Richardson, R.K.; Morris, E.R.; Dea, I.C.M. Xanthan-like ‘weak gel’ rheology from dispersions of ispaghula seed husk. Carbohyd. Polym. 1993, 22, 223–232. [Google Scholar] [CrossRef]
- Rodríguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Extraction of sulfated polysaccharides by autohydrolysis of brown seaweed Fucus vesiculosus. J. Appl. Phycol. 2013, 25, 31–39. [Google Scholar] [CrossRef]
- Guo, Q.; Cui, S.W.; Wang, Q.; Goff, H.D.; Smith, A. Microstructure and rheological properties of psyllium polysaccharide gel. Food Hydrocolloid. 2009, 23, 1542–1547. [Google Scholar] [CrossRef]
- Genovese, D.B.; Ye, A.; Singh, H. High Methoxyl Pectin/Apple Particles Composite Gels: Effect of Particle Size and Particle concentration on Mechanical Properties and Gel Structure. J. Text Stud. 2010, 41, 171–189. [Google Scholar] [CrossRef]




| Samples | Moisture | Ash | Sulphates |
|---|---|---|---|
| (%) | (%, d.b.) | ||
| Laminaria ochroleuca [17] | 9.20 ± 0.07 | 35.01 ± 0.31 | 2.21 ± 0.10 |
| Psyllium husk (160–315 μm) | 9.03 ± 0.31 h | 2.98 ± 0.05 g | 0.09 ± 0.00 e |
| Psy (control) | 95.83 ± 0.08 a | 2.91 ± 0.18 g | 0.20 ± 0.00 e |
| PsyL | 93.58 ± 0.06 b | 18.01 ± 1.83 f | 0.98 ± 0.00 d |
| Lo.Psy_25.75 | 91.73 ± 0.03 c | 24.75 ± 0.51 d | 1.75 ± 0.01 c |
| Lo.Psy_50.50 | 87.50 ± 0.06 e | 33.08 ± 0.24 b | 2.20 ± 0.07 a,b |
| Lo.Psy_75.25 | 84.48 ± 0.22 g | 36.02 ± 0.23 a | 1.98 ± 0.13 b,c |
| LoUS.Psy_25.75 | 91.66 ± 0.12 c | 21.91 ± 0.71 e | 1.78 ± 0.02 c |
| LoUS.Psy_50.50 | 87.78 ± 0.13 d | 30.34 ± 0.41 c | 2.14 ± 0.11 a,b |
| LoUS.Psy_75.25 | 85.18 ± 0.12 f | 34.71 ± 0.37 a,b | 2.25 ± 0.01 a |
| Samples | L * | a * | b * | ΔE * |
|---|---|---|---|---|
| Psyllium husk (160–315 μm) | 59.39 ± 0.91 a | 6.06 ± 0.18 a | 24.22 ± 0.37 a | - |
| Laminaria ochroleuca [17] | 56.36 ± 0.57 | −2.48 ± 0.04 | 14.49 ± 0.18 | - |
| AH liquor [17] | 31.42 ± 1.74 | 1.45 ± 0.26 | 2.88 ± 1.60 | - |
| Psy (control) | 33.12 ± 1.78 b,c | 1.32 ± 0.14 c | 9.85 ± 1.36 c | - |
| PsyL | 35.70 ± 2.86 b | 3.78 ± 1.50 b | 17.67 ± 5.54 b | 8.6 |
| Lo.Psy_25.75 | 30.08 ± 0.37 c,d | −0.16 ± 0.22 d | 12.82 ± 0.57 c | 4.5 |
| Lo.Psy_50.50 | 27.50 ± 0.77 d,e | −0.07 ± 0.16 d | 12.37 ± 1.07 c | 6.3 |
| Lo.Psy_75.25 | 25.14 ± 1.70 e | −0.45 ± 0.09 d | 10.90 ± 1.24 c | 8.2 |
| LoUS.Psy_25.75 | 29.98 ± 0.82 d | −0.06 ± 0.23 d | 14.10 ± 0.46 b,c | 5.5 |
| LoUS.Psy_50.50 | 26.96 ± 1.17 d,e | −0.40 ± 0.09 d | 12.38 ± 1.15 c | 6.9 |
| LoUS.Psy_75.25 | 25.87 ± 0.55 e | −0.64 ± 0.06 d | 11.60 ± 1.14 c | 7.7 |
| Samples | G′ | G″ | ||
|---|---|---|---|---|
| α′ | b′ | α″ | b″ | |
| Psy (Control) | 436.8 ± 15.4 | 0.153 ± 0.002 | 104.8 ± 7.0 | 0.071 ± 0.011 |
| PsyL | 323.0 ± 18.2 | 0.149 ± 0.005 | 79.6 ± 2.4 | 0.090 ± 0.005 |
| Lo.Psy_25.75 | 1689.3 ± 20.8 | 0.124 ± 0.000 | 344.2 ± 2.7 | 0.022 ± 0.000 |
| Lo.Psy_50.50 | 6819.9 ± 103.8 | 0.148 ± 0.002 | 1584.5 ± 28.3 | 0.049 ± 0.002 |
| Lo.Psy_75.25 | 0.5 ± 1816.6 | 0.158 ± 0.002 | 5485.0 ± 434.5 | 0.119 ± 0.001 |
| LoUS.Psy_25.75 | 1681.6 ± 3.5 | 0.121 ± 0.000 | 331.9 ± 2.8 | 0.019 ± 0.000 |
| LoUS.Psy_50.50 | 6239.3 ± 543.3 | 0.146 ± 0.002 | 1442.5 ± 122.0 | 0.049 ± 0.001 |
| LoUS.Psy_75.25 | 23,533.5 ± 1267.8 | 0.159 ± 0.001 | 5530.1 ± 331.8 | 0.116 ± 0.002 |
| Samples | Firmness (N) | Adhesiveness (N.s) | Cohesiveness | |
|---|---|---|---|---|
| Developed gel samples | Psy (control) | 0.296 ± 0.036 c,d,e | −0.034 ± 0.006 a | 0.581 ± 0.051 b |
| PsyL | 0.419 ± 0.031 b,c | −0.032 ± 0.003 a | 0.453 ± 0.024 c,d,e | |
| Lo.Psy_25.75 | 0.259 ± 0.028 c,d,e | −0.040 ± 0.012 a | 0.517 ± 0.037 b,c | |
| Lo.Psy_50.50 | 0.316 ± 0.020 c,d,e | −0.274 ± 0.038 a | 0.362 ± 0.044 e,f,g | |
| Lo.Psy_75.25 | 0.279 ± 0.039 c,d,e | −0.977 ± 0.075 c | 0.403 ± 0.026 d,e,f | |
| LoUS.Psy_25.75 | 0.261 ± 0.007 c,d,e | −0.069 ± 0.017 a | 0.538 ± 0.009 b,c | |
| LoUS.Psy_50.50 | 0.333 ± 0.026 b,c,d | −0.268 ± 0.042 a | 0.354 ± 0.028 f,g | |
| LoUS.Psy_75.25 | 0.319 ± 0.024 c,d,e | −0.978 ± 0.047 c | 0.391 ± 0.030 d,e,f,g | |
| Commercial products | Baby Food | 0.065 ± 0.008 d,e | −0.325 ± 0.034 a,b | 0.475 ± 0.023 c,d |
| Jelly Gum | 10.314 ± 0.386 a | −3.596 ± 0.562 e | 0.330 ± 0.028 f,g | |
| Guacamole | 0.091 ± 0.011 e | −0.638 ± 0.106 b,c | 0.748 ± 0.075 a | |
| Jam | 0.182 ± 0.003 c,d,e | −0.298 ± 0.015 a | 0.310 ± 0.015 g | |
| Pâté | 0.364 ± 0.032 b,c | −0.854 ± 0.081 c | 0.464 ± 0.038 c,d | |
| Pet Food | 0.587 ± 0.066 b | −2.173 ± 0.148 d | 0.371 ± 0.024 e,f,g | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fradinho, P.; Raymundo, A.; Sousa, I.; Domínguez, H.; Torres, M.D. Psyllium and Laminaria Partnership—An Overview of Possible Food Gel Applications. Appl. Sci. 2019, 9, 4356. https://doi.org/10.3390/app9204356
Fradinho P, Raymundo A, Sousa I, Domínguez H, Torres MD. Psyllium and Laminaria Partnership—An Overview of Possible Food Gel Applications. Applied Sciences. 2019; 9(20):4356. https://doi.org/10.3390/app9204356
Chicago/Turabian StyleFradinho, Patrícia, Anabela Raymundo, Isabel Sousa, Herminia Domínguez, and María Dolores Torres. 2019. "Psyllium and Laminaria Partnership—An Overview of Possible Food Gel Applications" Applied Sciences 9, no. 20: 4356. https://doi.org/10.3390/app9204356