Effective Adsorption of Methylene Blue dye onto Magnetic Nanocomposites. Modeling and Reuse Studies
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Magnetic γ-Fe2O3 Nanoparticles
2.3. Preparation of (A/γ-Fe2O3/f-CNT) Beads
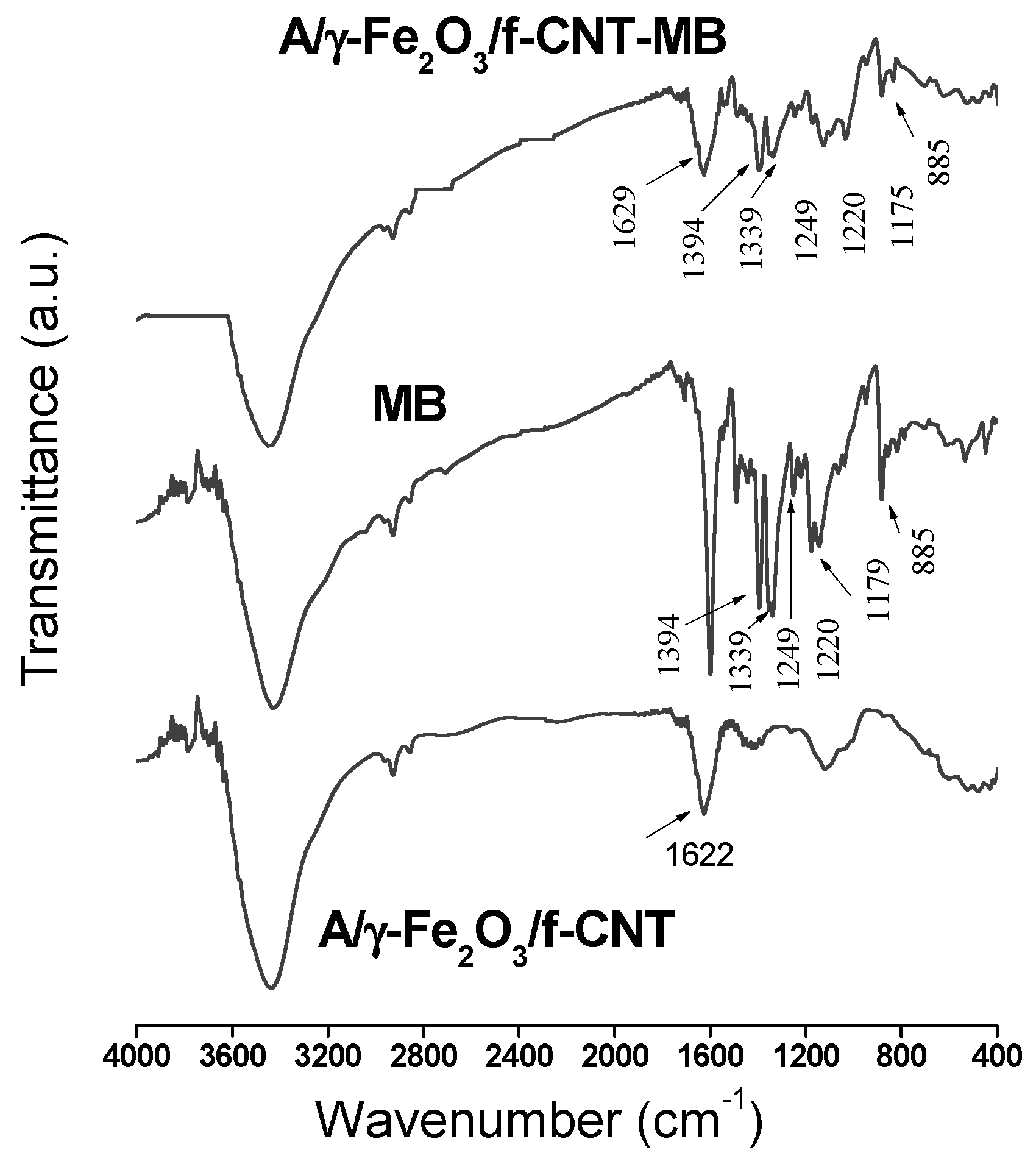
2.4. Characterization Techniques
2.5. Adsorption Experiments
2.6. Desorption of MB Dye and Reusability Studies
2.7. Analysis of the Experimental Data
3. Results and Discussion
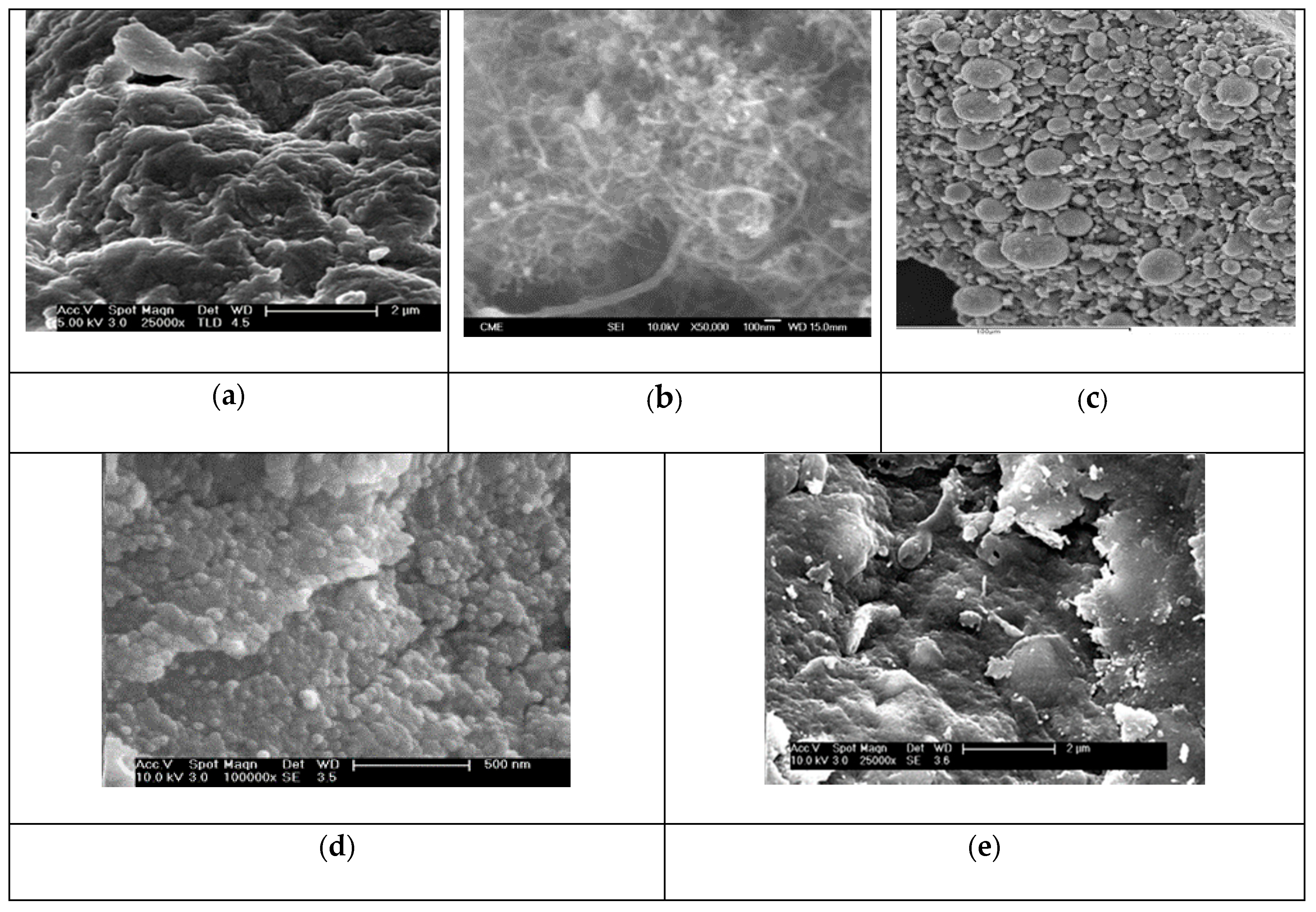
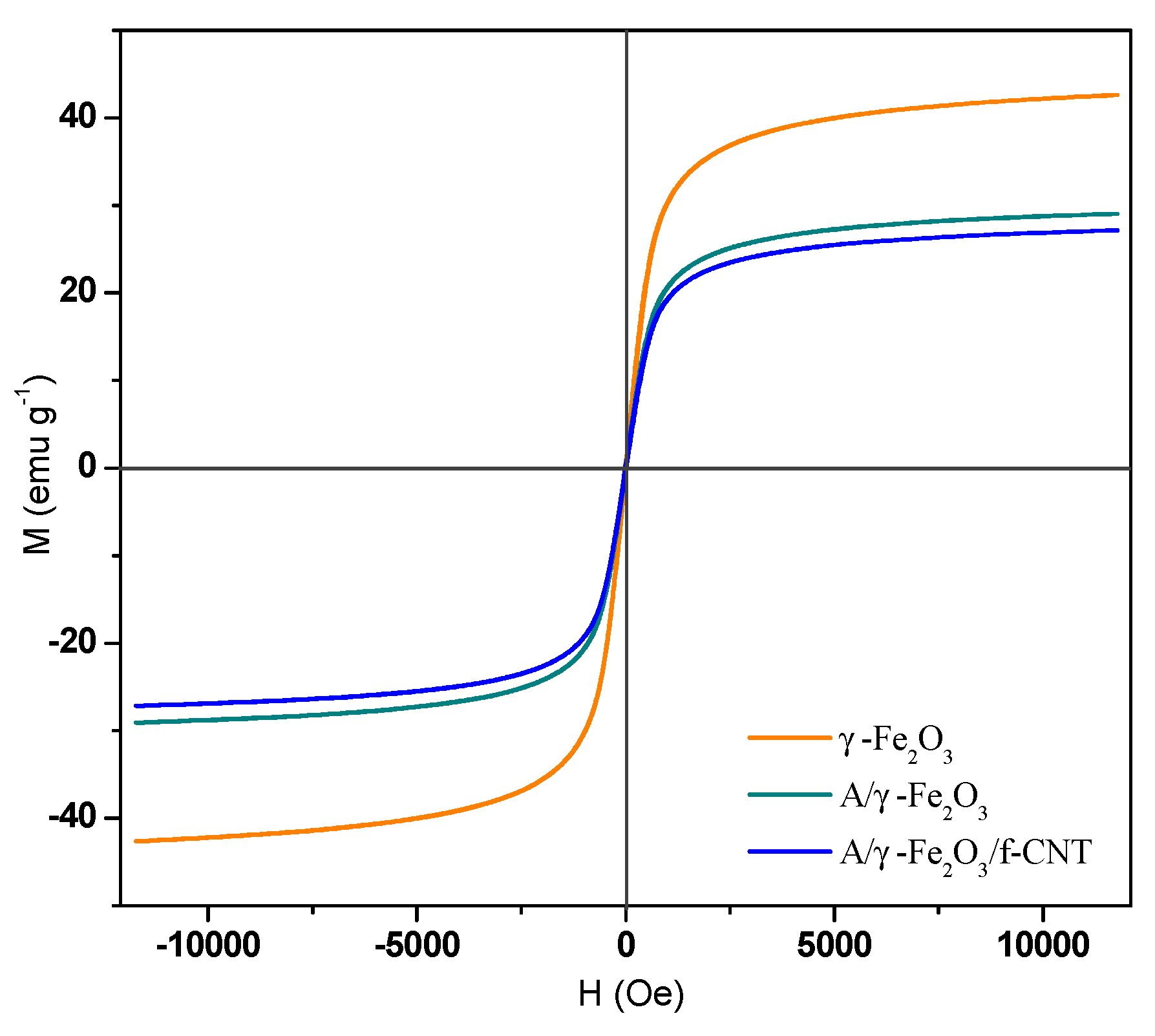
3.1. Characterization of the Tested Adsorbents
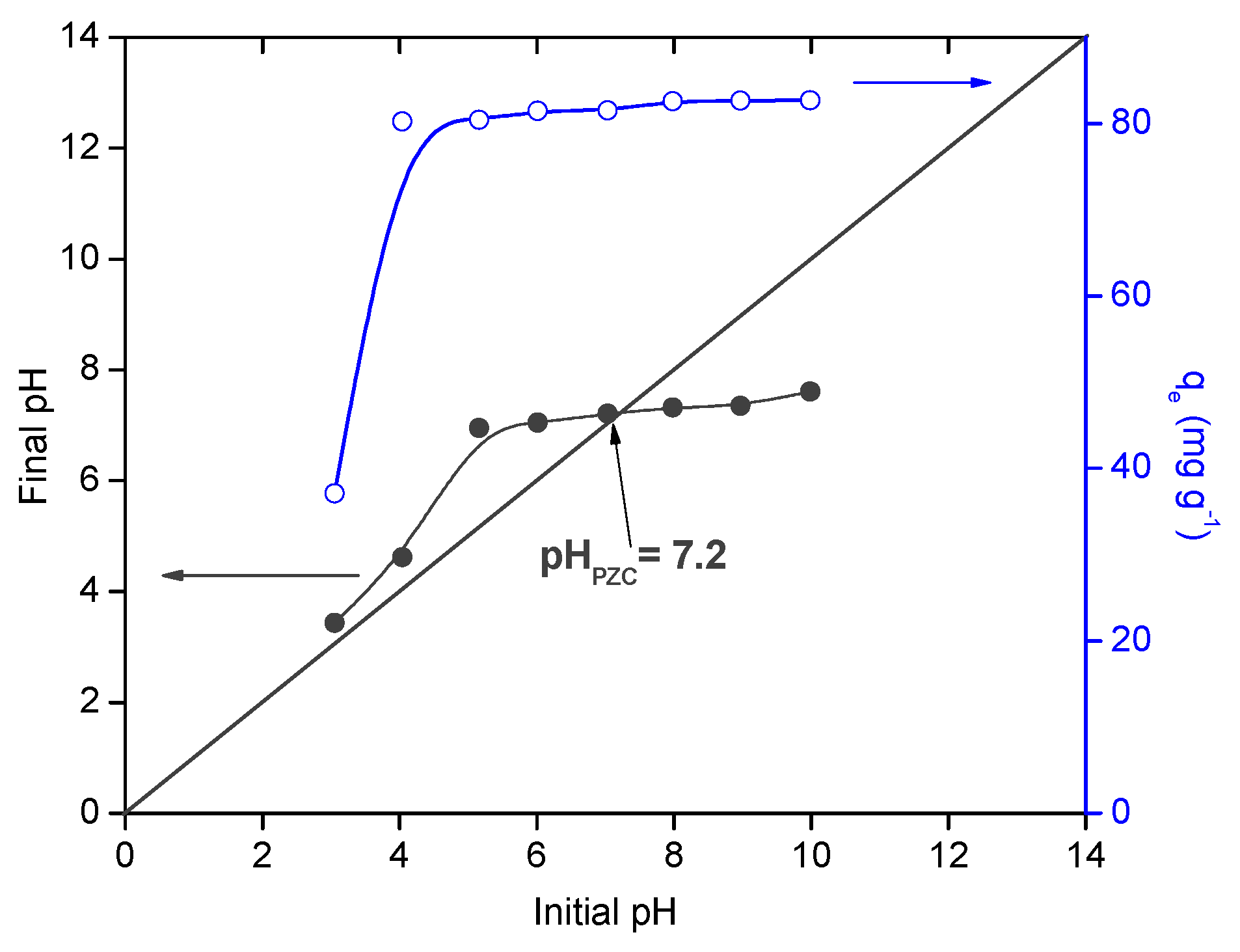
3.2. Effect of Initial PH in Adsorption Tests
3.3. Kinetic Studies
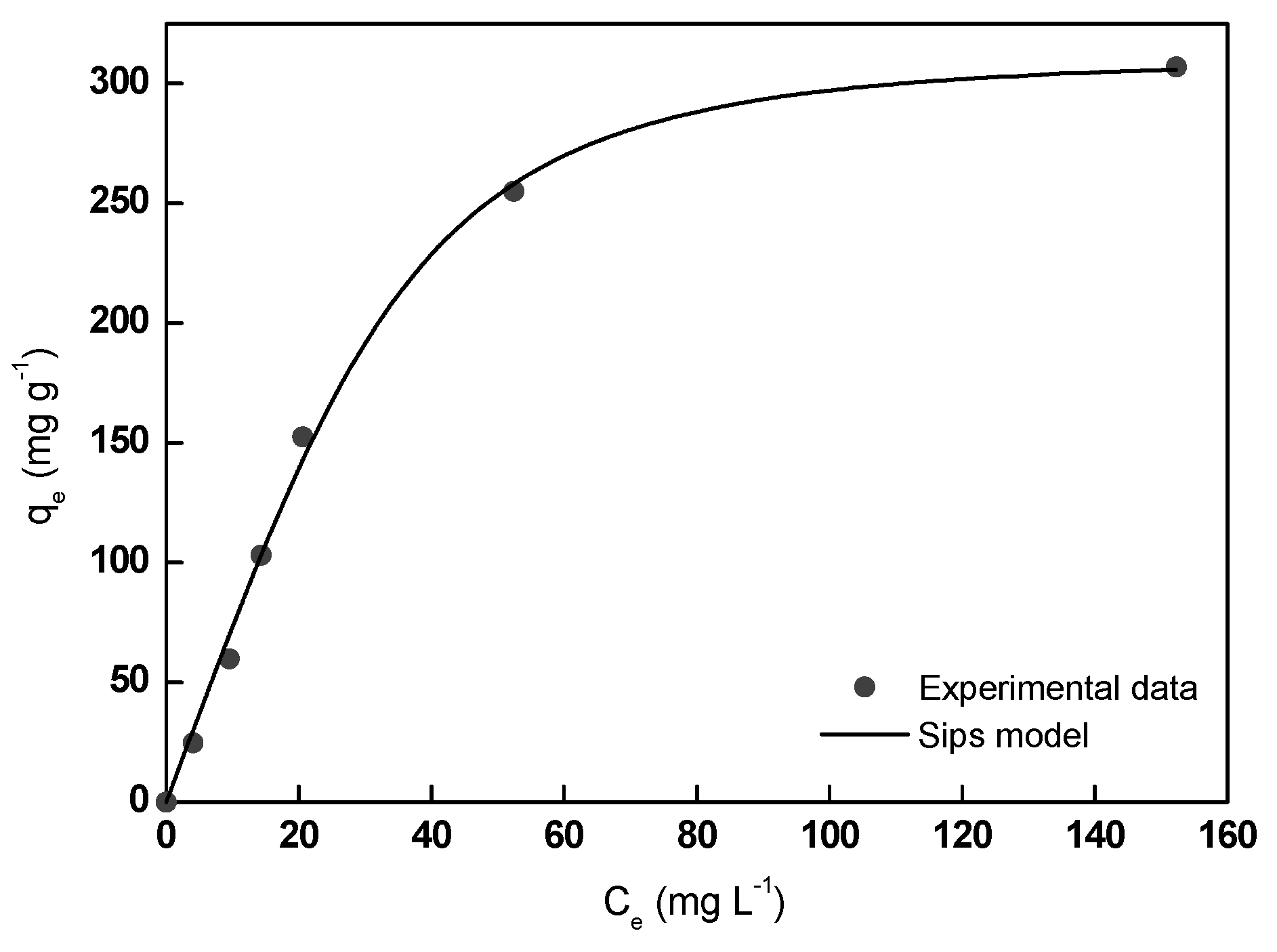
3.4. Adsorption Isotherm Studies
3.5. Statistical Physics Analysis (Monolayer Model)
3.6. Thermodynamic Adsorption
3.7. Proposed Adsorption Mechanism of MB onto A/γ-Fe2O3/f-CNT Composite Beads
3.8. Adsorbent Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Freeman, H.S.; Mock, G.N. Dye application manufacture of dye intermediates and dyes. In Kent and Riegel’s Handbook of Industrial Chemistry and Biotechnology, 11th ed.; Kent, J.A., Ed.; Springer: New York, NY, USA, 2010; pp. 499–590. [Google Scholar]
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Cogliano, V. Carcinogenicity of some aromatic amines, organic dyes, and related exposures. Lancet Oncol. 2008, 9, 322–323. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Chiha, M. Removal of methylene blue from aqueous solutions by wheat bran. Acta Chim. Slov. 2007, 54, 407–418. [Google Scholar]
- Ghosh, D.; Bhattacharyya, G. Adsorption of methylene blue on kaolinite. Appl. Clay Sci. 2002, 20, 295–300. [Google Scholar] [CrossRef]
- Benhouria, A.; Islam, M.A.; Zaghouane-Boudiaf, H.; Boutahala, M.; Hameed, B.H. Calcium alginate-bentonite-activated carbon composite beads as highly effective adsorbent for methylene blue. Chem. Eng. J. 2015, 270, 621–630. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Ifebajo, A.O.; Nisar, N.; Ajayi, O.A. High-performance magnetic chiken bone-based biochar for efficient removal of rhodamine-B dye and tetracycline: Competitive sorption analysis. Water Sci. Technol. 2017, 76, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Gomri, F.; Finqueneisel, G.; Zimny, T.; Korili, S.A.; Gil, A.; Boutahala, M. Adsorption of Rhodamine 6G and humic acids on composite bentonite–alginate in single and binary systems. Appl. Water Sci. 2018, 8. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Ifebajo, A.O. Highly efficient magnetic chicken bone biochar for removal of tetracycline and fluorescent dye from wastewater: Two-stage adsorber analysis. J. Environ. Manag. 2018, 209, 9–16. [Google Scholar] [CrossRef]
- Ruiz, V.; Blanco, C.; Santamaría, R.; Ramos-Fernández, J.M.; Martínez-Escandell, M.; Sepúlveda-Escribano, A.; Rodríguez-Reinoso, F. An activated carbon monolith as an electrode material for supercapacitors. Carbon 2009, 47, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Reinoso, F. The role of carbon materials in heterogeneous catalysis. Carbon 1998, 36, 159–175. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Gazi, M. Two-stage batch sorber design and optimization of biosorption conditions by Taguchi methodology for the removal of acid red 25 onto magnetic biomass. Korean J. Chem. Eng. 2015, 32, 1864–1878. [Google Scholar] [CrossRef]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-G.; Zhao, X.-H.; Yang, H.; Chen, X.-H.; Yang, Q.; Yu, L.-Y.; Jiang, J.-H.; Chen, X.-Q. Aqueous adsorption and removal of organic contaminants by carbon nanotubes. Sci. Total Environ. 2014, 482–483, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Ordóñez, S.; Vega, A. Adsorption of volatile organic compounds onto carbon nanotubes, carbon nanofibers, and high-surface-area graphites. J. Colloid Interfaces Sci. 2007, 305, 7–16. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Functionalization of Graphite Oxide with Magnetic Chitosan for the Preparation of a Nanocomposite Dye Adsorbent. Langmuir 2013, 29, 1657–1668. [Google Scholar] [CrossRef]
- Hu, J.; Shao, D.; Chen, C.; Sheng, G.; Li, J.; Wang, X.; Nagatsu, M. Plasma-Induced Grafting of Cyclodextrin onto Multiwall Carbon Nanotube/Iron Oxides for Adsorbent Application. J. Phys. Chem. B 2010, 114, 6779–6785. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of pollutants by porous carbon, carbon nanotubes and fullerene—An overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. [Google Scholar] [CrossRef]
- Kokate, M.; Garadkar, K.; Gole, A. One pot synthesis of magnetite-silica nanocomposites: Applications as tags, entrapment matrix and in water purification. J. Mater. Chem. A 2013, 1, 2022–2029. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, C.H.; Fiore, S.; Tong, D.S.; Zhang, H.; Li, C.S.; Ji, S.F.; Yu, W.H. Functional magnetic nanoparticle/clay mineral nanocomposites: Preparation, magnetism and versatile applications. Appl. Clay Sci. 2016, 127–128, 143–163. [Google Scholar] [CrossRef]
- Kalantari, K.; Ahmad, M.B.; Shameli, K.; Hussein, M.Z.B.; Khandanlou, R.; Khanehzaei, H. Size-controlled synthesis of Fe3O4 magnetic nanoparticles in the layers of montmorillonite. J. Nanomater. 2014, 2014, 739485. [Google Scholar] [CrossRef]
- Pershina, A.G.; Sazonov, A.E.; Filimonov, V.D. Magnetic nanoparticles-DNA interactions: Design and applications of nanobiohybrid systems. Russ. Chem. Rev. 2014, 83, 299–322. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358–5372. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-L.; Wang, B.; Zeng, G.-M.; Yang, C.-P.; Niu, C.-G.; Niu, Q.-Y.; Zhou, W.-J.; Liang, J. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J. Hazard. Mater. 2009, 164, 1517–1522. [Google Scholar] [CrossRef]
- Zahoor, M.; Mahramanlioglu, M. Adsorption of Imidacloprid on Powdered Activated Carbon and Magnetic Activated Carbon. Chem. Biochem. Eng. Q. 2011, 25, 55–63. [Google Scholar]
- Fu, Y.; Wang, J.; Liu, Q.; Zeng, H. Water-dispersible magnetic nanoparticle-graphene oxide composites for selenium removal. Carbon 2014, 77, 710–721. [Google Scholar] [CrossRef]
- Djebri, N.; Boukhalfa, N.; Boutahala, M.; Hauchard, D.; Chelali, N.E.; Kahoul, A. Calcium alginate-organobentonite-activated carbon composite beads as a highly effective adsorbent for bisphenol A and 2,4,5-trichlorophenol: Kinetics and equilibrium studies. Desalin. Water Treat. 2017, 83, 294–305. [Google Scholar] [CrossRef]
- Bee, A.; Talbot, D.; Abramson, S.; Dupuis, V. Magnetic alginate beads for Pb(II) ions removal from wastewater. J. Colloid Interfaces Sci. 2011, 362, 486–492. [Google Scholar] [CrossRef]
- Hassan, A.F.; Abdel-Mohsen, A.M.; Fouda, M.M.G. Comparative study of calcium alginate, activated carbon, and their composite beads on methylene blue adsorption. Carbohydr. Polym. 2014, 102, 192–198. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, S.; Cheng, X.; Wang, X.; Zheng, L. Removal of anionic azo dyes from aqueous solution using magnetic polymer multi-wall carbon nanotube nanocomposite as adsorbent. Chem. Eng. J. 2013, 223, 84–90. [Google Scholar] [CrossRef]
- Algothmi, W.M.; Bandaru, N.M.; Yu, Y.; Shapter, J.G.; Ellis, A.V. Alginate-graphene oxide hybrid gel beads: An efficient copper adsorbent material. J. Colloid Interfaces Sci. 2013, 397, 32–38. [Google Scholar] [CrossRef]
- Mahmoudi, N.M.; Hayati, B.; Arami, M.; Bahrami, H. Preparation, Characterization and dye adsorption properties of biocomposite (alginate/titania nanoparticle). Desalination 2011, 275, 93–101. [Google Scholar] [CrossRef]
- Djebri, N.; Boutahala, M.; Chelali, N.E.; Boukhalfa, N.; Zeroual, L. Enhanced removal of cationic dye by calcium alginate/organobentonite beads: Modeling, kinetics, equilibriums, thermodynamic and reusability studies. Int. J. Biol. Macromol. 2016, 92, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Duman, O.; Tunc, S.; Polat, T.G.; Bozoglan, B.K. Synthetic of magnetic oxidized multiwalled carbon nanotube-ĸ-carrageenan-Fe3O4 nanocomposite adsorbent and its application in cationic Methylene Blue dye adsorption. Carbohydr. Polym. 2016, 147, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Sujana, M.G.; Mishra, A.; Acharya, B.C. Hydrous ferric oxide doped alginate beads for fluoride removal: Adsorption kinetics and equilibrium studies. Appl. Surf. Sci. 2013, 270, 767–776. [Google Scholar] [CrossRef]
- Salam, M.A.; Burk, R. Synthesis and characterization of multi-walled carbon nanotubes modified with octadecylamine and polyethylene glycol. Arab. J. Chem. 2017, 10, 921–927. [Google Scholar] [CrossRef]
- Hai, H.T.; Kura, H.; Takahashi, M.; Ogawa, T. Facile synthesis of Fe3O4 nanoparticles by reduction phase transformation from γ-Fe2O3 nanoparticles in organic solvent. J. Colloid Interfaces Sci. 2010, 341, 194–199. [Google Scholar] [CrossRef]
- Ramos Guivar, J.A.; Martínez, A.I.; Osorio Anaya, A.; De los Santos Valladares, L.; León Félix, L.; Bustamante Domínguez, A. Structural and magnetic properties of monophasic maghemite (γ-Fe2O3) nanocrystalline poder. Adv. Nanopart. 2014, 3, 114–121. [Google Scholar] [CrossRef]
- Veintemillas-Verdaguer, S.; Morales, M.P.; Serna, C.J. Effect of the oxidation conditions on the maghemites produced by laser pyrolysis. Appl. Organomet. Chem. 2001, 15, 365–372. [Google Scholar] [CrossRef]
- Idris, A.; Hassan, N.; Mohd Ismail, N.S.; Misran, E.; Mohd Yusof, N.; Ngomsik, A.-F.; Bee, A. Photocatalytic magnetic separable beads for chromium (VI) reduction. Water Res. 2010, 44, 1683–1688. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Q.; Shen, X.; Xia, Y.; Tan, L.; Kong, Q. Pyrolysis products and thermal degradation mechanism of intrinsically flame-retardant calcium alginate fibre. Polym. Degrad. Stab. 2011, 96, 936–942. [Google Scholar] [CrossRef]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclética Quim. J. 2004, 29, 53–56. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Jacobs, N.V.; Luyt, A.S. Electrospun alginate nanofibres as potential biosorption agent of heavy metals in water treatment. Express Polym. Lett. 2017, 11, 652–663. [Google Scholar] [CrossRef]
- Ozaki, M.; Suzuki, H.; Takahashi, K.; Matijević, E. Reversible ordered agglomeration of hematite particles due to weak magnetic interactions. J. Colloid Interfaces Sci. 1986, 113, 76–80. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, M.W.; Woo, S.H. Enhanced mechanical strength of chitosan hydrogel beads by impregnation with carbon nanotubes. Carbon 2009, 47, 2933–2939. [Google Scholar] [CrossRef]
- Kang, Y.S.; Risbud, S.; Rabolt, J.F.; Stroeve, P. Synthesis and Characterization of Nanometer-Size Fe3O4 and γ-Fe2O3 Particles. Chem. Mater. 1996, 8, 2209–2211. [Google Scholar] [CrossRef]
- Boukhalfa, N.; Boutahala, M.; Djebri, N.; Idris, A. Kinetics, thermodynamics, equilibrium isotherms, and reusability studies of cationic dye adsorption by magnetic alginate/oxidized multiwalled carbon nanotubes composites. Int. J. Biol. Macromol. 2019, 123, 539–548. [Google Scholar] [CrossRef]
- Sui, K.; Li, Y.; Liu, R.; Zhang, Y.; Zhao, X.; Liang, H.; Xia, Y. Biocomposite fiber of calcium alginate/multi-walled carbon nanotubes with enhanced adsorption properties for ionic dyes. Carbohydr. Polym. 2012, 90, 399–406. [Google Scholar] [CrossRef]
- Ghemit, R.; Boutahala, M.; Kahoul, A. Removal of diclofenac from water with calcined ZnAlFe-CO3 layered double hydroxides: Effect of contact time, concentration, pH and temperature. Desalin. Water Treat. 2017, 83, 75–85. [Google Scholar]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. A 1906, 57, 385–470. [Google Scholar]
- Sips, R. Combined form of Langmuir and Freundlich equations. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Baia, H.; Zhang, T.; Ibarra-Galvan, V.; Song, S. Methylene blue removal from water using the hydrogel beads of poly(vinylalcohol)-sodium alginate-chitosan-montmorillonite. Carbohydr. Polym. 2018, 198, 518–528. [Google Scholar] [CrossRef]
- Melo, B.C.; Paulino, F.A.A.; Cardoso, V.A.; Pereira, A.G.B.; Fajardo, A.R.; Rodrigues, F.H.A. Cellulose nanowhiskers improve the methylene blue adsorption capacity of chitosan-g-poly(acrylic acid) hydrogel. Carbohyd. Polym. 2018, 181, 358–367. [Google Scholar] [CrossRef]
- Liu, L.; Wan, Y.; Xie, Y.; Zhai, R.; Zhang, B.; Liu, J. The removal of dye from aqueous solution using alginate-halloysite nanotube beads. Chem. Eng. J. 2012, 187, 210–216. [Google Scholar] [CrossRef]
- Shahryari, Z.; Goharrizi, A.S.; Azadi, M. Experimental study of methylene blue adsorption from aqueous solutions onto carbon nanotubes. Int. J. Water Res. Environ. Eng. 2010, 2, 016–028. [Google Scholar]
- Ma, J.; Yu, F.; Zhou, L.; Jin, L.; Yang, M.X.; Luan, J.S. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 2012, 4, 5749–5760. [Google Scholar] [CrossRef]
- Boukhalfa, N.; Boutahala, M.; Djebri, N.; Idris, A. Maghemite/alginate/functionalized multiwalled carbon nanotubes beads for methylene blue removal: Adsorption and desorption studies. J. Mol. Liq. 2019, 275, 431–440. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.S.; Costa, M.T.; Almeida, R.L.; Abud, A.K.S.; Soletti, J.L.; Dotto, G.L.; Tanabe, E.H.; Sellaoui, L.; Carvalho, S.H.V.; et al. Adsorption of methylene blue on agroindustrial wastes: Experimental investigation and phenomenological modelling. Prog. Biophys. Mol. Biol. 2019, 141, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gao, B.; Zimmerman, A.R.; Lee, X. Impregnation of multiwall carbon nanotubes in alginate beads dramatically enhances their adsorptive ability to aqueous methylene blue. Chem. Eng. Res. Des. 2018, 133, 235–242. [Google Scholar] [CrossRef]
- Pradhan, A.C.; Paul, A.; Rao, G.R. Sol-gel-cum-hydrothermal synthesis of mesoporous Co-Fe@Al2O3−MCM-41 for methylene blue remediation. J. Chem. Sci. 2017, 129, 381–395. [Google Scholar] [CrossRef]
- Ovchinnikov, O.V.; Evtukhova, A.V.; Kondratenjo, T.S.; Smirnov, M.S.; Khokhlov, V.Y.; Erina, O.V. Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vib. Vib. Spectrosc. 2016, 86, 181–189. [Google Scholar] [CrossRef]
- Talbot, D.; Abramson, S.; Griffete, N.; Bée, A. pH-sensitive magnetic alginate/γ-Fe2O3 nanoparticles for adsorption/desorption of a cationic dye from water. J. Water Process Eng. 2018, 25, 301–308. [Google Scholar] [CrossRef]
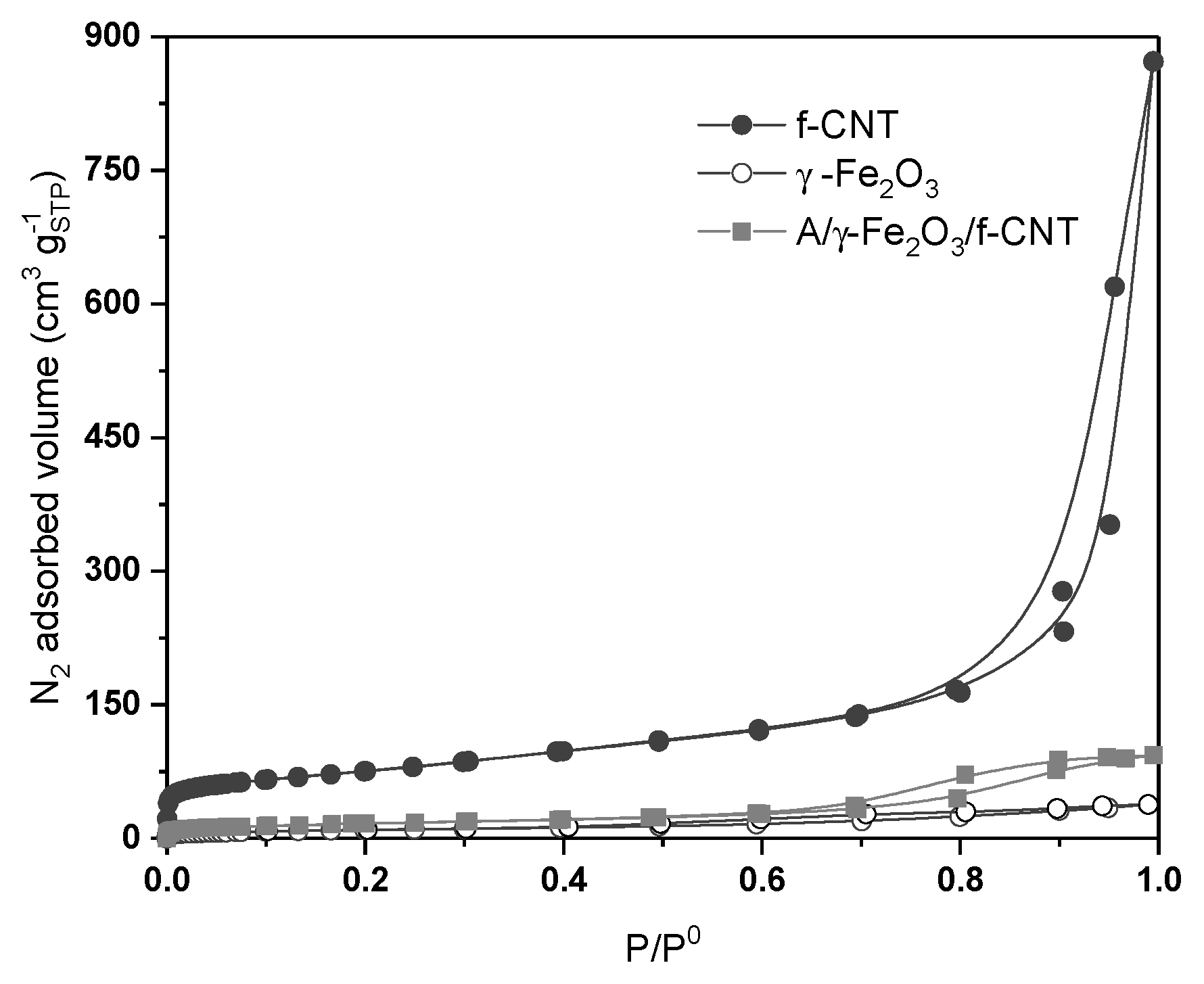




| SBET (m2 g−1) | Sext (m2 g−1) | VTotal (cm3 g−1) | Pore Diameter (nm) | |
|---|---|---|---|---|
| f-CNT | 265 | 331 | 1.35 | 2.5–3.5 a |
| γ-Fe2O3 | 35 | 31 | 0.06 | 1.0–1.6 b |
| A/γ-Fe2O3/f-CNT | 59 | 103 | 0.14 | 1.0–1.5 b |
| Model | Parameter | Value |
|---|---|---|
| Langmuir | KL (L mg−1) | 0.03 |
| qmax (mg g−1) | 396.7 | |
| R2 | 0.977 | |
| RMSE | 17.4 | |
| Freundlich | KF (L g−1) | 31.6 |
| 1/n | 0.5 | |
| R2 | 0.907 | |
| RMSE | 35.5 | |
| Sips | qmax (mg g−1) | 320.5 |
| Ks (L mg−1) × 103 | 6.4 | |
| ns | 0.6 | |
| R2 | 0.999 | |
| RMSE | 4.1 |
| Adsorbent | C0 (mg L−1) | qmax (mg g−1) | Model | Reference |
|---|---|---|---|---|
| Mesoporous activated carbon-alginate beads | 20–100 | 230.0 | Freundlich | [29] |
| Iron oxide/carbon nanocomposites | 118.2 | [16] | ||
| Poly(vinyl alcohol)-sodium alginate-chitosan-montmorillonite hydrogel beads | 137.2 | Freundlich | [56] | |
| Chitosan-g-poly(acrylic acid) hydrogel modified with cellulose nanowhiskers | 1968 | [57] | ||
| Alginate-halloysite nanotube beads | 250.0 | [58] | ||
| MWCNTs untreated | 132.6 | [59] | ||
| MWCNTs alkali-activated | 399.0 | [60] | ||
| Alginate-bentonite-activated carbon (ABA) | 25–500 | 756.9 | Freundlich | [5] |
| Alginate/acid-activated organobentonite beads (A-OAB) | 20–500 | 799.4 | Langmuir | [33] |
| Alginate (A) | 20–500 | 483.6 | Chapman | [33] |
| Functionalized MWCNTs | 20–1000 | 260.8 | Freundlich | [61] |
| γ-Fe2O3 | 20–1000 | 155.3 | Langmuir | [61] |
| Alginate/γ-Fe2O3 | 20–1000 | 438.6 | Langmuir | [61] |
| Alginate/maghemite/functionalized multiwalled carbon nanotubes beads (A/γ-Fe2O3/f-CNTs) | 5–270 | 320.5 | Langmuir | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Torrellas, S.; Boutahala, M.; Boukhalfa, N.; Munoz, M. Effective Adsorption of Methylene Blue dye onto Magnetic Nanocomposites. Modeling and Reuse Studies. Appl. Sci. 2019, 9, 4563. https://doi.org/10.3390/app9214563
Alvarez-Torrellas S, Boutahala M, Boukhalfa N, Munoz M. Effective Adsorption of Methylene Blue dye onto Magnetic Nanocomposites. Modeling and Reuse Studies. Applied Sciences. 2019; 9(21):4563. https://doi.org/10.3390/app9214563
Chicago/Turabian StyleAlvarez-Torrellas, Silvia, Mokhtar Boutahala, Nadia Boukhalfa, and Macarena Munoz. 2019. "Effective Adsorption of Methylene Blue dye onto Magnetic Nanocomposites. Modeling and Reuse Studies" Applied Sciences 9, no. 21: 4563. https://doi.org/10.3390/app9214563