Featured Application
Predicting the nutritive value of forage relies on forage samples with in vivo values. Obtaining in vivo values is time-consuming because many samples are required. Sometimes, the samples used to develop prediction methods were obtained a long time ago and stored at sub-optimal conditions (temperature and humidity). Consequently, possible modifications to the sample composition under sub-optimal storage conditions can be a source of error when developing prediction methods for the forage’s nutritive value. We found that changes in temperate forage samples after 29 years of storage did not modify their nutritive value. Samples stored under ambient temperatures over three decades did not modify their nutritive value compared to the samples stored and frozen at −20 °C.
Abstract
This study examined the effects of long-term storage conditions on the chemical composition, pepsin-cellulase dry matter digestibility (PCDMD), and visible (VIS)/near infrared spectra (NIR) of forage. Eighteen samples of different whole-crop maize varieties originally harvested in 1987 were used. After drying, these samples were analyzed in the laboratory for ash, crude protein (CP), structural carbohydrates, total soluble carbohydrates (TSC), starch and PCDMD, and the remaining samples were stored frozen (at −20°C) or at barn temperature (ambient temperatures ranged from −8.5 °C to 27.1 °C). In 2016, the samples were analyzed for ash, CP, structural carbohydrates, TSC, starch and PCDMD. The visible/NIR spectra of both storage methods were obtained. Chemical composition and PCDMD analyses revealed significant differences (p < 0.05) between the storage methods for TSC but not for the other parameters (p > 0.05). After sample harvesting in 1987, the analyses were compared with those in 2016. It was found that the post-harvest TSC and ash content were higher (p < 0.05) and lower (p < 0.05), respectively, during 2016. No significant differences were found for starch and PCDMD. Important differences between the VIS/NIR spectra of both storage methods were obtained in the VIS segment, particularly in the area between 630 and 760 nm. We concluded that storing dry forage samples at ambient temperature for a very long time (29 years) did not change their nutritive value compared to the values obtained before storage.
1. Introduction
Ruminant performance is known to be dependent on forage nutritive value and intake. The determination of nutritive value is based on digestibility and degradability measurements, which, in combination with intake measurements, are considered to be the reference methods for determining the feed value of forage [1]. These in vivo feed evaluation methods are considered the gold standard and are generally used in research programs [2]. However, these methods are expensive, involve intensive animal studies, and, therefore, are not routinely used to evaluate the nutritive or feed values of forages for ruminants. Generally, in vivo feed evaluations are used as a reference to compare and estimate the nutritive value of forages using less expensive chemical/laboratory-based methods [3] or in vitro methods, including an estimation of in vitro digestibility [4,5,6] or visible (VIS)/near-infrared (NIR) spectroscopy [7,8]. Research studies using NIR spectroscopy models to predict the nutritive value and/or intake of forages require a large number of samples [7,9]. The nutritive value of these samples obtained from previous in vivo trials are needed [7,9]. However, this is a time-consuming process, and, in some cases, these values were obtained for in vivo trials carried out before the development of the model.
In vivo trials are followed by grinding, analysis, and storage of forage samples. The samples are then exposed to atmospheric oxygen, fluctuating temperatures, and certain enzymes released during storage, causing the destruction of the stored samples’ nutrients [10]. It is recommended for dried forage samples to be stored at low temperatures [11], in the absence of direct heat, sunlight [12], and oxygen. However, in practice, samples are usually stored at ambient conditions because this is cheaper and freezers are sometimes not available for this function. This practice may change the chemical composition of the samples stored for a long period of time, making them unsuitable in the development of new predictive methods for their nutritive value. Landau et al. [13] found some changes in the NIR spectra of fecal samples after a 3-year storage period. However, to our knowledge, the potential changes in the chemical composition and nutritive value of samples stored under different conditions for many years have never been extensively addressed.
The objective of this study is to evaluate (i) changes in the chemical composition and nutritive value of forage samples (whole maize forage) stored at frozen or ambient temperatures for a very long time and (ii) potential changes in the VIS/NIR spectra of forage samples according to the storage method. We hypothesized that the storage environment could influence the chemical composition of the forage samples, their spectra, and, consequently, their nutritive value.
2. Materials and Methods
2.1. Samples
The samples studied correspond to an experiment carried out in 1987 on 125 samples of maize hybrids [12]. Eighteen samples cut in 1987 were selected according to the phenological stage of the cut (between the milky and vitreous stages) and the location of growth [14] to give a dry matter (DM) content between 0.22 and 0.35. After harvest, the samples were oven-dried at 80 °C for 48 h and ground in a hammer mill through a 1 mm screen. Two subsamples were then produced. The first sample of about 1 kg was placed in a plastic airtight container and stored in a barn at room temperature (minimal and maximal temperatures ranged from −8.5 °C to 27.1 °C). The second sample of about 500 g was placed in an airtight plastic bag and stored at −20 °C.
2.2. Chemical and Biological Analyses
After collection in 1987, the samples were analyzed for ash and crude protein (Kjeldahl nitrogen × 6.25) (CP) using mercuric oxide as a catalyzer, neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) according to the method of Van Soest [15] and Van Soest and Wine [16]. In 2016, barn-stored and frozen-stored samples were analyzed for ash [17] (method 942.05) and CP [15] (Kjeldahl nitrogen; method 976.06 using selenium (2%), cooper sulphate (1.5%), and sodium sulphate (96.5%) as catalyzers). For NDF, the procedure proposed by Van Soest et al. [18] was used, and we used the method proposed by Van Soest and Robertson for ADF and ADL [19]. The neutral detergent fiber was assayed with a heat-stable amylase to avoid interference between the starch and cell wall content [20]. In 1987 and 2016, the samples were also analyzed for starch as per Ewers (quoted by Radley) [21], total soluble carbohydrates (TSC) as per Somogyi [22], and pepsin–cellulase dry matter digestibility (PCDMD) following the procedure proposed by Aufrère and Michalet Doreau [6].
2.3. Acquisition of Visible/Near Infrared Spectra
In 2016, after homogenizing the samples, the ground frozen and barn-stored forages were placed in a 50 mm-diameter ring cup and scanned in reflectance mode at 2-nm intervals from 400 to 2500 nm using a Foss NIRSystems model 6500 scanning VIS/NIR spectrometer (Foss NIRSystems, Silver Spring, MD, USA). Spectra and reference values were recorded using ISIscan version 2.21 software (Infrasoft International, State College, PA, USA). Each spectrum was averaged from 32 scans. A reference scan (using the internal ceramic reference tile) was taken before each sample. The reflectance (R) values were converted into absorbance (A) values using the formula A = log (1/R). The raw NIR spectroscopy data were pre-processed using the standard normal variate and de-trending scatter correction procedure [23]. The spectra were then transformed using a mathematical second-order gap derivative (2,8,8,1), where the first digit is the order of the derivative, the second is the gap over which the derivative is calculated, the third is the number of datapoints in the first smoothing, and the fourth is the number of datapoints in the second smoothing. Then, the average spectrum for the samples under the same storage treatment (frozen or barn storage) was calculated. Finally, the difference between the average spectra of the storage methods was computed.
2.4. Statistical Analyses
Data on the forage chemical composition and PCDMD were tested by ANOVA using the mixed procedure of the SAS statistical package [24]. The model used the included storage method and sample as its factors. The storage method was considered to be a repeated measure and the sample to be a random variable. The effect of the storage method after 29 years (frozen vs. barn temperature) was studied for all determinations, whereas the effect of time was only tested for ash, TSC, starch, and PCDMD because the methods of analysis performed in 1987 differed from those performed in 2016 for all other determinations. The levels of both effects were compared by orthogonal contrasts.
3. Results
The means, standard deviation, and minimum and maximum values for PCDMD and chemical composition determinations obtained in 1987 for the 18 maize samples used in this experiment are given in Table 1. The coefficient of variation between the samples ranged from 5.25 for PCDMD to 49.5 for starch.

Table 1.
Descriptive statistics (standard deviation (SD), minimum value (min), maximum value (max), and coefficient of variation (CV) for the ash, crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF), acid detergent lignin (ADL), total soluble carbohydrates (TSC), starch contents, and pepsin-cellulase dry matter digestibility (PCDMD) within the set of samples (n = 18) analyzed in 1987.
There were no differences between the storage methods (frozen vs. barn temperature over 29 years) for ash, CP, NDF, ADF, ADL, starch, and PCDMD. The TSC content was lower for the samples stored at barn temperature for 29 years (p < 0.001) (81 g/kg DM) than for the samples stored frozen for 29 years (93 g/kg DM). The ash content was significantly different between the samples analyzed in 1987 and 2016 (55 vs. 56.5 g/kg DM respectively; p < 0.01), but no difference was obtained between the storage methods (56 vs. 57 g/kg DM for samples stored at barn temperature or frozen, respectively; p > 0.05). The TSC significantly differed (113 vs. 87 g/kg DM) between 1987 and 2016 (p < 0.001). The TSC stored at barn temperature decreased by 28% in relation to the TSC content obtained in 1987 just after harvesting the samples, whereas the TSC of the samples stored frozen for 29 years decreased by only 18% in relation to the original value measured in 1987. The starch content (p > 0.05) did not vary over time in storage nor between frozen vs. barn-temperature samples (Table 2).

Table 2.
Contents of ash, crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF), acid detergent lignin (ADL), total soluble carbohydrates (TSC), starch and pepsin–cellulase digestibility (PCDMD), and standard error of the mean (SEM) for the whole-maize forage samples analyzed in 1987 and in 2016 (stored frozen or at barn temperature).
The averaged second-derivative NIR spectrum for frozen and barn-stored whole-maize forage samples is presented in Figure 1a. Whole-maize forage samples presented bands with peaks at 660, 728, 750, 1420, 1920, 2022, 2248, 2314, and 2412 nm. The different spectra between the second-derivative NIR spectra for the frozen and barn-stored samples is presented in Figure 1b. The average spectra for both storage methods showed substantial differences at 660, 698, 728, 1420, 1920, and 2066 nm.
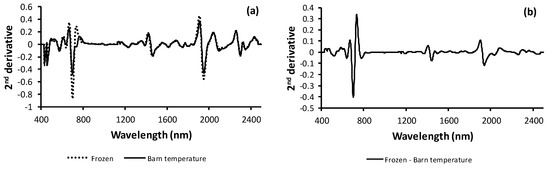
Figure 1.
Visible and near/infrared second-derivative spectra for the average whole-maize forage samples stored frozen or at barn temperature over 29 years (a) and the differences between the average second-derivative spectra of the average frozen or barn-dried whole-maize forage samples (b).
4. Discussion
The samples used in this experiment were chosen to represent a broad range of the variability in the nutritive value of temperate forages (PCDMD between 0.59 and 0.71) reported in the literature [3]. It is common practice for research centers to store samples at room temperature after in vivo trials, and several studies using samples stored under these conditions have been published [6,25,26]. However, studies that used stored freeze-dried samples have also been published [27]. In this study, we used whole-maize forage samples to investigate the changes over time for a number of chemical and biological determinants related to the nutritive value of forages for ruminants. The chemical composition of the samples used in the current study is characterized by high TSC, starch, and structural carbohydrates contents, and moderate ash and CP contents [3]. The samples used in this study were dried at 80 °C, which is not an optimal drying condition to preserve the chemical composition of fresh forages [28] because of the potential formation of Maillard reaction products. These drying conditions could influence the extent of the changes during very-long-term storage time, as Maillard products remain very stable over the long-term [20].
Other studies have investigated the effects of storage time on the nutritive value of hays [29,30], fresh forages, and silages [31,32], but, to our knowledge, this is the first work to investigate changes in the nutritive value of ground forages after a long storage period in contrasting conditions. Earlier studies [29,30] found that in-storage changes in forage chemical composition and nutritive value are mainly related to the moisture content of the samples. In our study, the DM content of the samples in 2016 was higher than 0.88, regardless of the storage method (0.91 ± 0.005 for barn-stored samples and 0.89 ± 0.010 for frozen samples), which means that the extent of changes in the chemical composition and nutritive value of the forage may be limited.
The TSC content was lower in the samples stored at barn temperatures for 29 years than in the samples frozen during the same interval. Soluble carbohydrates are sensitive to storage conditions. Greenhill et al. [33] reported that the loss of DM (mainly nonstructural carbohydrates) increased along with high storage temperatures, high moisture content, and time in storage. Smith [10] claimed that TSC is likely to show less change when dried samples are stored at low temperatures (which fits with the results found here), as heat drying does not inactivate all the enzymes related to the degradation of nonstructural carbohydrates.
Our results indicate no influence of the storage method on ash, CP, structural carbohydrate, and starch contents. Other authors, however, have reported differences in these parameters when forage samples were submitted to different drying procedures before analysis [34,35]. Changes in the structural carbohydrate content are related to the formation of Maillard reaction products, which interfere with these assays. Maillard reaction products are affected by the type, amount of nonstructural carbohydrates [28], heat [36], water activity [37], and pH [38]. In our experiment, although differences in the temperatures between storage methods were produced over time, the barn temperatures were not high enough to produce Maillard products in the forage samples and thus increased the structural carbohydrate contents of the barn-stored samples relative to the frozen samples. The drying conditions of the fresh samples (80 °C) in 1987 could have modified the chemical composition of the samples and made them less sensitive to the effects of ambient temperature in subsequent years. Blackman and Templeman [39] reported the presence of active starch-hydrolyzing enzymes after drying samples at 95 °C. However, in the current study, the storage methods did not affect the starch content. Blackman and Templeman [39] found that, although starch-hydrolyzing enzymes were not inactivated when samples were dried at 95 °C, most of the starch disappeared in 3 weeks. Therefore, samples prepared under the same conditions were not expected to show in-storage changes in starch content. Changes in ash and CP contents were explained by the modifications produced in other components, mainly TSC [30,34]. The current study found that the decrease in the TSC content of barn-stored samples was not sufficient to increase the ash and CP contents. Finally, these changes in the chemical compositions of the samples were not able to modify the PCDMD of samples stored differently.
The comparison between the average NIR spectra of the frozen and barn-stored samples showed that the bands with the highest differences were obtained in the VIS segment (660–700 nm). This might be due to the potential loss of color, mainly because of chlorophyll pigment degradation, in samples stored at barn temperature. The differences observed between the frozen and barn-stored second-derivative spectra at around 1420 and 1920 nm could be related to the different moisture contents of both preservation methods (i.e., 0.911 for the barn-stored samples and 0.890 for the frozen samples). The absorbance values at 2028 and 2250 nm might be related to the –C=O- and –OH molecular groups, respectively [40]. The carbonyl and hydroxyl molecular groups are characteristic of TSC, confirming that the main storage method-related changes in the forage samples are due to TSC changes.
The storage duration effect was analyzed only for ash, TSC, starch, and PCDMD, as the analytical method used for CP and structural carbohydrates was different in 1987 and 2016 [18,41,42], and this may cause a confounding effect on the storage duration. The TSC and ash content for the samples across 29 years of storage changed, whereas the starch content did not vary between 1987 and 2016. The two methods (frozen and barn-dried) showed similar results, which could be explained by the fact that TSC-hydrolyzing enzymes are not fully heat-inactivated [10]. However, the possibility of presence of starch-hydrolyzing enzymes after drying in 1987 [39] would be negligible or they could be biologically inactivated. Finally, it needs to be noted that all changes produced by prolonged storage of the whole-maize forage samples did not affect their digestibility.
5. Conclusions
This study suggests that the nutritive value of whole maize samples oven-dried at 80 °C, ground down to 1 mm, and stored under barn-temperature conditions, is similar to that of the samples stored frozen at −20 °C for a very long period of time. The results of this study show that the chemical composition of the samples stored over the long term changed, especially the TSC fraction and other components related to NIR absorption in the visible segment (400–700 nm). However, these changes are not sufficient to modify the nutritive value of forages in comparison with their post-harvest and pre-storage values.
Author Contributions
D.A. carried out the experimental design, data interpretation, manuscript writing, and editing. F.P. was involved in the experimental design, data recovery, data analysis, and manuscript revision. C.B. was involved in the data analysis and manuscript revision. V.M. was involved in the data analysis and manuscript revision. C.G. was involved in the data analysis and manuscript revision. G.M. contributed to the interpretation of results, manuscript writing, and manuscript revision. All authors read and approved the final manuscript.
Funding
This work was partially performed thanks to the program “Investissement d’Avenir” (16-IDEX-0001 CAP 20-25) funded by the Agence Nationale de la Recherche of the French government.
Acknowledgments
The authors thank the Unité de Recherche Pluridisciplinaire Prairies Plantes Fourragères, and particularly Philippe Barre for running the sample starch analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Demarquilly, C.; Chenost, M.; Giger, S. Pertes fécales et digestibilité des aliments et des rations. In Nutrition des Ruminants Domestiques. Ingestion et Digestion; Jarrige, R., Ruckebusch, Y., Demarquilly, C., Farce, M.H., Journet, M., Eds.; INRA Éditions: Paris, France, 1995; pp. 601–647. [Google Scholar]
- Andueza, D.; Picard, F.; Pradel, P.; Theodoridou, K. Feed Value of Barn-Dried Hays from Permanent Grassland: A Comparison with Fresh Forage. Agronomy 2019, 9, 273. [Google Scholar] [CrossRef]
- Baumont, R.; Tran, G.; Chapoutot, P.; Maxin, G.; Sauvant, D.; Heuzé, V.; Lemosquet, S.; Lamadon, A. INRA feed tables used in France and temperate areas. In INRA Feeding System for Ruminants; Nozière, P., Sauvant, D., Delaby, L., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; pp. 441–548. [Google Scholar]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. Estimation of the digestibility and metabolizable energy content of ruminant feeding stuffs from the gas-production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Aufrere, J.; Michalet-Doreau, B. In vivo digestibility and prediction of digestibility of some by-products. In Feeding Value of By-Products and Their Use by Beef Cattle; Boucqui, V., Fiems, L.O., Cottyn, B.G., Eds.; Commission of the European Communities Publishing: Brussels, Belgium; Luxembourg, 1983; pp. 25–34. [Google Scholar]
- Andueza, D.; Picard, F.; Jestin, M.; Andrieu, J.; Baumont, R. NIRS prediction of the feed value of temperate forages: Efficacy of four calibration strategies. Animal 2011, 5, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Andueza, D.; Picard, F.; Martin-Rosset, W.; Aufrere, J. Near-Infrared Spectroscopy Calibrations Performed on Oven-Dried Green Forages for the Prediction of Chemical Composition and Nutritive Value of Preserved Forage for Ruminants. Appl. Spectrosc. 2016, 70, 1321–1327. [Google Scholar] [CrossRef]
- Andueza, D.; Picard, F.; Dozias, D.; Aufrère, J. Fecal near-infrared reflectance spectroscopy prediction of the feed value of temperate forages for ruminants and some parameters of the chemical composition of feces: Efficiency of four calibration strategies. Appl. Spectrosc. 2017, 71, 2164–2176. [Google Scholar] [CrossRef]
- Smith, D. Influence of drying and storage conditions on nonstructural carbohydrate analysis of herbage tissue—Review. J. Br. Grassl. Soc. 1973, 28, 129–134. [Google Scholar] [CrossRef]
- Lashley, M.A.; Chitwood, M.C.; Harper, C.A.; Moorman, C.E.; DePerno, C.S. Collection, handling and analysis of forages for concentrate selectors. Wildl. Biol. Pract. 2014, 10, 6–15. [Google Scholar] [CrossRef]
- Ball, D.M.; Collins, M.; Lacefield, G.D.; Martin, N.P.; Mertens, D.A.; Olson, K.E.; Putnam, D.H.; Undersander, D.J.; Wolf, M.W. Understanding Forage Quality; American Farm Bureau Federation Publication 1-01: Park Ridge, IL, USA, 2001; pp. 1–17. [Google Scholar]
- Landau, S.; Giger-Reverdin, S.; Rapetti, L.; Dvash, L.; Dorleans, M.; Ungar, E.D. Data mining old digestibility trials for nutritional monitoring in confined goats with aids of fecal near infra-red spectrometry. Small Rumin. Res. 2008, 77, 146–158. [Google Scholar] [CrossRef]
- Aufrere, J.; Graviou, D.; Demarquilly, C.; Andrieu, J.; Emile, J.C.; Giovanni, R.; Maupetit, P. Estimation of organic-matter digestibility of whole maize plants by laboratory methods. Anim. Feed Sci. Technol. 1992, 36, 187–204. [Google Scholar] [CrossRef]
- Van Soest, P.J. Use of detergents in analysis of fibrous feeds. 2. A rapid method for determination of fiber and lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar]
- Van Soest, P.J.; Wine, R.H. Use of detergents in analysis of fibrous feeds. 4. Determination of plant cell-wall constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; p. 1298. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber neutral detergent fiber, and nonstarch poysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B. Systems of analysis for evaluating fibrous feeds. In IDRC No 134; Pidgen, W.J., Balch, C.C., Graham, M., Eds.; International Development Research Centre: Ottawa, ON, Canada, 1980; pp. 49–60. [Google Scholar]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994; p. 476. [Google Scholar]
- Radley, J.A. Starch and Its Derivatives, 3rd ed.; Chapman and Hall Ltd.: London, UK, 1953; Volume II, p. 465. [Google Scholar]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- SAS. SAS/STAT UsersGuide, Version 6.12; Statistical Analysis System Institute: Cary, NC, USA, 1998. [Google Scholar]
- Decruyenaere, V.; Lecomte, P.; Demarquilly, C.; Auffere, J.; Dardenne, P.; Stilmant, D.; Buldgen, A. Evaluation of green forage intake and digestibility in ruminants using near infrared reflectance spectroscopy (NIRS): Developing a global calibration. Anim. Feed Sci. Technol. 2009, 148, 138–156. [Google Scholar] [CrossRef]
- Tran, H.; Salgado, P.; Tillard, E.; Dardenne, P.; Nguyen, X.T.; Lecomte, P. “Global” and “local” predictions of dairy diet nutritional quality using near infrared reflectance spectroscopy. J. Dairy Sci. 2010, 93, 4961–4975. [Google Scholar] [CrossRef]
- Foskolos, A.; Calsamiglia, S.; Chrenkovy, M.; Weisbjerg, M.R.; Albanell, E. Prediction of rumen degradability parameters of a wide range of forages and non-forages by NIRS. Animal 2015, 9, 1163–1171. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J.; Hemken, R.W. Relative susceptibility of forages to heat damage as afected by moisture, temperature and pH. J. Dairy Sci. 1973, 56, 137–143. [Google Scholar] [CrossRef]
- Coblentz, W.K.; Fritz, J.O.; Bolsen, K.K.; Cochran, R.C. Quality changes in alfalfa hay during storage in bales. J. Dairy Sci. 1996, 79, 873–885. [Google Scholar] [CrossRef]
- Turner, J.E.; Coblentz, W.K.; Scarbrough, D.A.; Coffey, K.P.; Kellogg, D.W.; McBeth, L.J.; Rhein, R.T. Changes in nutritive value of bermudagrass hay during storage. Agron. J. 2002, 94, 109–117. [Google Scholar] [CrossRef]
- Nelson, M.L.; Bozich, M.J. Effect of storage temperature and time on fiber content of fresh and ensiled alfalfa. J. Anim. Sci. 1996, 74, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.; Kung, L. The effects of dry matter and length of storage on the composition and nutritive value of alfalfa silage. J. Dairy Sci. 2016, 99, 5466–5469. [Google Scholar] [CrossRef] [PubMed]
- Greenhill, W.L.; Couchman, J.F.; De Freitas, J. Storage of hay. III.—Effect of temperature and moisture on loss of dry matter and changes in composition. J. Sci. Food Agric. 1961, 12, 293–297. [Google Scholar] [CrossRef]
- Pelletier, S.; Tremblay, G.F.; Bertrand, A.; Belanger, G.; Castonguay, Y.; Michaud, R. Drying procedures affect non-structural carbohydrates and other nutritive value attributes in forage samples. Anim. Feed Sci. Technol. 2010, 157, 139–150. [Google Scholar] [CrossRef]
- Parissi, Z.M.; Papachristou, T.G.; Nastis, A.S. Effect of drying method on estimated nutritive value of browse species using an in vitro gas production technique. Anim. Feed Sci. Technol. 2005, 123, 119–128. [Google Scholar] [CrossRef]
- Cleale, R.M.; Klopfenstein, T.J.; Britton, R.A.; Satterlee, L.D.; Lowry, S.R. Induced nonenzymatic browning of soybean-meal. 1. Effects of factors controlling nonenzymatic browning on in vitro ammonia release. J. Anim. Sci. 1987, 65, 1312–1318. [Google Scholar] [CrossRef]
- Labuza, T.P.; Saltmarch, M. Kinetics of browning and protein Qquality loss in whey powders during steady state and nonsteady state storage conditions. J. Food Sci. 1982, 47, 92–96. [Google Scholar] [CrossRef]
- Cleale, R.M.; Britton, R.A.; Klopfenstein, T.J.; Bauer, M.L.; Harmon, D.L.; Satterlee, L.D. Induced nonenzymatic browning of soybean-meal. 2. Ruminal scape and net portal absorption of soybean protein treated with xylose. J. Anim. Sci. 1987, 65, 1319–1326. [Google Scholar] [CrossRef]
- Blackman, G.E.; Templeman, W.G. The Interaction of Light Intensity and Nitrogen Supply in the Growth and Metabolism of Grasses and Clover (Trifolium repens): III. Analytical Methods for the Estimation of Some Nitrogen and Carbohydrate Fractions. Ann. Bot. 1940, 4, 119–134. [Google Scholar] [CrossRef]
- Osborne, B.G.; Fearn, T. Near Infrared Spectroscopy in Food Analysis; Longman Scientific & Technical/John Wiley & Sons, Inc.: New York, NY, USA, 1988. [Google Scholar]
- Saez-Plaza, P.; Michalowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An Overview of the Kjeldahl Method of Nitrogen Determination. Part I. Early History, Chemistry of the Procedure, and Titrimetric Finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Saez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michalowski, T.; Asuero, A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).