Mutated Channelrhodopsins with Increased Sodium and Calcium Permeability
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmids and RNA Generation for Xenopus Laevis Oocyte Expression
2.2. Two-Electrode Voltage-Clamp Recordings of Xenopus Laevis Oocytes
2.3. Light Stimulation
2.4. Protein Quantification by Fluorescence
2.5. Fluorescence Imaging
2.6. Data Processing
3. Results
3.1. Mutating the Conserved Aspartate of TM Helix 4 Influences the Expression, Photocurrent, and Kinetics
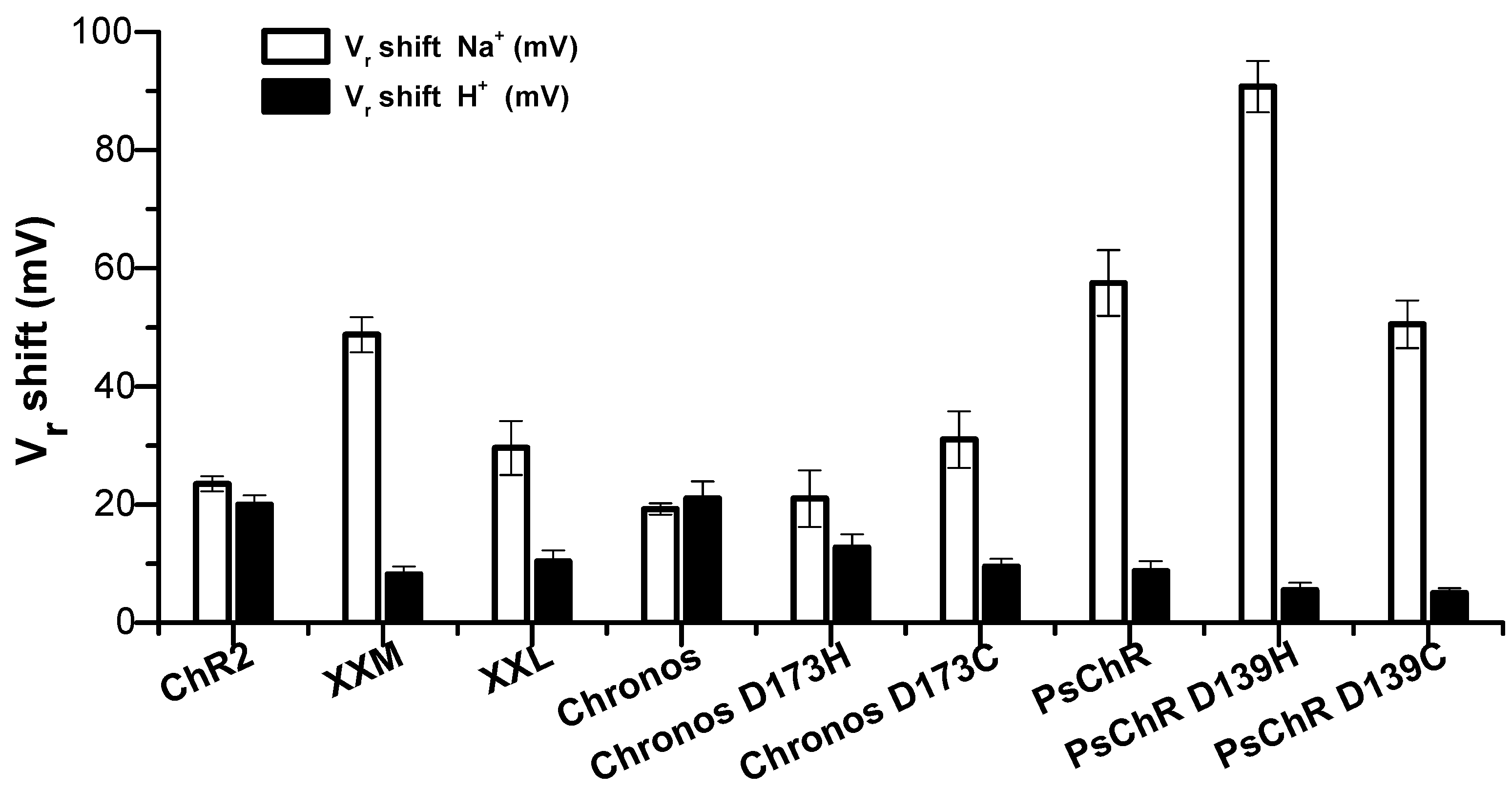
3.2. Mutation of the Aspartate in TM4 Influences the Na+ Permeability
3.3. Mutation of the Aspartate in TM4 Influences the K+ Permeability
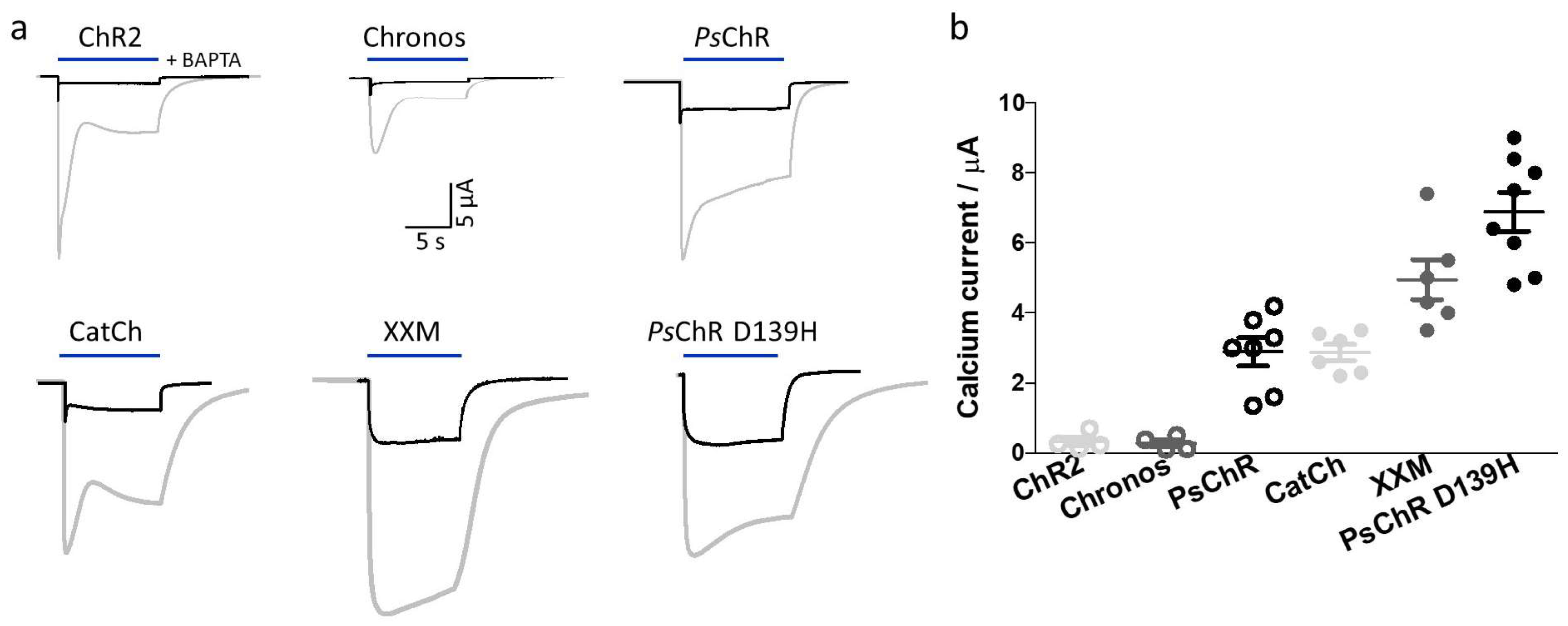
3.4. Mutation of the Aspartate in TM4 Influences the Ca2+ Permeability
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

References
- Nagel, G.; Ollig, D.; Fuhrmann, M.; Kateriya, S.; Mustl, A.M.; Bamberg, E.; Hegemann, P. Channelrhodopsin-1: A light-gated proton channel in green algae. Science 2002, 296, 2395–2398. [Google Scholar] [CrossRef]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef] [PubMed]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gutierrez, D.V.; Hanson, M.G.; Han, J.; Mark, M.D.; Chiel, H.; Hegemann, P.; Landmesser, L.T.; Herlitze, S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl. Acad. Sci. USA 2005, 102, 17816–17821. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Brauner, M.; Liewald, J.F.; Adeishvili, N.; Bamberg, E.; Gottschalk, A. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr Biol. 2005, 15, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Bi, A.D.; Cui, J.J.; Ma, Y.P.; Olshevskaya, E.; Pu, M.L.; Dizhoor, A.M.; Pan, Z.H. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron. 2006, 50, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, T.; Kakuda, M.; Araki, R.; Yawo, H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci. Res. 2006, 54, 85–94. [Google Scholar] [CrossRef]
- Kleinlogel, S.; Feldbauer, K.; Dempski, R.E.; Fotis, H.; Wood, P.G.; Bamann, C.; Bamberg, E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat. Neurosci. 2011, 14, 513–518. [Google Scholar] [CrossRef]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.J.; et al. Independent optical excitation of distinct neural populations. Nat. Methods 2014, 11, 972. [Google Scholar] [CrossRef]
- Wietek, J.; Wiegert, J.S.; Adeishvili, N.; Schneider, F.; Watanabe, H.; Tsunoda, S.P.; Vogt, A.; Elstner, M.; Oertner, T.G.; Hegemann, P. Conversion of Channelrhodopsin into a Light-Gated Chloride Channel. Sci. 2014, 344, 409–412. [Google Scholar] [CrossRef]
- Govorunova, E.G.; Sineshchekov, O.A.; Janz, R.; Liu, X.Q.; Spudich, J.L. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Sci. 2015, 349, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Yizhar, O.; Gunaydin, L.A.; Hegemann, P.; Deisseroth, K. Bi-stable neural state switches. Nat. Neurosci. 2009, 12, 229–234. [Google Scholar] [CrossRef]
- Radu, I.; Bamann, C.; Nack, M.; Nagel, G.; Bamberg, E.; Heberle, J. Conformational changes of channelrhodopsin-2. J. Am. Chem. Soc. 2009, 131, 7313–7319. [Google Scholar] [CrossRef]
- Bamann, C.; Gueta, R.; Kleinlogel, S.; Nagel, G.; Bamberg, E. Structural Guidance of the Photocycle of Channelrhodopsin-2 by an Interhelical Hydrogen Bond. Biochem. 2010, 49, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Nack, M.; Radu, I.; Gossing, M.; Bamann, C.; Bamberg, E.; von Mollard, G.F.; Heberle, J. The DC gate in Channelrhodopsin-2: crucial hydrogen bonding interaction between C128 and D156. Photochem. Photobiol. Sci. 2010, 9, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.E.; Zhang, F.; Yizhar, O.; Ramakrishnan, C.; Nishizawa, T.; Hirata, K.; Ito, J.; Aita, Y.; Tsukazaki, T.; Hayashi, S.; et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nat. 2012, 482, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Volkov, O.; Kovalev, K.; Polovinkin, V.; Borshchevskiy, V.; Bamann, C.; Astashkin, R.; Marin, E.; Popov, A.; Balandin, T.; Willbold, D.; et al. Structural insights into ion conduction by channelrhodopsin 2. Sci. 2017, 358, eaan8862. [Google Scholar] [CrossRef]
- Dawydow, A.; Gueta, R.; Ljaschenko, D.; Ullrich, S.; Hermann, M.; Ehmann, N.; Gao, S.; Fiala, A.; Langenhan, T.; Nagel, G.; et al. Channelrhodopsin-2-XXL, a powerful optogenetic tool for low-light applications. Proc. Natl. Acad. Sci. USA 2014, 111, 13972–13977. [Google Scholar] [CrossRef]
- Scholz, N.; Guan, C.; Nieberler, M.; Grotemeyer, A.; Maiellaro, I.; Gao, S.; Beck, S.; Pawlak, M.; Sauer, M.; Asan, E.; et al. Mechano-dependent signaling by Latrophilin/CIRL quenches cAMP in proprioceptive neurons. eLife 2017, 6, e28360. [Google Scholar] [CrossRef]
- Govorunova, E.G.; Sineshchekov, O.A.; Li, H.; Janz, R.; Spudich, J.L. Characterization of a highly efficient blue-shifted channelrhodopsin from the marine alga Platymonas subcordiformis. J. Biol. Chem. 2013, 288, 29911–29922. [Google Scholar] [CrossRef]
- Ullrich, S.; Gueta, R.; Nagel, G. Degradation of channelopsin-2 in the absence of retinal and degradation resistance in certain mutants. Biol. Chem. 2013, 394, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, V.; Zhang, F.; Ramakrishnan, C.; Mattis, J.; Prakash, R.; Diester, I.; Goshen, I.; Thompson, K.R.; Deisseroth, K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 2010, 141, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Boton, R.; Dascal, N.; Gillo, B.; Lass, Y. Two calcium-activated chloride conductances in Xenopus laevis oocytes permeabilized with the ionophore A23187. J. Physiol. 1989, 408, 511–534. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Weitemier, A.Z.; Zeng, X.; He, L.; Wang, X.; Tao, Y.; Huang, A.J.Y.; Hashimotodani, Y.; Kano, M.; Iwasaki, H.; et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Sci. 2018, 359, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Allman, S.; Rietdorf, K.; Bultynck, G. Deleterious effects of calcium indicators within cells; an inconvenient truth. Cell Calcium 2018, 73, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Yu-Strzelczyk, J.; Pauls, D.; Constantin, O.M.; Gee, C.E.; Ehmann, N.; Kittel, R.J.; Nagel, G.; Gao, S. Synthetic Light-Activated Ion Channels for Optogenetic Activation and Inhibition. Front. Neurosci. 2018, 12, 643. [Google Scholar] [CrossRef] [PubMed]



| Expression * | Is | Closing Time (ms) | EPD50 (µW/mm2) | ||
|---|---|---|---|---|---|
| τ1 (%‡) | τ2 (%‡) | ||||
| ChR2 | 1× † | 1× † | 7.5 ± 0.5 (>98) | - | 710 |
| XXM | 2.7 × | 30 × | 80 ± 5.5 (8) | 1100 ± 110 (92) | 90 |
| XXL | 3 × | 48 × | - | 71000 ± 2900 (>96) | 5.4 |
| Chronos | 1 × † | 1 × † | 2.8 ± 0.3 (>99) | - | 500 |
| Chronos D173H | 2.2 × | 1.2 × | 1400 ± 160 (56) | - | 84 |
| Chronos D173C | 2.6 × | 10 × | 22 ± 1.3 (85) | 130 ± 16 (15) | 190 |
| PsChR | 1 × † | 1 × † | 8.5 ± 0.6 (>98) | - | 810 |
| PsChR D139H | 2.2 × | 6 × | 37 ± 2.7 (14) | 810 ± 54 (86) | 160 |
| PsChR D139C | 3.1 × | 11 × | 3.4 ± 0.8 (4) | 74000 ± 4300 (96) | 3.2 |
| Reversal Potential Shift (mV) | Permeability Ratio | ||||||
|---|---|---|---|---|---|---|---|
| Na+ | H+ † | K + | H+ ‡ | Na+/H+ | K+/H+ | Na+/K+ | |
| ChR2 | 24 ± 0.6 | 20 ± 0.7 | 21 ± 0.6 | 22 ± 0.8 | 3.1 × 10−7 | 2.5 × 10−7 | 1.2 |
| XXM | 49 ± 1.5 | 8.2 ± 0.6 | 32 ± 1.0 | 11 ± 1.1 | 12 × 10−7 | 5 × 10−7 | 2.2 |
| XXL | 30 ± 2 | 10 ± 0.8 | 27 ± 0.8 | 10 ± 0.4 | 4.5 × 10−7 | 3.8 × 10−7 | 1.2 |
| PsChR | 58 ± 2.8 | 8.8 ± 5.5 | 54 ± 5.7 | 20 ± 1.3 | 18 × 10−7 | 16 × 10−7 | 1.2 |
| PsChR D139H | 91 ± 2.2 | 5.5 ± 0.6 | 65 ± 5.5 | 11 ± 0.6 | 90 × 10−7 | 26 × 10−7 | 3.5 |
| PsChR D139C | 51 ± 2.1 | 5 ± 0.4 | 40 ± 1.7 | 6.0 ± 0.6 | 13 × 10−7 | 8 × 10−7 | 1.7 |
| Chronos | 19 ± 0.4 | 21 ± 1.1 | 3.8 ± 0.6 | 53 ± 1.8 | 2.3 × 10−7 | 0.33 × 10−7 | 7.1 |
| Chronos D173H | 21 ± 2.4 | 14 ± 1.1 | 3.3 ± 0.6 | 38 ± 3.6 | 2.6 × 10−7 | 0.28 × 10−7 | 9.4 |
| Chronos D173C | 31 ± 2.4 | 9.5 ± 0.6 | 7.2 ± 0.7 | 37 ± 2.6 | 5 × 10−7 | 0.67 × 10−7 | 7.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Nagel, G.; Gao, S. Mutated Channelrhodopsins with Increased Sodium and Calcium Permeability. Appl. Sci. 2019, 9, 664. https://doi.org/10.3390/app9040664
Duan X, Nagel G, Gao S. Mutated Channelrhodopsins with Increased Sodium and Calcium Permeability. Applied Sciences. 2019; 9(4):664. https://doi.org/10.3390/app9040664
Chicago/Turabian StyleDuan, Xiaodong, Georg Nagel, and Shiqiang Gao. 2019. "Mutated Channelrhodopsins with Increased Sodium and Calcium Permeability" Applied Sciences 9, no. 4: 664. https://doi.org/10.3390/app9040664
APA StyleDuan, X., Nagel, G., & Gao, S. (2019). Mutated Channelrhodopsins with Increased Sodium and Calcium Permeability. Applied Sciences, 9(4), 664. https://doi.org/10.3390/app9040664





