Low-Cost CuIn1−xGaxSe2 Ultra-Thin Hole-Transporting Material Layer for Perovskite/CIGSe Heterojunction Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
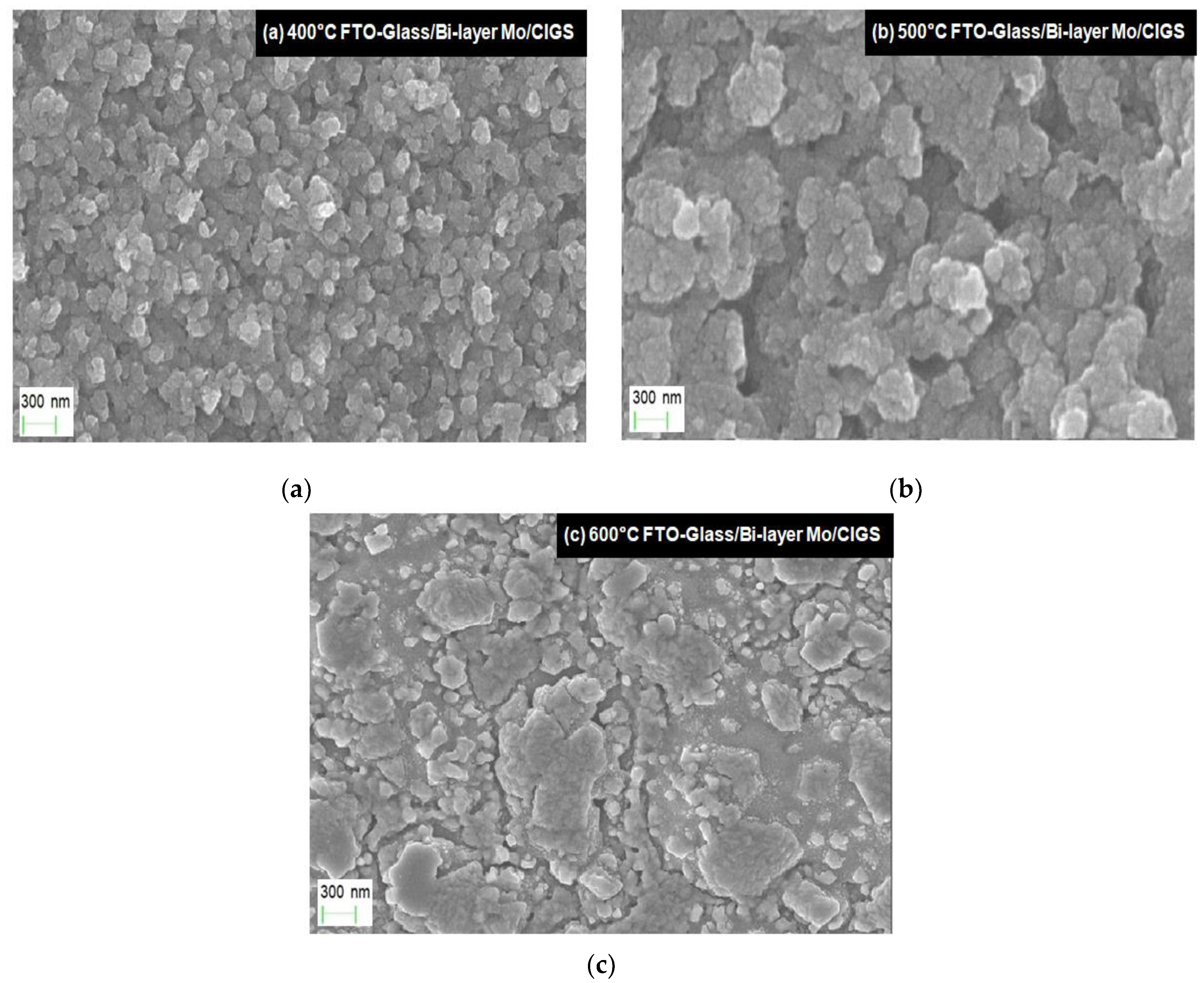
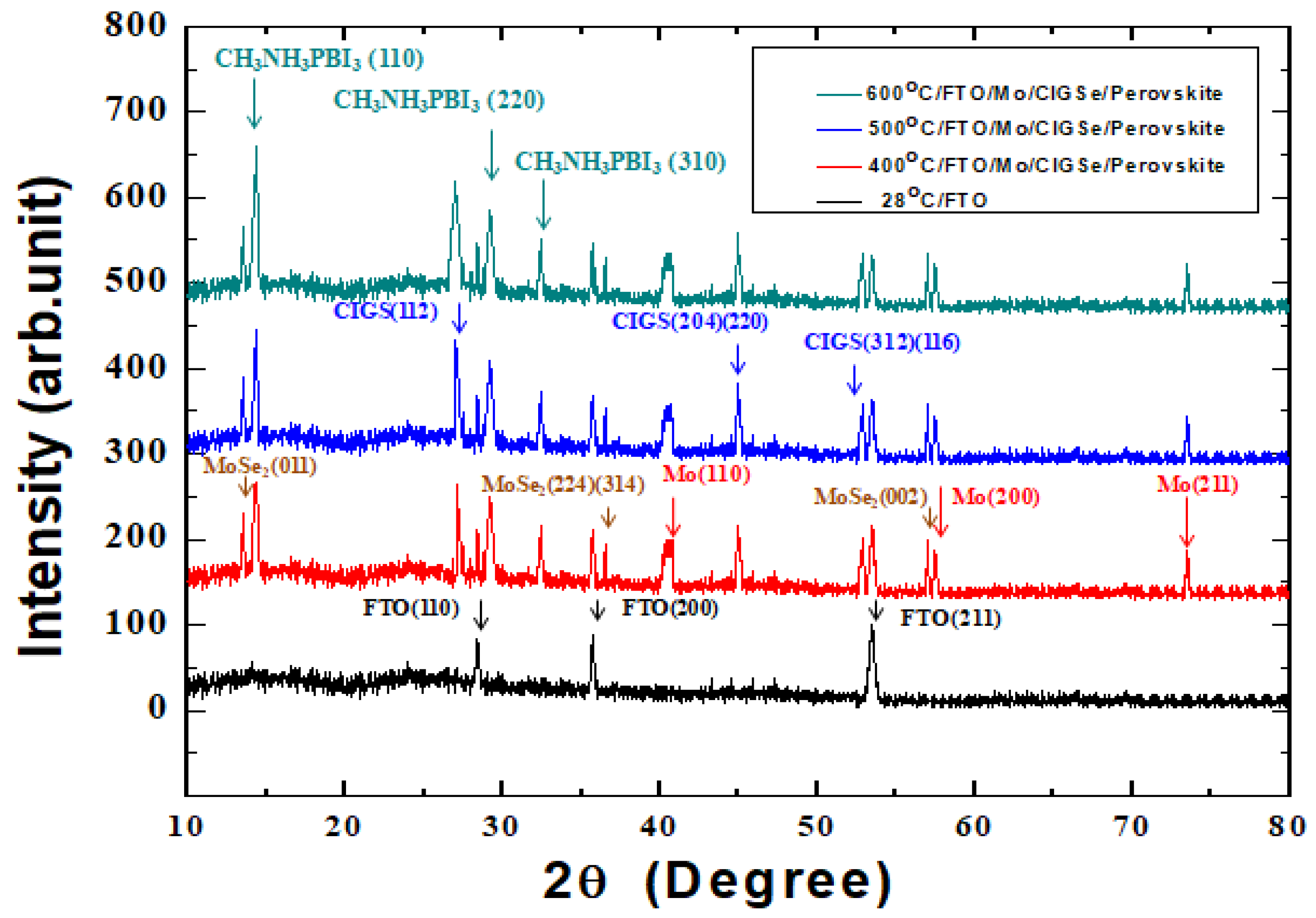
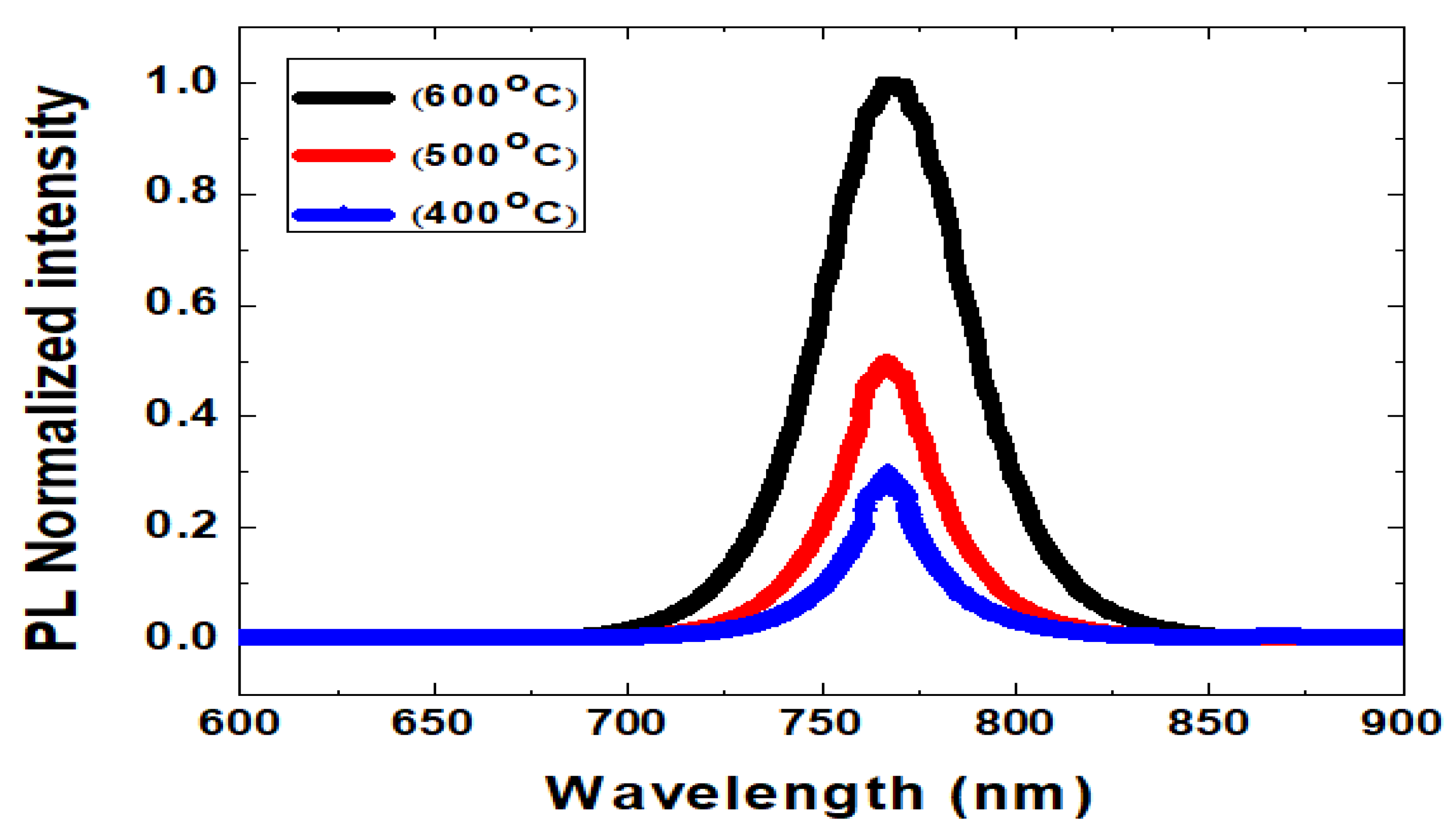
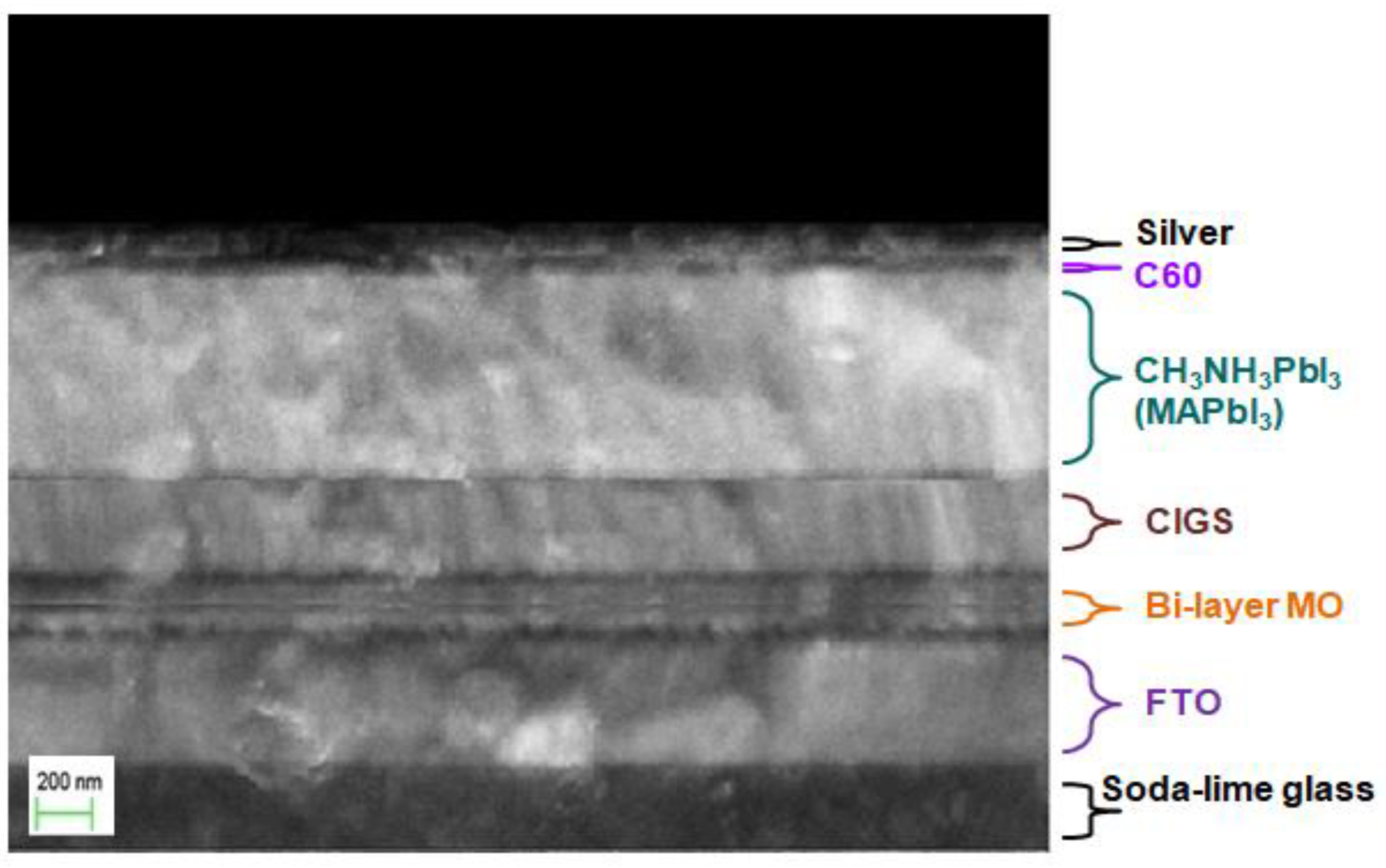
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Correa-Baena, J.B.; Abate, A.; Saliba, M.; Tress, W.; Jacobsson, T.J.; Gratzelc, M.; Hagfeldt, A. The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 2017, 10, 710–727. [Google Scholar] [CrossRef]
- Dubey, A.; Adhikari, N.; Mabrouk, S.; Wu, F.; Chen, K.; Yang, S.; Qiao, Q. A strategic review on processing routes towards highly efficient perovskite solar cells. J. Mater. Chem. A 2018, 6, 2406–2431. [Google Scholar] [CrossRef]
- Meng, L.; You, J.; Guo, T.; Yang, Y. Recent advances in the inverted planar structure of perovskite solar cells. Acc. Chem. Res. 2016, 49, 155–165. [Google Scholar] [CrossRef]
- Dubey, A.; Adhikari, N.; Venkatesan, S.; Gu, S.; Khatiwada, D.; Wang, Q.; Mohammad, L.; Kumar, M.; Qiao, Q. Solution processed pristine PDPP3T polymer as hole-transport layer for efficient perovskite solar cells with slower degradation. Solar Energy Mater. Solar Cells 2016, 145, 193–199. [Google Scholar] [CrossRef]
- Wu, W.; Chen, D.; Caruso, R.A.; Cheng, Y. Recent progress in hybrid perovskite solar cells based on n-type materials. J. Mater. Chem. A 2017, 5, 10092–10109. [Google Scholar] [CrossRef]
- Ahmadian-Yazdi, M.; Habibi, M.; Esiamian, M. Excitation of wet perovskite films by ultrasonic vibration improves the device performance. Appl. Sci. 2018, 8, 308. [Google Scholar] [CrossRef]
- Zuo, C.; Bolink, H.J.; Han, H.; Huang, J.; Cahen, D.; Ding, L. Advances in perovskite solar cells. Adv. Sci. 2016, 3, 1500324. [Google Scholar] [CrossRef]
- Wang, R.; Wu, X.; Xu, K.; Zhou, W.; Shang, Y.; Tang, H.; Chen, H.; Ning, Z. Highly efficient inverted structural quantum dot solar cells. Adv. Mater. 2018, 30, 1704882. [Google Scholar] [CrossRef]
- Pierini, F.; Lanzi, M.; Nakielski, P.; Pawlowska, S.; Urbanek, O.; Zembrzycki, K.; Kowalewski, T.A. Single-material organic solar cells based on electro spun fullerene-grafted polythiophene nanofibers. Macromolecules 2017, 50, 4972–4981. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Chondroudis, K.; Kagan., C.R. Design, structure, and optical properties of organic inorganic perovskites containing an oligothiophenechromophor. Prog. Inorg. Chem. 1999, 38, 6246–6256. [Google Scholar] [CrossRef]
- Mitzi, D.B. Synthesis, structure, properties of organic-inorganic perovskites and related materials. Prog. Inorg. Chem. 2007, 48, 1–121. [Google Scholar]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 396. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Jang, I.H.; Pellet, N.; Gratzel, M.; Park, N. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nanotechnol. 2014, 9, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Sani, F.; Shafie, S.; Lim, H.N.; Musa, A.O. Advancement on lead-free organic-inorganic halide perovskite solar cells: A review. Materials 2018, 11, 1008. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Gratzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometaltrihalide perovskite absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Jeng, J.Y.; Chiang, Y.F.; Lee, M.H.; Peng, S.R.; Guo, T.F.; Chen, P.; Wen, T.C. CH3NH3PbI3 perovskite/fullerene planar-heterojunction hybrid solar cells. Adv. Mater. 2013, 25, 3727–3732. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Hong, Z.; Li, G.; Yang, Y. Recent progress in polymer solar cells: Manipulation of polymer: Fullerene morphology and the formation of efficient inverted polymer solar cells. Adv. Mater. 2009, 21, 1434–1449. [Google Scholar] [CrossRef]
- Choi, H.; Mai, C.; Kim, H.; Jeong, J.; Song, S.; Bazan, G.C.; Kim, J.Y.; Heeger, A.J. Conjugated polyelectrolyte hole transport layer for inverted-type perovskite solar cells. Nat. Commun. 2015, 6, 7348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Klein, T.R.; Kim, D.H.; Yang, M.; Berry, J.J.; van Hest, M.F.; Zhu, K. Scalable fabrication of perovskite solar cells. Nat. Rev. Mater. 2018, 3, 18017. [Google Scholar] [CrossRef]
- Christians, J.A.; Schulz, P.; Tinkham, J.S.; Schloemer, T.H.; Harvey, S.P.; Tremolet de Villers, B.J.; Sellinger, A.; Berry, J.J.; Luther, J.M. Tailored interfaces of unencapsulated perovskite solar cells for >1000 hour operational stability. Nat. Energy 2018, 3, 68–74. [Google Scholar] [CrossRef]
- Snaith, H.J.; Hacke, P. Enabling reliability assessments of pre-commercial perovskite photovoltaics with lessons learned from industrial standards. Nat. Energy 2018, 3, 459–465. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; McMeekin, D.P.; Snaith, H.J.; Nicholas, R.J. Research update: Strategies for improving the stability of perovskite solar cells. APL Mater. 2016, 4, 091503. [Google Scholar] [CrossRef]
- Kim, J.; Yang, J.; Yu, J.H.; Baek, W.; Lee, C.; Son, H.J.; Hyeon, T.; Ko, M.J. Highly efficient copper–indium–selenide quantum dot solar cells: Suppression of carrier recombination by controlled ZnS overlayers. ACS Nano 2015, 9, 11286–11295. [Google Scholar] [CrossRef]
- Lian, L.; Xia, Y.; Zhang, C.; Xu, B.; Yang, L.; Liu, H.; Zhang, D.; Wang, K.; Gao, J.; Zhang, J. In situ tuning the reactivity of selenium precursor to synthesize wide range size, ultra large-scale, and ultrastable PbSe quantum dots. Chem. Mater. 2018, 30, 982–989. [Google Scholar] [CrossRef]
- Wen, X.; Chen, C.; Lu, S.; Li, K.; Kondrotas, R.; Zhao, Y.; Chen, W.; Gao, L.; Wang, C.; Zhang, J.; et al. Vapor transport deposition of antimony selenide thin film solar cells with 7.6% efficiency. Nat. Commun. 2018, 9, 2179. [Google Scholar] [CrossRef]
- Sapkota, Y.R.; Mazumdar, D. Bulk transport properties of bismuth selenide thin films grown by magnetron sputtering approaching the two-dimensional limit. J. Appl. Phys. 2018, 124, 105306. [Google Scholar] [CrossRef]
- Wei, S.H.; Zunger, A. Band offsets and optical bowings of chalcopyrites and Zn-based II-VI alloys. J. Appl. Phys. 1995, 78, 3846–3856. [Google Scholar] [CrossRef]
- Van Lare, C.; Yin, G.; Polman, A.; Schmid, M. Light coupling and trapping in ultrathin Cu(In,Ga)Se2 solar cells using dielectric scattering patterns. ACS Nano 2015, 9, 9603–9613. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Zhang, Y.; Song, W.; Li, B.; Liu, W.; Sun, Y. Structure, morphology and properties of thinned Cu(In, Ga)Se2 films and solar cells. Semicond. Sci. Technol. 2012, 27, 3. [Google Scholar] [CrossRef]
- Saifullah, M.; Ahn, S.; Gwak, J.; Ahn, S.; Kim, K.; Cho, J.; Park, J.H.; Eo, Y.J.; Cho, A.; Yoo, J.U.; et al. Development of semitransparent CIGS thin-film solar cells modified with a sulfurized-AgGa layer for building applications. J. Mater. Chem. A 2016, 4, 10542–10551. [Google Scholar] [CrossRef]
- Wu, G.M.; Lin, H.H.; Lu, H.C. Work function and valance band structure of tin-doped indium oxide thin film for OLED. Vacuum 2008, 82, 1371. [Google Scholar] [CrossRef]
- Yin, G.; Brackmann, V.; Hoffmann, V.; Schmid, M. Enhanced performance of ultra-thin Cu(In,Ga)Se2 solar cells deposited at low process temperature. Solar Energy Mater. Solar Cells 2005, 132, 142–147. [Google Scholar] [CrossRef]
- Martin, F.; Muralt, P.; Dubois, M.A. Process optimization for the sputter deposition of molybdenum thin films as electrode for AlN thin films. J. Vacuum Sci. Technol. A 2006, 24, 946–952. [Google Scholar] [CrossRef]
- Li, Z.H.; Cho, E.S.; Kwon, S.J. Molybdenum thin film deposited by in-line DC magnetron sputtering as a back contact for Cu(In,Ga)Se2 solar cells. Appl. Surf. Sci. 2011, 257, 9682–9688. [Google Scholar] [CrossRef]
- Ma, X.; Liu, D.; Yang, L.; Zuo, S.; Zhou, M. Molybdenum (Mo) back contacts for CIGS solar cell. Proc. of SPIE 2013, 9068, 906814. [Google Scholar] [CrossRef]
- Jaegaermann, W.; Loher, T.; Pettenkofer, C. Surface properties of chalcopyrite semiconductors. Cryst. Res. Cryst. Technol. 1996, 31, 273. [Google Scholar]
- Alvarez-Garcia, J.; Perez-Rodriguez, A.; Romano-Rodriguez, A.; Morante, J.R.; Calvo-Barrio, L.; Scheer, R.; Klenk, R. Microstructure and secondary phases in co-evaporated CuInS2 films: Dependence on growth temperature and chemical composition. J. Vac. Sci. Technol. A 2001, 19, 232–239. [Google Scholar] [CrossRef]
- Wada, T.; Kohara, N.; Nishiwaki, S.; Negami, T. Characterization of the Cu (In, Ga) Se2/Mo interface in CIGS solar cells. Thin Solid Films 2001, 387, 118–122. [Google Scholar] [CrossRef]
- Schmid, D.; Ruckh, M.; Schock, H.W. A comprehensive characterization of the interfaces in Mo/ CIS/CdS/ZnOsolarcell structures. Solar Energy Mater. Solar Cells 1996, 41, 281–294. [Google Scholar] [CrossRef]
- Klinkert, T.; Patriarche, G.; Theys, B.; Theys, B. New insights into the Mo/Cu(In,Ga)Se2 interface in thin film solar cells: Formation and properties of the MoSe2 interfacial layer. J. Chem. Phys. 2016, 145, 154702. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Kohara, N.; Negami, T.; Nishitani, M. Chemical and structural characterization of Cu(In, Ga)Se2/Mo interface in Cu(In, Ga)Se2 solar cells. Jpn. J. Appl. Phys. 1996, 35, L1253–L1256. [Google Scholar] [CrossRef]
- Duchatelet, A.; Savidand, G.; Vannier, R.N.; Lincot, D. Optimization of MoSe2 formation for Cu(In,Ga)Se2-based solar cells by using thin superficial molybdenum oxide barrier layers. Thin Solid Films 2013, 545, 94–99. [Google Scholar] [CrossRef]
- Zhang, X.; Kobayashi, M.; Yamada, A. Comparison of Ag(In,Ga)Se2/Mo and Cu(In,Ga)Se2/Mo interfaces in solar cells. ACS Appl. Mater. Interfaces 2017, 9, 16215–16220. [Google Scholar] [CrossRef]
- Schmid, M.; Klenk, R.; Lux-Steiner, M.C.; Topic, M. Modeling plasmonic scattering combined with thin-film optics. Nanotechnology 2011, 22, 025204. [Google Scholar] [CrossRef]
- Schmid, M.; Klaer, J.; Klenk, R.; Topic, M.J. Stability of plasmonicmetalnanoparticles integrated in the back contact of ultra-thin Cu(In,Ga)S2 solar cells. Thin Solid Films 2013, 527, 308–313. [Google Scholar] [CrossRef]
- Amin, N.; Chelvanathan, P.; Hossain, M.I.; Sopian, K. Numerical modelling of ultra thin Cu(In,Ga)Se2 solar cells. Energy Procedia 2011, 15, 291–298. [Google Scholar] [CrossRef]
- Gloeckler, M.; Sites, J.R. Potential of submicrometer thickness Cu(In,Ga)Se2 solar cells. J. Appl. Phys. 2005, 98, 103703. [Google Scholar] [CrossRef]
- Xu, L.; Deng, L.; Cao, J.; Wang, X.; Chen, W.; Jiang, Z. Solution-processed Cu(In,Ga)(S,Se)2 nanocrystal as inorganic hole-transporting material for efficient and stable perovskite solar cells. Nanoscale Res. Lett. 2017, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.S.; Chang, J.A.; Lee, Y.H.; Sarkar, A.; Nazeeruddin, M.K. Efficient inorganic-organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonic 2013, 7, 486–491. [Google Scholar] [CrossRef]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells. Nano. Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Li, W.; Meng, F.; Wang, L.; Dong, H.; Qiu, Y. Study on the stability of CH3NH3PbI3 (MAPbI3) films and the effect of post-modification by aluminum oxide in all-solid-state hybrid solar cells. J. Mater. Chem. A 2014, 2, 705–710. [Google Scholar] [CrossRef]
- Christians, J.A.; Fung, R.C.; Kamat, P.V. An inorganic hole conductor for organo-lead halide perovskite solar cells: Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 2013, 136, 758–764. [Google Scholar] [CrossRef]
- Chen, W.Y.; Deng, L.L.; Dai, S.M.; Wang, X.; Tian, C.B.; Zhan, X.X.; Xie, S.Y.; Huang, R.B.; Zheng, L.S. Low-cost solution-processed copper iodide as an alternative to PEDOT:PSS hole transport layer for efficient and stable inverted planar heterojunction perovskite solar cells. J. Mater. Chem. A 2015, 3, 19353–19359. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Huang, Y.; Wei, J.; Liu, F.; Shao, Z.; Hu, L.; Chen, S.; Yang, S.; Tang, J.; et al. Efficient inorganic solid solar cells composed of perovskite and PbS quantum dots. Nanoscale 2015, 7, 9902–9907. [Google Scholar] [CrossRef]
- Kim, J.H.; Liang, P.W.; Williams, S.T.; Cho, N.; Chueh, C.C.; Glaz, M.S.; Ginger, D.S.; Jen, A.K. High-performance and environmentally stable planar heterojunction perovskite solar cells based on a solution-processed copper-doped nickel oxide hole-transporting layer. Adv. Mater. 2015, 27, 695–701. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, J.; Huang, Y.; Li, Y.; Shao, Z.; Xu, Y.; Dai, S. Colloidal CuInS2 quantum dots as inorganic hole-transporting material in perovskite solar cells. ACS Appl. Mater. Interfaces 2015, 7, 17482–17488. [Google Scholar]
- Chen, J.; Park, N. Inorganic hole transporting materials for stable and high efficiency perovskite solar cells. J. Phys. Chem. C 2018, 8, 01177. [Google Scholar] [CrossRef]
- Yoshida, K.; Oku, T.; Suzuki, A.; Akiyama, T.; Yamasaki, Y. Fabrication and characterization of phthalocyanine/C60 solar cells with inverted structure. Adv. Chem. Eng. Sci. 2012, 2, 461–464. [Google Scholar] [CrossRef]
- Kim, D.Y.; So, F.; Gao, Y. Aluminum phthalocyanine chloride/C60 organic photovoltaic cells with high open-circuit voltages. Solar Energy Mater. Solar Cells 2009, 93, 1688–1691. [Google Scholar] [CrossRef]
- Bakr, N.A.; Khodair, Z.T.; Mahdi, H.I. Influence of thiourea concentration on some physical properties of chemically sprayed Cu2ZnSnS4 thin films. Intl. J. Mater. Sci. Appl. 2016, 5, 261–270. [Google Scholar]
- Sahoo, A.K.; Wu, G.M. Effects of argon flow rate on electrical properties of amorphous indium gallium zinc oxide thin-film transistors. Thin Solid Films 2016, 605, 129–135. [Google Scholar] [CrossRef]
- Yin, G.; Knight, M.W.; van Lare, M.; Magdalena, M.; Garcia, S.; Polman, A.; Schmid, M. Optoelectronic enhancement of ultrathin CuIn1–xGaxSe2solar cells by nanophotonic contacts. Adv. Opt. Mater. 2017, 5, 1600637. [Google Scholar] [CrossRef]
- Jheng, B.T.; Liu, P.; Wu, M. Efficiency enhancement of non-selenized Cu(In,Ga)Se2 solar cells employing scalable low-cost antireflective coating. Nanoscale Res. Lett. 2014, 9, 331. [Google Scholar] [CrossRef]



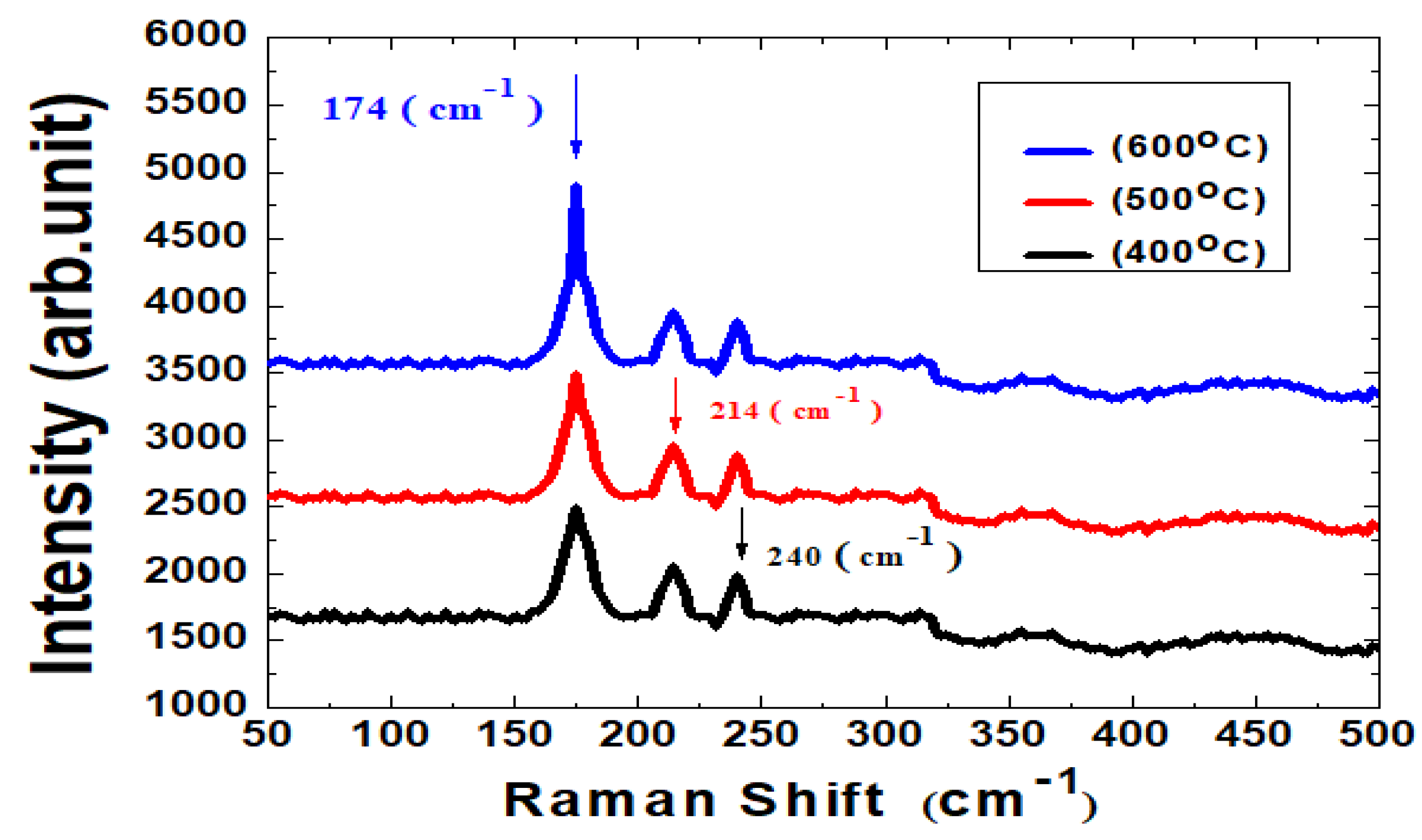
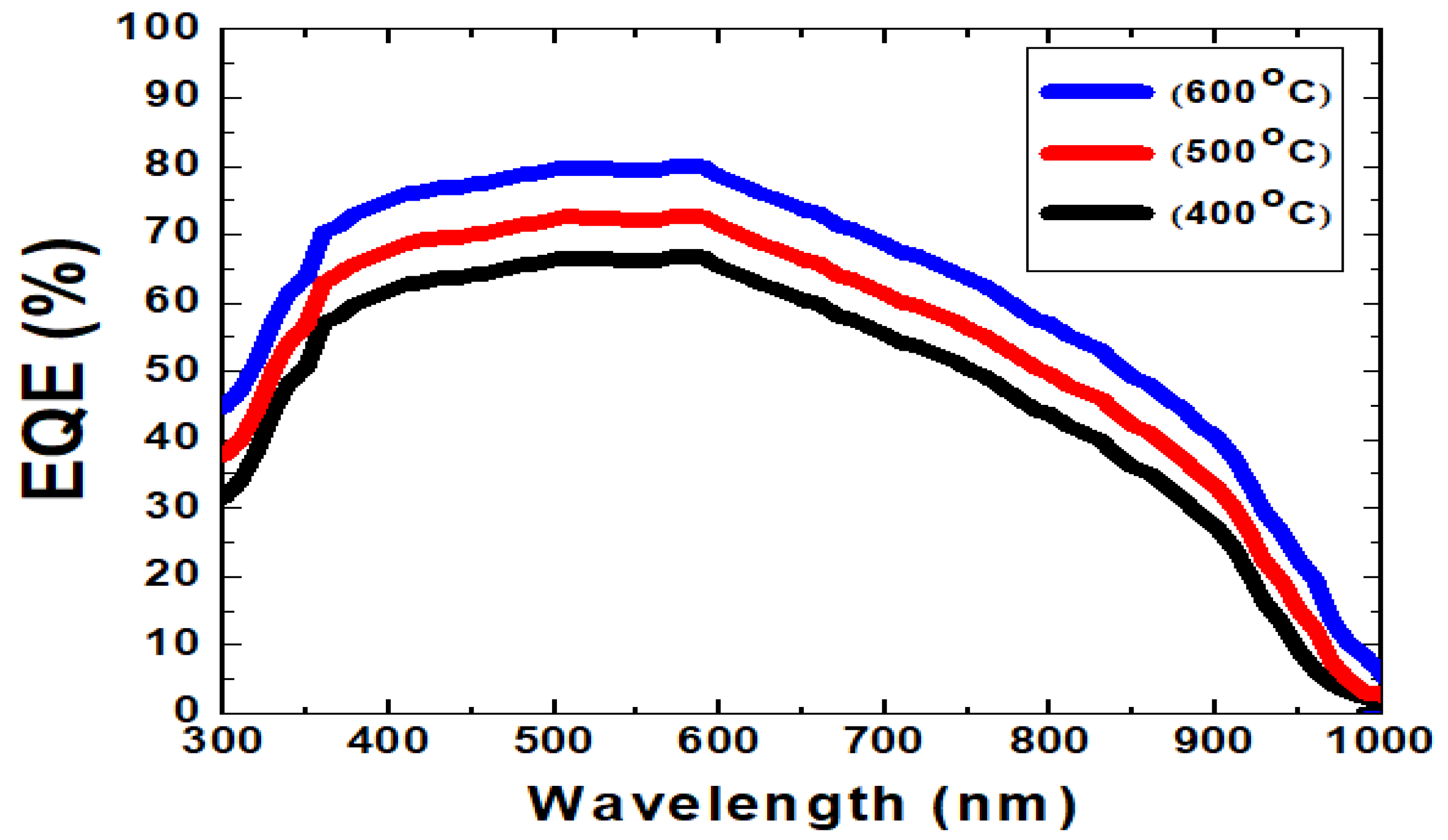
| HTM Thickness (nm) | VOC (V) | JSC (mA/cm2) | FF (%) | Eff (%) | RS (Ω) | Pmax (mW) |
|---|---|---|---|---|---|---|
| 100 | 0.96 | 20.4 | 67.4 | 13.2 | 16.9 | 1.32 |
| 200 | 1.02 | 20.9 | 66.6 | 14.2 | 15.8 | 1.42 |
| 300 | 1.00 | 20.9 | 65.6 | 13.7 | 16.2 | 1.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, L.-B.; Tseng, C.-C.; Wu, G.; Feng, W.-S.; Jeng, M.-J.; Chen, L.-C.; Lee, K.-L.; Popko, E.; Jacak, L.; Gwozdz, K. Low-Cost CuIn1−xGaxSe2 Ultra-Thin Hole-Transporting Material Layer for Perovskite/CIGSe Heterojunction Solar Cells. Appl. Sci. 2019, 9, 719. https://doi.org/10.3390/app9040719
Chang L-B, Tseng C-C, Wu G, Feng W-S, Jeng M-J, Chen L-C, Lee K-L, Popko E, Jacak L, Gwozdz K. Low-Cost CuIn1−xGaxSe2 Ultra-Thin Hole-Transporting Material Layer for Perovskite/CIGSe Heterojunction Solar Cells. Applied Sciences. 2019; 9(4):719. https://doi.org/10.3390/app9040719
Chicago/Turabian StyleChang, Liann-Be, Chzu-Chiang Tseng, Gwomei Wu, Wu-Shiung Feng, Ming-Jer Jeng, Lung-Chien Chen, Kuan-Lin Lee, Ewa Popko, Lucjan Jacak, and Katarzyna Gwozdz. 2019. "Low-Cost CuIn1−xGaxSe2 Ultra-Thin Hole-Transporting Material Layer for Perovskite/CIGSe Heterojunction Solar Cells" Applied Sciences 9, no. 4: 719. https://doi.org/10.3390/app9040719





