Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives
Abstract
:1. Introduction
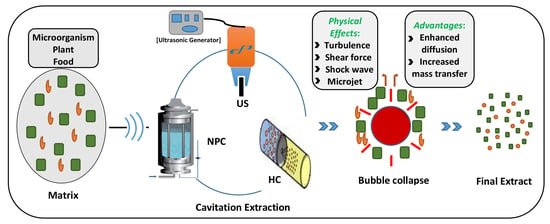
2. Principles of Cavitation-Based Extraction
- (1)
- Increased mass transfer rate and enhanced solvent penetration into the cells, due to the temperature and pressure generated during bubble collapse events resulting in thinning of membranes and disruption of cells.
- (2)
- Enhanced diffusion caused by microscopic level turbulence, intense inter-particle collision, and agitation in microporous particles of the matrix, due to the implosion of cavitating bubbles.
- (3)
- Enhanced diffusion of solvent into the matrix, due to hydration and swelling of the matrix with the enlargement of pores.
- (4)
- Generation of highly reactive free radicals and the associated radical driven cell disruption.
- (5)
- The increased surface area of matrix following disintegration by shock waves and microjets.
3. Extraction of Vital Products
3.1. Bioactive Compounds
3.2. Oils
3.3. Lipids
3.4. Proteins
3.5. Dyes and Pigments
3.6. Aromas and Flavors
4. Effect of Essential Factors on CE
4.1. Solvent Characteristics
4.2. Reactor Type and Its Design
4.3. Temperature
4.4. Intensity and Pressure
4.5. Frequency
5. Novel Combined Extraction Techniques
5.1. Cavitation and Microwave Assisted Extraction
5.2. Cavitation and Enzyme Assisted Extraction
5.3. Ultrasound and Supercritical Fluid Assisted Extraction
6. Advantages and Disadvantages of CE
- (1)
- Effective mixing capability;
- (2)
- Operation at a lower temperature;
- (3)
- Easy operation and the elimination of multiple process steps;
- (4)
- Selective extraction and enhanced yield.
7. Future Perspectives
- (1)
- Use of multi-frequency and multiple transducer sonoreactor.
- (2)
- Developing a continuous flow cell reactor, while introducing ultrasonic transducers in periodic spaces.
- (3)
- Eliminating erosion of transducer material via suitable building materials.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Espinal-Ruiz, M.; Restrepo-Sánchez, L.; Narváez-Cuenca, C.; Mcclements, D.J. Impact of pectin properties on lipid digestion under simulated gastrointestinal conditions: Comparison of citrus and banana passion fruit (Passiflora tripartita var. mollissima) pectins. Food Hydrocoll. 2015, 52, 329–342. [Google Scholar] [CrossRef]
- Picó, Y. Ultrasound-assisted extraction for food and environmental samples. TrAC Trends Anal. Chem. 2013, 43, 84–99. [Google Scholar] [CrossRef]
- Panda, D.; Manickam, S. Recent advancements in the sonophotocatalysis (SPC) and doped-sonophotocatalysis (DSPC) for the treatment of recalcitrant hazardous organic water pollutants. Ultrason. Sonochem. 2017, 36, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Manickam, S. Hydrodynamic cavitation assisted degradation of persistent endocrine-disrupting organochlorine pesticide Dicofol: Optimization of operating parameters and investigations on the mechanism of intensification. Ultrason. Sonochem. 2019, 51, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Manickam, S. Sonochemical degradation of endocrine-disrupting organochlorine pesticide Dicofol: Investigations on the transformation pathways of dechlorination and the influencing operating parameters. Chemosphere 2018, 204, 101–108. [Google Scholar]
- McDonnell, C.; Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology for Bioactives and Contaminants. Compr. Anal. Chem. 2017, 76, 111–129. [Google Scholar]
- Dong, J.; Liu, Y.; Liang, Z.; Wang, W. Investigation on ultrasound-assisted extraction of salvianolic acid B from Salvia miltiorrhiza root. Ultrason. Sonochem. 2010, 17, 61–65. [Google Scholar] [CrossRef]
- Guamán-balcázar, M.C.; Setyaningsih, W.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of resveratrol from functional foods: Cookies and jams. Appl. Acoust. 2016, 103, 207–213. [Google Scholar] [CrossRef]
- Gonzalez-Centeno, M.R.; Comas-Serra, F.; Femenia, A.; Rossello, C.; Simal, S. Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (Vitis vinifera L.): Experimental kinetics and modeling. Ultrason. Sonochem. 2015, 22, 506–514. [Google Scholar] [CrossRef]
- Parniakov, O.; Apicella, E.; Koubaa, M.; Barba, F.J.; Grimi, N.; Lebovka, N.; Pataro, G.; Ferrari, G.; Vorobiev, E. Ultrasound-assisted green solvent extraction of high-added value compounds from microalgae Nannochloropsis spp. Bioresour. Technol. 2015, 198, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Das, A.B.; Goud, V.V.; Das, C. Extraction of phenolic compounds and anthocyanin from black and purple rice bran (Oryza sativa L.) using ultrasound: A comparative analysis and phytochemical profiling. Ind. Crops Prod. 2017, 95, 332–341. [Google Scholar] [CrossRef]
- Lazar, L.; Talmaciu, A.L.; Volf, I.; Popa, V.I. Kinetic modeling of the ultrasound-assisted extraction of polyphenols from Picea abies bark. Ultrason. Sonochem. 2016, 32, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Qu, W.; Ma, H.; Atungulu, G.G.; McHugh, T.H. Continuous and pulsed ultrasound-assisted extractions of antioxidants from pomegranate peel. Ultrason. Sonochem. 2011, 18, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Hammi, K.M.; Jdey, A.; Abdelly, C.; Majdoub, H.; Ksouri, R. Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Zizyphus lotus fruits using response surface methodology. Food Chem. 2015, 184, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, L.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Li, Y.; Chen, Z. Ultrasound-assisted extraction from defatted oat (Avena sativa L.) bran to simultaneously enhance phenolic compounds and b—Glucan contents: Compositional and kinetic studies. J. Food Eng. 2018, 222, 1–10. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.; Yue, X.; Liang, J.; Jiang, J.; Gao, X.; Yue, P. Optimization of Ultrasound-Assisted Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Karim, R.; Mirhosseini, H.; Hamid, A.A. Optimization of pulsed ultrasound-assisted technique for extraction of phenolics from pomegranate peel of Malas variety: Punicalagin and hydroxybenzoic acids. Food Chem. 2016, 206, 156–166. [Google Scholar] [CrossRef]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.; Dangles, O.; Chemat, F. Ultrasound-assisted extraction of polyphenols (flavanone glycosides) from orange (Citrus sinensis L.) peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 241, 338–345. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Li, W.; Xiao, D.; Li, Z.; Wang, J. Negative-pressure cavitation extraction of secoisolariciresinol diglycoside from flaxseed cakes. Molecules 2015, 20, 11076–11089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zu, Y.; Fu, Y.; Luo, M.; Gu, C.; Wang, W.; Yao, X. Negative pressure cavitation extraction and antioxidant activity of biochanin A and genistein from the leaves of Dalbergia odorifera T. Chen. Sep. Purif. Technol. 2011, 83, 91–99. [Google Scholar] [CrossRef]
- Kong, Y.; Wei, Z.; Fu, Y.; Gu, C.; Zhao, C.; Yao, X.; Efferth, T. Negative—Pressure cavitation extraction of cajaninstilbene acid and pinostrobin from pigeon pea [Cajanus cajan (L.) Millsp.] leaves and evaluation of antioxidant activity. Food Chem. 2011, 128, 596–605. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, S.; Zu, Y.; Fu, Y.; Kong, Y.; Gao, Y.; Zhao, J.; Efferth, T. Negative pressure cavitation extraction and antioxidant activity of genistein and genistin from the roots of pigeon pea [Cajanus cajan (L.) Millsp.]. Sep. Purif. Technol. 2010, 74, 261–270. [Google Scholar] [CrossRef]
- Cheung, Y.; Wu, J. Kinetic models and process parameters for ultrasound-assisted extraction of water-soluble components and polysaccharides from a medicinal fungus. Biochem. Eng. J. 2013, 79, 214–220. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, Y.; Yang, H.; Mao, L. Extraction of Angelica sinensis polysaccharides using ultrasound-assisted way and its bioactivity. Int. J. Biol. Macromol. 2016, 88, 44–50. [Google Scholar] [CrossRef]
- Zhu, W.; Xue, X.; Zhang, Z. Ultrasonic-assisted extraction, structure and antitumor activity of polysaccharide from Polygonum multiflorum. Int. J. Biol. Macromol. 2016, 91, 132–142. [Google Scholar] [CrossRef]
- Oliveira, C.F.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F. Extraction of pectin from passion fruit peel assisted by ultrasound. LWT Food Sci. Technol. 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, J.; Liu, Y.; Zhao, M.; Wang, J. Ultrasound assisted extraction of polysaccharides from Lentinus edodes and its anti-hepatitis B activity in vitro. Int. J. Biol. Macromol. 2018, 107, 2217–2223. [Google Scholar] [CrossRef]
- Rao, P.R.; Rathod, V.K. Mapping study of an ultrasonic bath for the extraction of andrographolide from Andrographis paniculata using ultrasound. Ind. Crops Prod. 2015, 66, 312–318. [Google Scholar] [CrossRef]
- Hierro, J.N.; Herrera, T.; García-Risco, M.R.; Fornari, T.; Reglero, G.; Martin, D. Ultrasound-assisted extraction and bioaccessibility of saponins from edible seeds: Quinoa, lentil, fenugreek, soybean and lupin. Food Res. Int. 2018, 109, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Lee, A.Y.; Lee, A.R.; Choi, G.; Kim, H.K. Optimization of ultrasound-assisted extraction of glycyrrhizic acid from licorice using response surface methodology. Integr. Med. Res. 2017, 6, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Rendón, M.C.V. Phycocyanin Extraction from Spirulina Platensis with Hydrodynamic Cavitation and Its Determination by a Spectrometric Method. Ph.D. Thesis, Monterrey Institute of Technology and Higher Education, Monterrey, Mexico, 2015. [Google Scholar]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhang, M.; Wang, Z. Improved extraction of oil from chickpea under ultrasound in a dynamic system. J. Food Eng. 2010, 98, 13–18. [Google Scholar] [CrossRef]
- Juliano, P.; Bainczyk, F.; Swiergon, P.; Supriyatna, M.M.; Guillaume, C.; Ravetti, L.; Canamasas, P.; Cravotto, G.; Xu, X. Extraction of olive oil assisted by high-frequency ultrasound standing waves. Ultrason. Sonochem. 2017, 38, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, C.; Wang, B.; Yagoub, A.; Ma, H.; Zhang, X.; Wu, M. Study of ultrasonic cavitation during extraction of the peanut oil at varying frequencies. Ultrason. Sonochem. 2017, 37, 106–113. [Google Scholar] [CrossRef]
- Khoei, M.; Chekin, F. The ultrasound-assisted aqueous extraction of rice bran oil. Food Chem. 2016, 194, 503–507. [Google Scholar] [CrossRef]
- Sicaire, A.; Vian, M.A.; Fine, F.; Carré, P.; Tostain, S.; Chemat, F. Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrason. Sonochem. 2016, 31, 319–329. [Google Scholar] [CrossRef]
- Adam, F.; Abert-Vian, M.; Peltier, G.; Chemat, F. “Solvent-free” ultrasound—Assisted extraction of lipids from fresh microalgae cells: A green, clean and scalable process. Bioresour. Technol. 2012, 114, 457–465. [Google Scholar] [CrossRef]
- Piasecka, A.; Krzeminska, I.; Tys, J. Physical methods of microalgal biomass pretreatment. Int. Agrophys. 2014, 28, 341–348. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Drogui, P.; Surampalli, R.Y. Ultrasonication assisted lipid extraction from oleaginous microorganisms. Bioresour. Technol. 2014, 158, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Han, J. Simultaneous treatment (cell disruption and lipid extraction) of wet microalgae using hydrodynamic cavitation for enhancing the lipid yield. Bioresour. Technol. 2015, 186, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Setyawan, M.; Budiman, A.; Mulyono, P. Optimum Extraction of Algae-oil from Microalgae using Hydrodynamic Cavitation. Int. J. Renew. Energy Res. 2018, 8, 451–458. [Google Scholar]
- Karki, B.; Lamsal, B.P.; Jung, S.; Leeuwen, J.; Pometto, A.L.; Grewell, D.; Khanal, S.K. Enhancing protein and sugar release from defatted soy flakes using ultrasound technology. J. Food Eng. 2010, 96, 270–278. [Google Scholar] [CrossRef]
- Zhu, K.; Sun, X.; Zhou, H. Optimization of ultrasound-assisted extraction of defatted wheat germ proteins by reverse micelles. J. Cereal Sci. 2009, 50, 266–271. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.J.; Fryer, P.J.; Zuidam, N.J. Intensification of protein extraction from soybean processing materials using hydrodynamic cavitation. Innov. Food Sci. Emerg. Technol. 2017, 41, 47–55. [Google Scholar] [CrossRef]
- Luengo, E.; Condón-Abanto, S.; Condón, S.; Álvarez, I.; Raso, J. Improving the extraction of carotenoids from tomato waste by application of ultrasound under pressure. Sep. Purif. Technol. 2014, 136, 130–136. [Google Scholar] [CrossRef]
- Sivakumar, V.; Vijaeeswarri, J.; Anna, J.L. Effective natural dye extraction from different plant materials using ultrasound. Ind. Crops Prod. 2011, 33, 116–122. [Google Scholar] [CrossRef]
- Zou, Y.; Xie, C.; Fan, G.; Gu, Z.; Han, Y. Optimization of ultrasound-assisted extraction of melanin from Auricularia auricula fruit bodies. Innov. Food Sci. Emerg. Technol. 2010, 11, 611–615. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.; Farhat, F.; Thiéry, V.; Piot, J.; Bérard, J.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Cardoso, L.C.; Serrano, C.M.; Rodríguez, M.R.; de la Ossa, E.J.M.; Lubián, L.M. Extraction of carotenoids and fatty acids from microalgae using supercritical technology. Am. J. Anal. Chem. 2012, 3, 877–883. [Google Scholar] [CrossRef]
- Lebovka, N.; Vorobiev, E.; Chemat, F. Enhancing Extraction Processes in the Food Industry, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 173–193. [Google Scholar]
- Mnayer, D.; Fabiano-Tixier, A.; Petitcolas, E.; Ruiz, K.; Hamieh, T.; Chemat, F. Extraction of green absolute from thyme using ultrasound and sunflower oil. Resour. Technol. 2017, 3, 12–21. [Google Scholar] [CrossRef]
- Dong, L.; Fu, Y.; Zu, Y.; Li, J.; Li, X.; Efferth, T. Negative pressure cavitation accelerated processing for extraction of main bioactive flavonoids from Radix Scutellariae. Chem. Eng. Process. Intens. 2011, 50, 780–789. [Google Scholar] [CrossRef]
- Dong, L.; Fu, Y.; Zu, Y.; Luo, M.; Wang, W.; Li, X.; Li, J. Application of cavitation system to accelerate the endogenous enzymatic hydrolysis of baicalin and wogonoside in Radix Scutellariae. Food Chem. 2012, 131, 1422–1429. [Google Scholar] [CrossRef]
- Mu, F.; Yang, L.; Wang, W.; Luo, M.; Fu, Y.; Guo, X.; Zu, Y. Negative-pressure cavitation extraction of four main vinca alkaloids from Catharanthusroseus leaves. Molecules 2012, 17, 8742–8752. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yang, L.; Yao, X.; Mu, F.; Zhang, D.; Song, Z.; Qiao, Q.; Fu, Y.; Zu, Y. Optimization of enzyme-assisted negative pressure cavitation extraction of five main indole alkaloids from Catharanthus roseus leaves and its pilot-scale application. Sep. Purif. Technol. 2014, 125, 66–73. [Google Scholar] [CrossRef]
- Zhang, D.; Zu, Y.; Fu, Y.; Luo, M.; Wang, W.; Gu, C.; Zhao, C.; Jiao, J.; Efferth, T. Enzyme pretreatment and negative pressure cavitation extraction of genistein and apigenin from the roots of Pigeon pea [Cajanus cajan (L.) Millsp.] and the evaluation of antioxidant activity. Ind. Crops Prod. 2012, 37, 311–320. [Google Scholar] [CrossRef]
- Yan, M.; Chen, C.; Zhao, B.; Zu, Y.; Fu, Y.; Liu, W.; Efferth, T. Enhanced extraction of astragalosides from Radix astragali by negative pressure cavitation-accelerated enzyme pretreatment. Bioresour. Technol. 2010, 101, 7462–7471. [Google Scholar] [CrossRef]
- Zhao, B.; Fu, Y.; Wang, W.; Zu, Y.; Gu, C.; Luo, M.; Efferth, T. Enhanced extraction of isoflavonoids from Radix Astragali by incubation pretreatment combined with negative pressure cavitation and its antioxidant activity. Innov. Food Sci. Emerg. Technol. 2011, 12, 577–585. [Google Scholar] [CrossRef]
- Duan, M.; Luo, M.; Zhao, C.; Wang, W.; Zu, Y.; Zhang, D.; Yao, X.; Fu, Y. Ionic liquid-based negative pressure cavitation-assisted extraction of three main flavonoids from the Pigeonpea roots and its pilot-scale application. Sep. Purif. Technol. 2013, 107, 26–36. [Google Scholar] [CrossRef]
- Qi, X.; Peng, X.; Huang, Y.; Li, L.; Wei, Z.; Zu, Y.; Fu, Y. Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind. Crops Prod. 2015, 70, 142–148. [Google Scholar] [CrossRef]
- Jiao, J.; Wei, F.; Gai, Q.; Wang, W.; Luo, M.; Fu, Y. A pilot-scale homogenization-assisted negative pressure cavitation extraction of Astragalus polysaccharides. Int. J. Biol. Macromol. 2014, 67, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhang, D.; Luo, M.; Jin, S.; Zu, Y.; Efferth, T. Negative pressure cavitation-microwave assisted preparation of extract of Pyrola incarnata Fisch. rich in hyperin, 2’-O-galloylhyperin and chimaphilin and evaluation of its antioxidant activity. Food Chem. 2015, 169, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Xu, W.; Yao, X.; Zhang, D.; Zhang, Y.; Fu, Y.; Zu, Y. Homogenate-assisted negative pressure cavitation extraction of active compounds from Pyrola incarnata Fisch. and the extraction kinetics study. Innov. Food Sci. Emerg. Technol. 2015, 27, 86–93. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, X.; Duan, M.; Luo, M.; Wang, W.; Fu, Y.; Zu, Y.; Efferth, T. An effective negative pressure cavitation-microwave assisted extraction for determination of phenolic compounds in P. calliantha H. Andr. Analyst 2013, 138, 4631–4641. [Google Scholar] [CrossRef]
- Wang, T.; Guo, N.; Wang, S.; Kou, P.; Zhao, C.; Fu, Y. Ultrasound-negative pressure cavitation extraction of phenolic compounds from blueberry leaves and evaluation of its DPPH radical scavenging activity. Food Bioprod. Process. 2018, 108, 69–80. [Google Scholar] [CrossRef]
- Dias, A.L.; Sergio, C.S.; Santos, P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Ultrasound-assisted extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L.): Effects on the vegetable matrix and mathematical modeling. J. Food Eng. 2017, 198, 36–44. [Google Scholar] [CrossRef]
- Hamed, S.F.; Wagdy, S.M.; Megahed, M.G. Chemical characteristics and antioxidant capacity of Egyptian and Chinese sunflower seeds: A case study. Life Sci. J. 2012, 9, 421–429. [Google Scholar]
- Li, Y.; Fabiano-Tixier, A.S.; Tomao, V.; Cravotto, G.; Chemat, F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013, 20, 12–18. [Google Scholar] [CrossRef]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Jerman, T.; Trebse, P.; Vodopivec, B.M. Ultrasound-assisted solid liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem. 2010, 123, 175–182. [Google Scholar] [CrossRef]
- Bajerová, P.; Adam, M.; Bajer, T.; Ventura, K. Comparison of various techniques for the extraction and determination of antioxidants in plants. J. Sep. Sci. 2014, 37, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Golash, N.; Gogate, P.R. Degradation of dichlorvos containing wastewaters using sonochemical reactors. Ultrason. Sonochem. 2012, 19, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108–120. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: A review. Ultrason. Sonochem. 2014, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.P.; Gogate, P.R. Intensification of degradation of Rhodamine B using hydrodynamic cavitation in the presence of additives. Sep. Purif. Technol. 2010, 75, 385–391. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Q.; Ngoh, G.C.; Yusoff, R.; Teoh, W.H. Sequential ultrasound-microwave assisted acid extraction (UMAE) of pectin from pomelo peels. Int. J. Biol. Macromol. 2016, 93, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zheng, Z.; Li, H.; Cao, R.; Zheng, Y.; Yu, H.; Xiao, J.; Miao, S.; Zheng, B. Optimization of ultrasonic-microwave assisted extraction of oligosaccharides from lotus (Nelumbo nucifera Gaertn.) seeds. Ind. Crops Prod. 2017, 107, 546–557. [Google Scholar] [CrossRef]
- Lu, C.; Wang, H.; Lv, W.; Ma, C.; Xu, P.; Zhu, J.; Xie, J.; Liu, B.; Zhou, Q. Ionic liquid-based ultrasonic/microwave-assisted extraction combined with UPLC for the determination of anthraquinones in rhubarb. Chromatographia 2011, 74, 139–144. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, J.; Diao, W.; Wang, C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata). Carbohydr. Polym. 2014, 113, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Tchabo, W.; Ma, Y.; Engmann, F.N.; Zhang, H. Ultrasound-assisted enzymatic extraction (UAEE) of phytochemical compounds from mulberry (Morus nigra) must and optimization study using response surface methodology. Ind. Crops Prod. 2015, 63, 214–225. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Hu, D.; Xiao, K.; Wu, J. Efficient extraction of pectin from sisal waste by combined enzymatic and ultrasonic process. Food Hydrocoll. 2018, 79, 189–196. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, S.; Wang, M.; He, L. PEG-based ultrasound-assisted enzymatic extraction of polysaccharides from Ginkgo biloba leaves. Int. J. Biol. Macromol. 2015, 80, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Goula, A.M.; Papatheodorou, A.; Karasavva, S.; Kaderides, K. Ultrasound-Assisted Aqueous Enzymatic Extraction of Oil from Pomegranate Seeds. Waste Biomass Valorization 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Dias, A.L.; Sergio, C.S.; Santos, P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Effect of ultrasound on the supercritical CO2 extraction of bioactive compounds from dedo de moca pepper (Capsicum baccatum L. var. pendulum). Ultrason. Sonochem. 2016, 31, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Santos-Zea, L.; Antunes-Ricardo, M.; Gutierrez-Uribe, J.A.; García-Pérez, J.V.; Benedito, J. Effect of ultrasound transducer design on the acoustically-assisted supercritical fluid extraction of antioxidants from oregano. Ultrason. Sonochem. 2018, 47, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Reátegui, J.L.; Machado, A.P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Extraction of antioxidant compounds from blackberry (Rubus sp.) bagasse using supercritical CO2 assisted by ultrasound. J. Supercrit. Fluids 2014, 94, 223–233. [Google Scholar] [CrossRef]
- Santos, P.; Aguiar, A.C.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Supercritical carbon dioxide extraction of capsaicinoids from malagueta pepper (Capsicum frutescens L.) assisted by ultrasound. Ultrason. Sonochem. 2015, 22, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.; García-Reverter, J.; Casas, E.; Riera, E. Effect of high power ultrasounds on mass-transfer zone in supercritical fluid extraction processes. In Proceedings of the 20th International Congress on Acoustics, Sydney, Australia, 23–27 August 2010. [Google Scholar]

| Category | Vital Products | Matrix | Conditions | Yield | Ref. |
|---|---|---|---|---|---|
| Bioactive compounds | Phenolic compounds | Defatted oat | f (kHz): 40; P (W): 200-600; T (°C): 70; ED (min): 25 | 184.16 mg/g | [16] |
| Blueberry wine pomace | P (W): 400; T (°C): 61.03; ED (min): 23.67; L/S: 21.70:1 | 16.41 mg/g | [17] | ||
| Pomegranate peel | f (kHz): 24; I (W/cm2): 105; ED (min): 10; L/S: 70:1 | 320.26 mg/g | [18] | ||
| Mandarin peel | f (kHz): 38.5; P (W): 56.71; T (°C): 48; ED (min): 40 | 26.51% | [20] | ||
| Mulberry pulp | f (kHz): 24; P (W): 200; T (°C): 64; ED (min): 10; L/S: 11:1.5; pH: 7 | 1.21 mg/g | [21] | ||
| Polysaccharides | Fungus | f (kHz): 20; I (W/cm2): 44.1; T (°C): 70; ED (min): 40; L/S: 70:1 | 0.180 ± 0.028 g/g | [26] | |
| Angelica sinensis | P (W): 180; T (°C): 90; ED (min): 45; L/S: 7:1 | 6.96% | [27] | ||
| Polygonum multiflorum | P (W): 140; T (°C): 62; ED (min): 80; L/S: 20:1 | 5.49% | [28] | ||
| Passion fruit | f (kHz): 20; I (W/cm2): 664; T (°C): 85; ED (min): 10; L/S: 30:1; pH: 2 | 12.67% | [29] | ||
| Lentinus edodes | P (W): 290; T (°C): 45; ED (min): 21; L/S: 20:1 | 9.75% | [30] | ||
| Traditional medicine | Andrographolide | Andrographis paniculata | f (kHz): 22; P (W): 134; T (°C): 40; ED (min): 10; L/S: 40:1 | 29.97 mg/g | [31] |
| Saponin | Edible seeds (quinoa, lentil, fenugreek, soybean, lupin) | - | 5.51 ± 1.18, 10.63 ± 1.86, 12.90 ± 0.91, 4.08 ± 0.7, 4.55 ± 0.36 g/100g | [32] | |
| Glycyrrhizic acid | Licorice | f (kHz): 44; P (W): 250; T (°C): 69; Extraction time (min): 34 | 3.414% | [33] | |
| Oil | Olive | f (kHz): 40 and 585; P (W): 242; T (°C): 29; ED (min): 50 | - | [37] | |
| Peanut | f (kHz): 40; Power density (W/L): 115; ED (min): 60; L/S: 6:1 | - | [38] | ||
| Rice bran | f (kHz): 60; T (°C): 45; ED (min): 70; pH: 12 | - | [39] | ||
| Protein | Defatted soy flakes | f (kHz): 20; P (W): 1280; ED (min): 2; pH: 8.5 | 78% | [46] | |
| Defatted wheat germ | f (kHz): 20; P (W): 363; ED (min): 24 | 57% | [47] | ||
| Dyes and pigments | Carotenoids | Tomato waste | f (kHz): 20; T (°C): 45; ED (min): 6 | 13.59 ± 1.06 mg/g | [49] |
| Dyes | Plants (Acacia decurrens, Tagetes erecta, Punica granatum, Mirabilis jalpa, Celosia cristata) | f (kHz): 20; P (W): 80; T (°C): 45; ED (h): 3 | 4.5, 26, 20, 26, 16 % | [50] | |
| Melanin | Auricularia auricula fruit bodies | f (kHz): 40; P (W): 250; T (°C): 63; ED (min): 36; L/S: 43:1; pH:12 | 120.05 mg/100 g | [51] | |
| Pigments: Fucoxanthin, Chlorophyll | Marine microalgae | P (W): 12.2; T (°C): 8.5; ED (min): 15 | 4.49 ± 0.08, 4.95 ± 0.27 μg/mg | [52] | |
| Aroma and Flavor | Green absolute | Thyme | f (kHz): 20; P (W): 98; T (°C): 50; ED (min): 22; L/S: 10:1 | 5.92 g/100 g | [55] |
| HC Reactor | Extract | Matrix | Conditions | Yield | Ref. |
|---|---|---|---|---|---|
| Venturi | lipids | Microalgae Nannochloropsis salina (wet) | Cv: 1.17; ED (min): 25.05 | 25.9–99% | [44] |
| Microalgae Nannochloropsis sp. (dry) | Cv: 0.126; T (°C): 42; ED (h): 2 | 93% | [45] | ||
| Protein | Soybean | Inlet pressure (MPa): 100 | 82% | [48] | |
| Phycocyanin | Spirulina Platensis | - | - | [34] | |
| NPC | Secoisolariciresinol diglucoside | Flaxseed cakes | NP (MPa): −0.04; T (°C): 35; ED (min): 35; L/S: 13.16:1 | 16.25 mg/g | [22] |
| Flavonoids: genistin, genistein | Pigeon pea roots | NP (MPa): −0.05; Room temp.; ED (min): 45; L/S: 44:1 | 0.418, 0.398 mg/g | [25] | |
| Cajaninstilbene acid, Pinostrobin | Pigeon pea leaves | NP (MPa): −0.075; T (°C): 45; ED (min): 30; L/S: 30:1 | 5.675 ± 0.127, 0.538 ± 0.014 mg/g | [24] | |
| Baicalin, Wogonoside, Baicalein, Wogonin | Radix Scutellariae | NP (MPa): −0.07; Room temp.; ED (min): 60; L/S: 40:1 | 128.89 ± 2.32, 25.07 ± 1.42, 28.28 ± 1.71, 7.55 ± 0.80 mg/g | [56] | |
| Baicalein, Wogonin | Radix Scutellariae | NP (MPa): −0.07; T (°C): 50; ED (min): 60; L/S: 20:1 | 95.8 ± 1.67, 70.65 ± 0.67 mg/g | [57] | |
| Biochanin A, Genistein | Dalbergia odorifera T. Chen leaves | NP (MPa): −0.05; Room temp.; ED (min): 20; L/S: 24:1 | 1.583, 0.933 mg/g | [23] | |
| Alkaloids (vindoline, catharanthine, vincristine, vinblastine) | Catharanthus roseus leaves | NP (MPa): −0.075; ED (min): 30; L/S: 20:1 | 0.5783, 0.2843, 0.018, 0.126 mg/g | [58] | |
| NPC/Enzyme | Alkaloids (vindoline, catharanthine, vincristine, vinblastine, anhydravinblastine) | Catharanthus roseus leaves | NPC: NP (MPa): −0.075; ED (min): 30; L/S: 20:1. Enzyme: Incubation T (°C): 35.87; Incubation time (h): 8.62; pH: 4.73 | 4.940 ± 0.215, 6.283 ± 0.307, 0.049 ± 0.002, 0.066 ± 0.003, 0.038 ± 0.001 mg/g | [59] |
| Flavonoids: genistein, apigenin | Pigeon pea roots | NPC: NP (MPa): −0.04; T (°C): 30; ED (min): 20; L/S: 40:1. Enzyme: Incubation time (h): 6; pH: 5 | 0.628, 0.359 mg/g | [60] | |
| Astragalosides III, Astragalosides IV | Radix Astragali (Astragalus) | NP (MPa): −0.08; Incubation T (°C): 45; ED (min): 30; L/S: 50:1 | 0.103, 0.325 mg/g | [61] | |
| Isoflavonoids (Calycosin, Formononetin) | Radix Astragali (Astragalus) | NPC: NP (MPa): −0.080; ED (min): 30; L/S: 25:1. Enzyme: Incubation T (°C): 35; Time (min): 60; pH: 4 | 0.650 ± 0.015, 0.307 ± 0.013 mg/g | [62] | |
| NPC/IL | Flavonoids: genistin, genistein, apigenin | Pigeon pea roots | NP (MPa): −0.07; T (°C): 74; ED (min): 15; L/S: 20:1 | 0.482 ± 0.008, 0.496 ± 0.017, 0.291 ± 0.015 mg/g | [63] |
| NPC/DES | Flavonoids: (9 type) | Equisetum palustre L. | NP (MPa): −0.07; T (°C): 60; ED (min): 20; L/S ratio: 25:1 | 57.14–89.25% | [64] |
| NPC/Homogenate | Polysaccharides | Radix Astragali (Astragalus) | Homogenate time (s): 70; NP (MPa): −0.068; T (°C): 64.8; ED (min): 53; L/S: 13.4 | 16.74% | [65] |
| NPC/MW | hyperin, 2′-O-galloylhyperin, chimaphilin | Pyrola (P. incarnata Fisch) | Microwave power (W): 700; NP (MPa): −0.05; T (°C): 50; ED (min): 12; L/S: 30:1 | 1.339 ± 0.029, 4.831 ± 0.117, 0.329 ± 0.011 mg/g | [66] |
| NPC/Homogenate | Homogenate time (s): 120; NP (MPa): −0.05; T (°C): 50; ED (min): 25; L/S: 30:1 | 1.205 ± 0.054, 4.961 ± 0.108, 0.291 ± 0.016 mg/g | [67] | ||
| NPC/MW | Phenolic compounds | Pyrola | Microwave power (W): 700; NP (MPa): −0.07; T (°C): 40; ED (min): 15; L/S: 20:1 | 0.406–5.977 mg/g | [68] |
| NPC/US | Total Phenols, flavonoids, procyanidins | Blueberry leaves | NP (MPa): −0.07; T (°C): 50; ED (min): 15; L/S: 30:1 | 352.12 ± 12.8, 111 ± 4.11, 211±7.81 mg/g | [69] |
| Type | Extract | Matrix | Conditions | Yield | Ref. |
|---|---|---|---|---|---|
| US/MW | Pectin | Pomelo peels | MW: P (W): 643.44; Irradiation time (min): 6.40. US: f (kHz): 40; Sonication time (min): 27.52. Solvent: water; pH: 1.80 | 38% | [81] |
| Oligosaccharides | lotus seeds | MW: P (W): 250 US: f (kHz): 25; P (W): 300.46; ED (min): 5.42; L/S: 10:1; Solvent: water | 11.009 ± 0.019% | [82] | |
| Anthraquinones | Rhubarb | MW: P (W): 500. US: P (W): 300; ED (min): 2; L/S: 15:1 | 28 mg/g | [83] | |
| US/Enzyme | Polysaccharides | Pumpkin | US:f (kHz): 20; P (W): 440; T (°C): 51.5; ED (min): 20; L/S: 5.70:1; pH: 5; Solvent: water | 4.33 ± 0.15% | [84] |
| Phytochemical: Total phenolics, Total flavonoids, Total anthocyanins | Mulberry must | US:f (kHz): 34; P (W): 60 W; T (°C): 20; Solvent: water. Enzyme: Enzyme concentration: 0.010% (v/w); ED (min): 12 | 298.06; 379.24; 55.14 (mg/100 mL) | [85] | |
| Pectin | Sisal waste | US:f (kHz): 20; P: 450 W. Enzyme: Enzyme loading: 88 U/g; T (°C): 50; ED (h): 20; L/S: 15:1; pH: 4 | 31.1% | [86] | |
| Polysaccharides | Gingko biloba leaves | Solvent: Polyethylene glycol; T (°C): 51.88; ED (min): 37.13; pH: 4.34 | 7.29 ± 0.21% | [87] | |
| Oil | Pomegranate Seeds | US:f (kHz): 20; P (W): 130; T (°C): 55. Enzyme: Enzyme loading: 2% w/w; ED (min): 10; pH: 5. L/S: 6:1; Solvent: Water. | 95.8% | [88] | |
| SFE-US | Bioactive compounds | Dedo de moça pepper | Solvent flow rate: 1.7569×10−4 kg/s; Pressure (MPa): 20. US: P (W): 800; T (°C): 40; ED (min): 60 | 45% | [89] |
| Antioxidants | Oregano | Solvent flow rate: 1 ± 0.1 kg/h; Pressure (MPa): 35. US: f (kHz): 30; Power density (W/L): 150; T (°C): 35; ED (min): 60 | 26.4 ± 1.1 μmol TE/g | [90] | |
| Antioxidants | Blackberry bagasse | Solvent flow rate: 2.77×10−4 kg/s; Pressure (MPa): 25. US: P (W): 400; T (°C): 50; ED (min): 120; L/S: 400 | 9.87 ± 0.40% | [91] | |
| Capsaicinoids | Malagueta pepper | Solvent flow rate: 1.673×10−4 kg/s; Pressure (MPa): 15. US: f (kHz): 20; P (W): 360; T (°C): 40; ED (min): 60; L/S: 600 ± 2 | 75.3% | [92] | |
| Oil | Almond | Solvent flow rate: 15 kg/h; Pressure (MPa): 33. US: f (kHz): 20; P (W): 75; T (°C): 45; ED (h): 5; L/S: 50 | 18% | [93] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, D.; Manickam, S. Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Appl. Sci. 2019, 9, 766. https://doi.org/10.3390/app9040766
Panda D, Manickam S. Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives. Applied Sciences. 2019; 9(4):766. https://doi.org/10.3390/app9040766
Chicago/Turabian StylePanda, Debabrata, and Sivakumar Manickam. 2019. "Cavitation Technology—The Future of Greener Extraction Method: A Review on the Extraction of Natural Products and Process Intensification Mechanism and Perspectives" Applied Sciences 9, no. 4: 766. https://doi.org/10.3390/app9040766