Controlling the Oxygen Electrocatalysis on Perovskite and Layered Oxide Thin Films for Solid Oxide Fuel Cell Cathodes
Abstract
:1. Introduction
| Material | D*/cm2∙s−1 @ 600 °C | k*/cm∙s−1 @ 600 °C | Reference |
|---|---|---|---|
| La0.8Sr0.2MnO3−δ | 10−17 | 1.12 × 10−10 | [8] |
| La0.5Sr0.5MnO3−δ | 4.22 × 10−18 | 10−10 | [8] |
| La0.8Sr0.2CoO3−δ | 1.69 × 10−13 | 1.38 × 10−8 | [8] |
| La0.6Sr0.4CoO3−δ | 2.86 × 10−9 | 9.09 × 10−8 | [21] |
| La0.5Sr0.5CoO3−δ | 2.51 × 10−10 | 3.29 × 10−7 | [8] |
| La0.6Sr0.4Co0.2Fe0.8O3−δ | 5.83 × 10−10 | 2.35 × 10−7 | [23] |
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ | 1.28 × 10−7 | 5.21 × 10−5 | [24] |
| La2NiO4+δ | 1.35 × 10−8 | 1.02 × 10−6 | [13] |
| La1.9Sr0.1NiO4+δ | 4.66 × 10−9 | 3.56 × 10−8 | [13] |
| La1.8Sr0.2NiO4+δ | 2.02 × 10−10 | 3.51 × 10−9 | [13] |
| La2CuO4+δ | 2.76 × 10−9 | 5.79 × 10−7 | [11] |
| La2Ni0.9Co0.1O4+δ | 1.07 × 10−8 | 6.52 × 10−7 | [14] |
| La2Ni0.9Co0.1O4+δ | 1.34 × 10−8 | 7.89 × 10−7 | [14] |
| La2CoO4+δ | 2.98 × 10−8 | 3.91 × 10−6 | [25] |
2. Oxygen Reduction in SOFCs
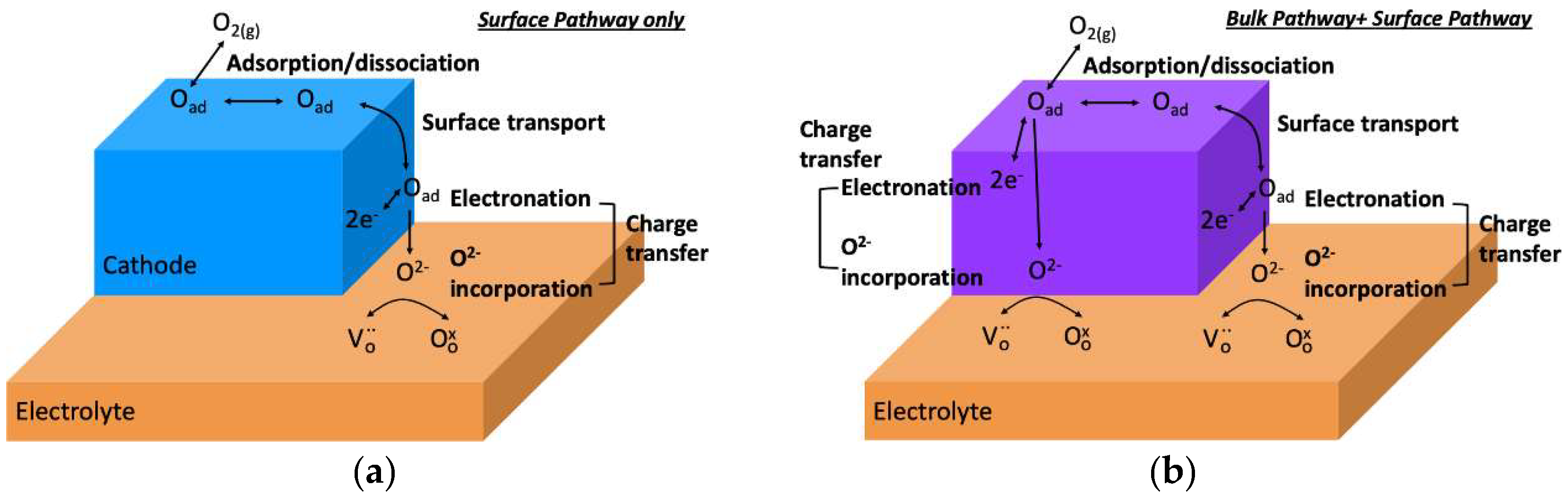
2.1. Oxygen Reduction Reaction Steps and Pathways in SOFC Cathodes
2.2. Oxygen Diffusion and Surface Exchange Kinetics in MIEC Cathodes
3. Thin Film Cathodes
3.1. ABO3 Oxide Thin Films
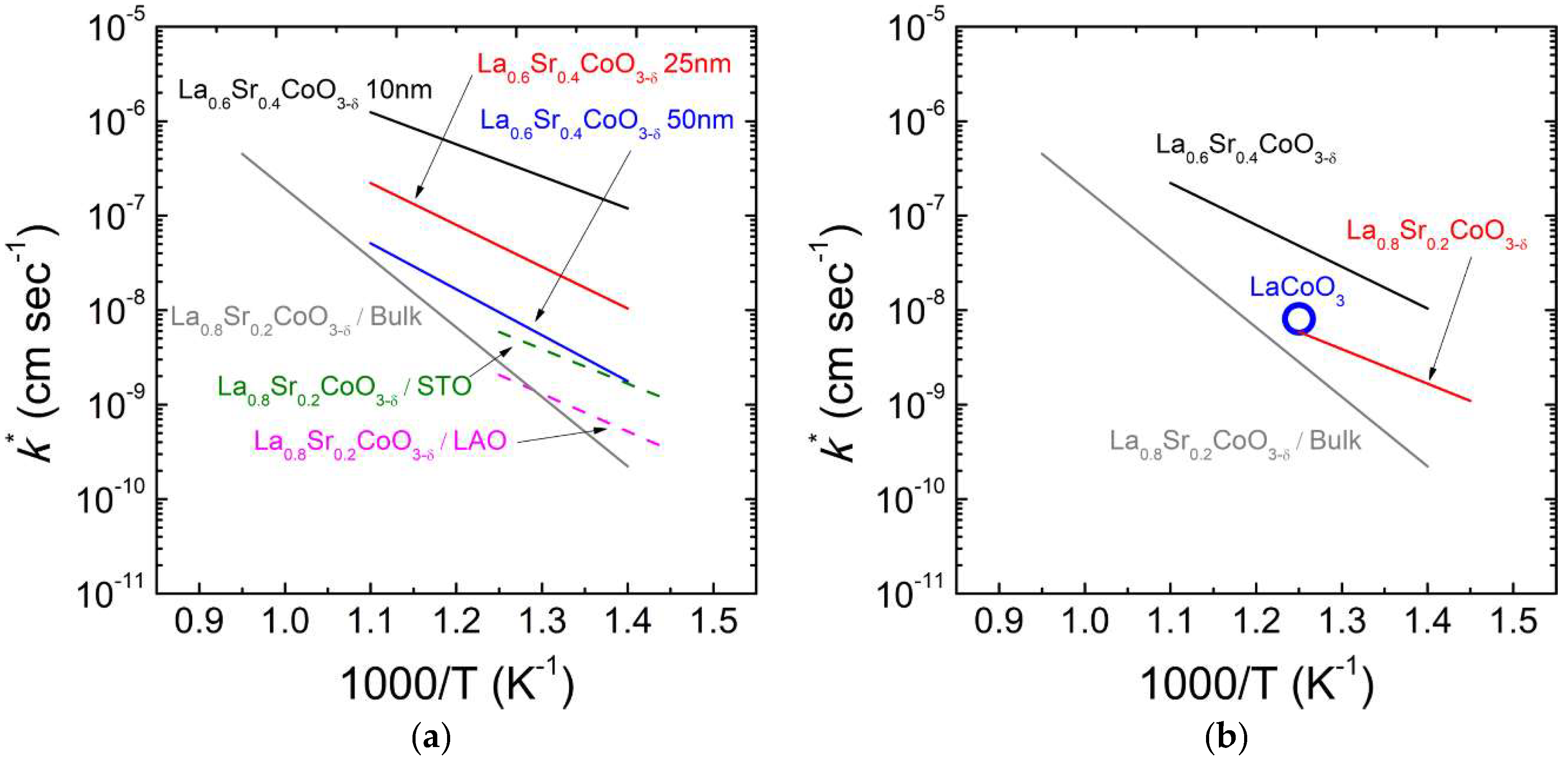
3.1.1. Cobaltites
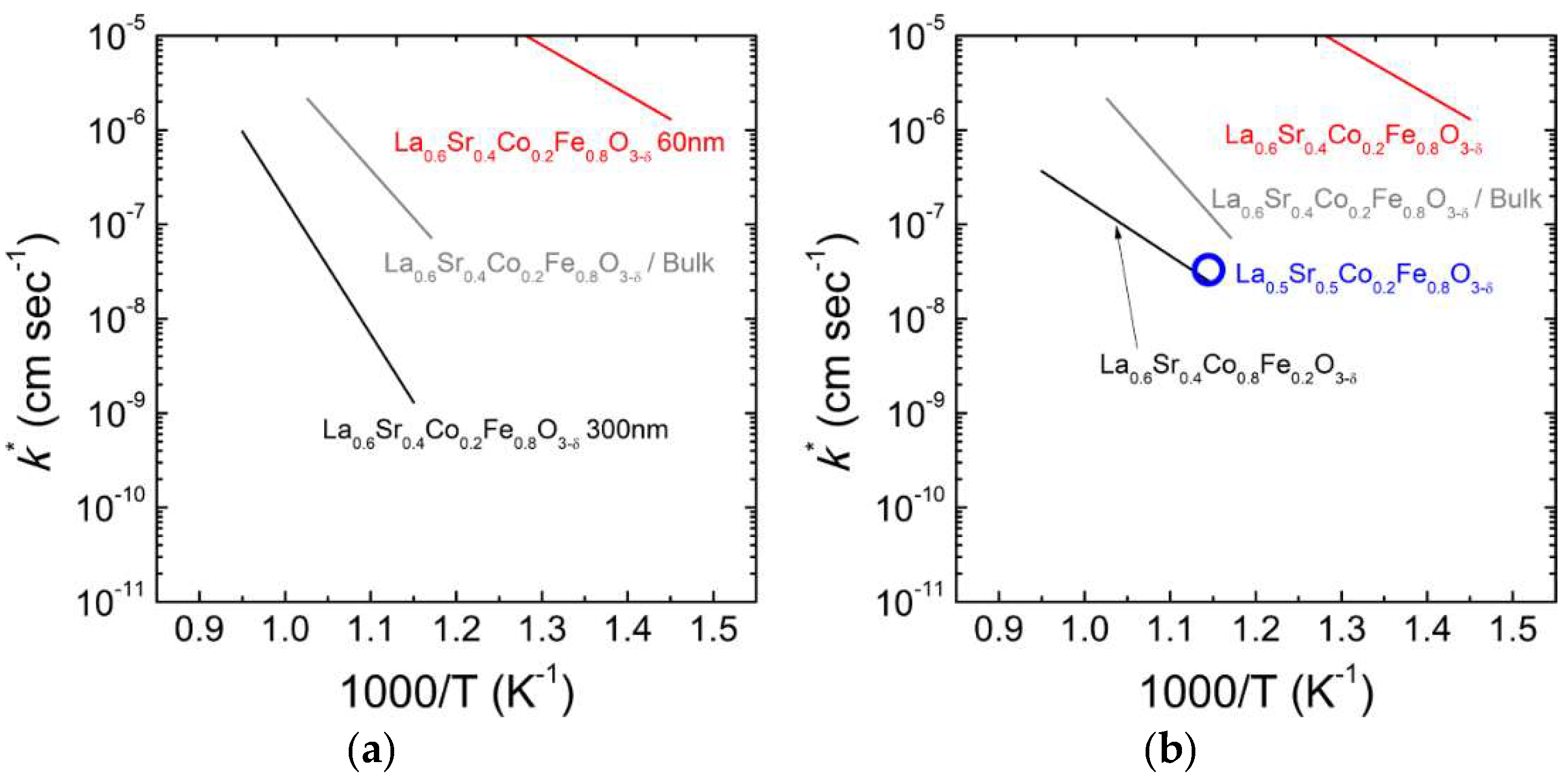
3.1.2. Ferrites
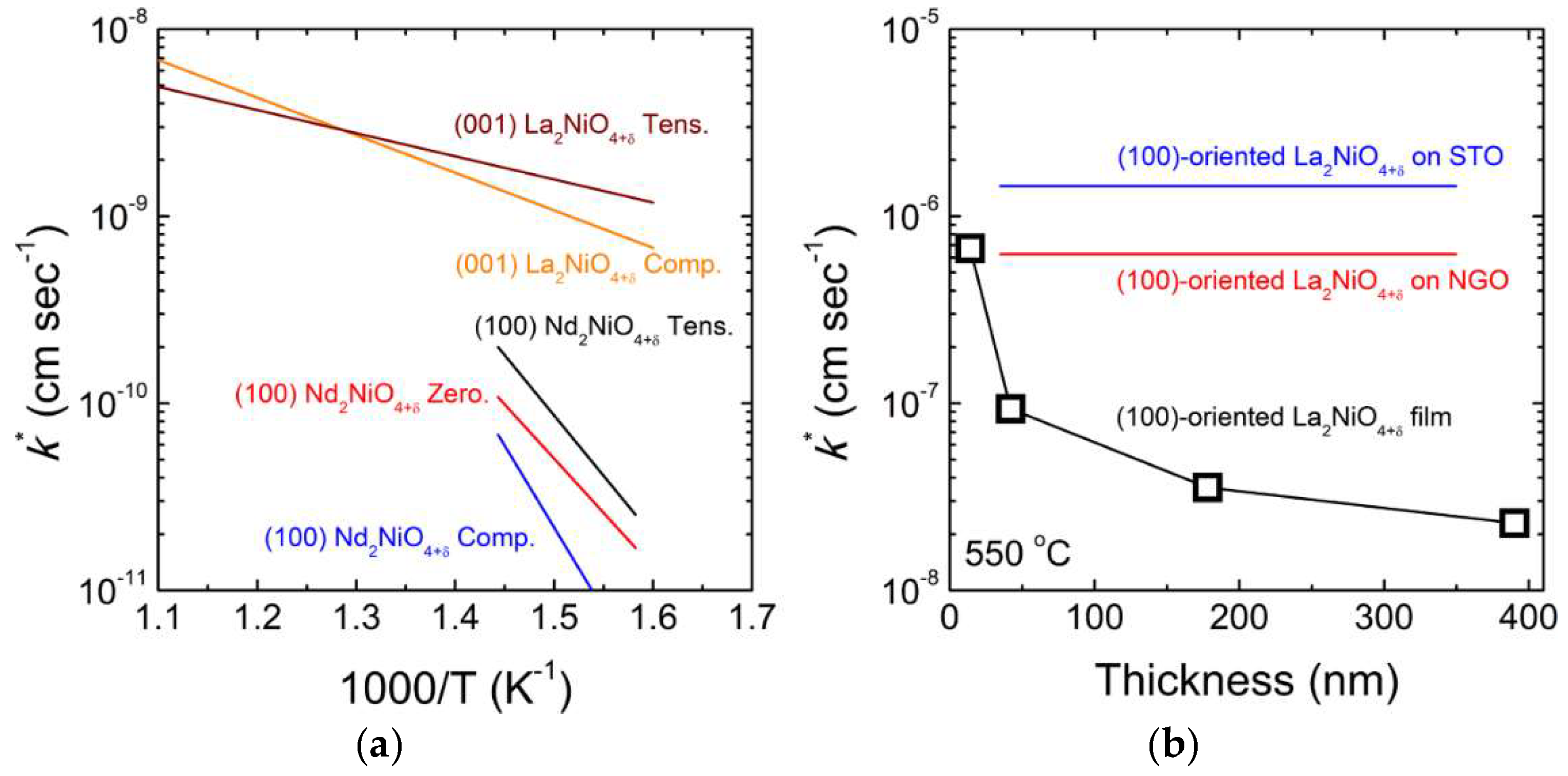
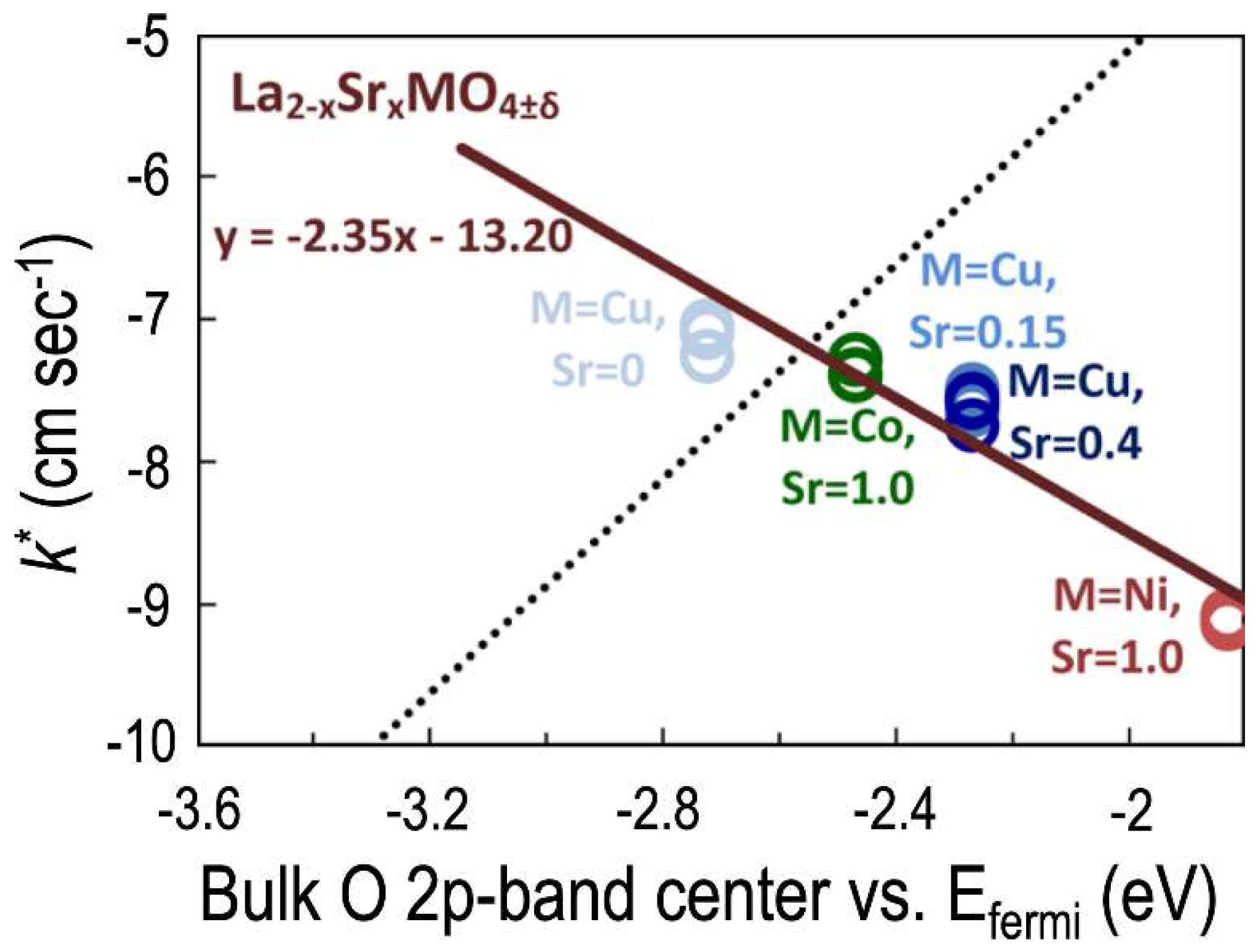
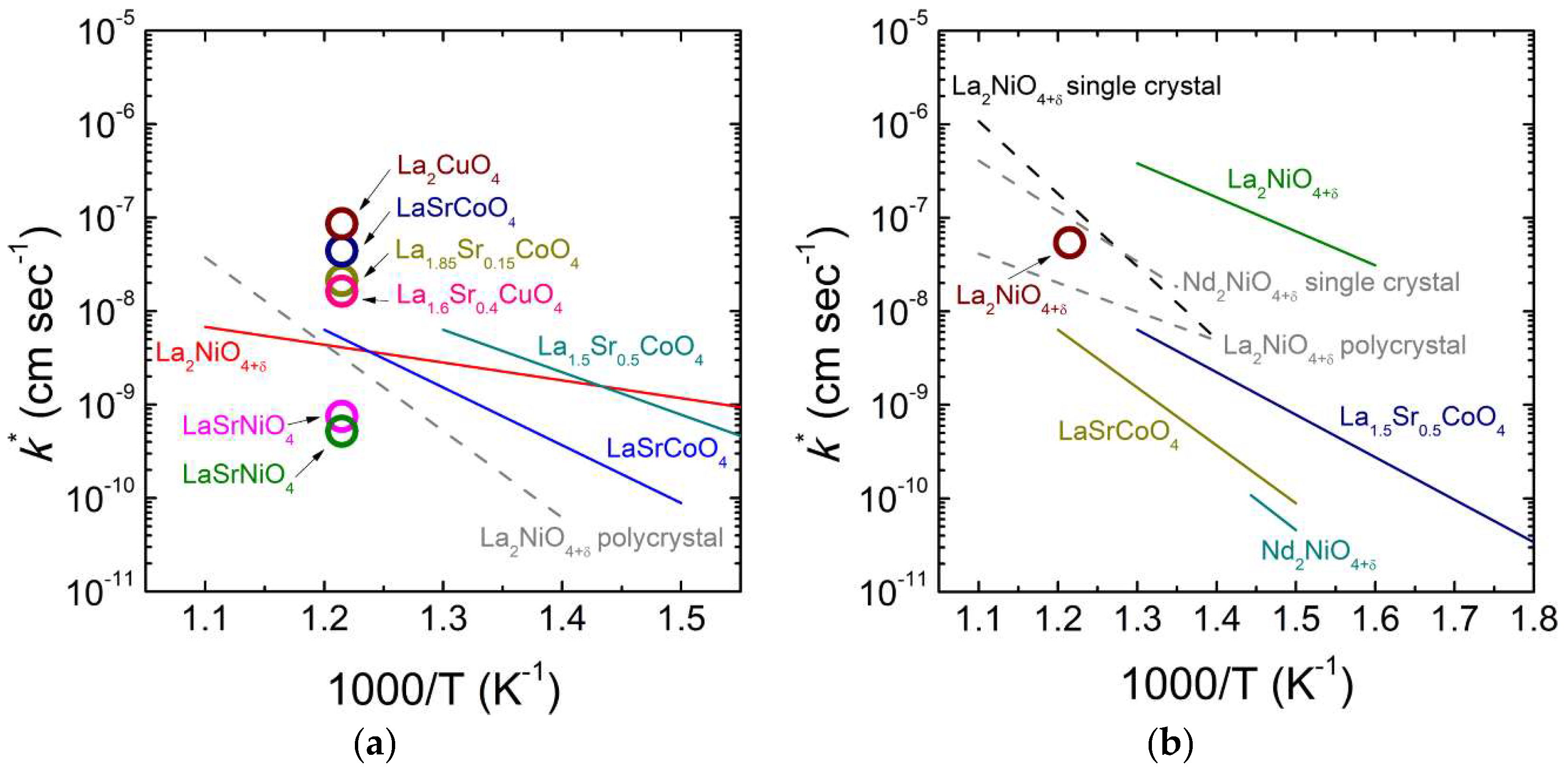
3.2. Ruddlesden-Popper (RP) Oxides
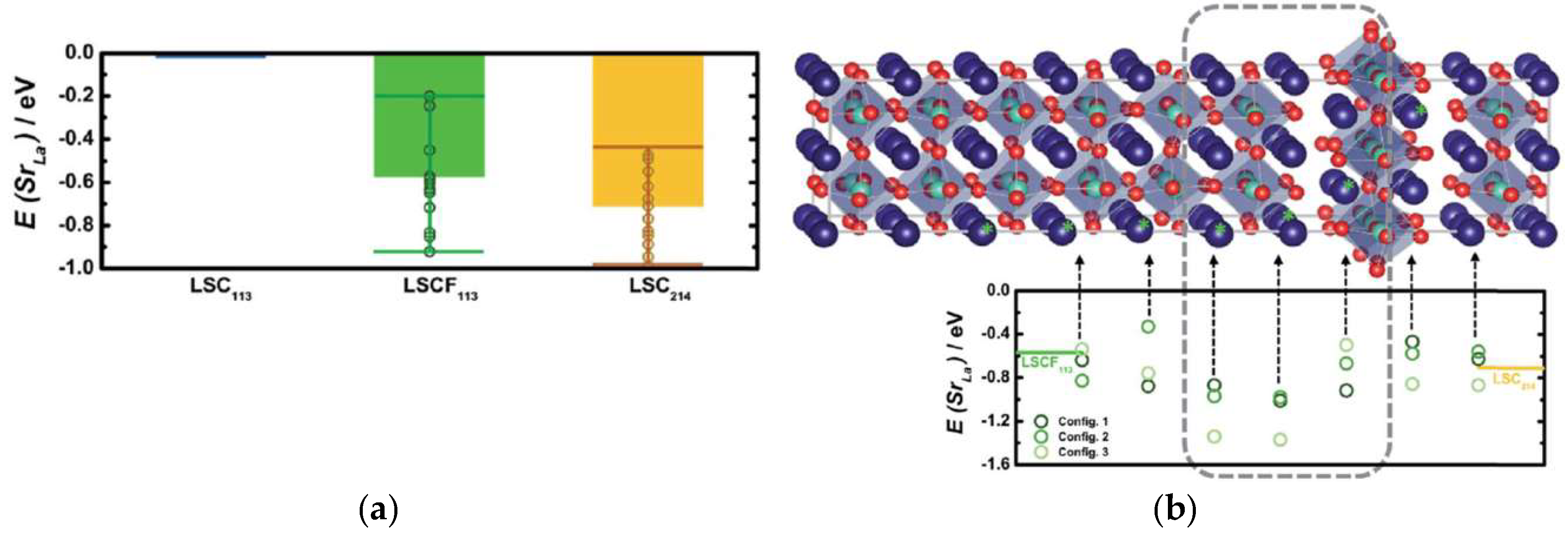
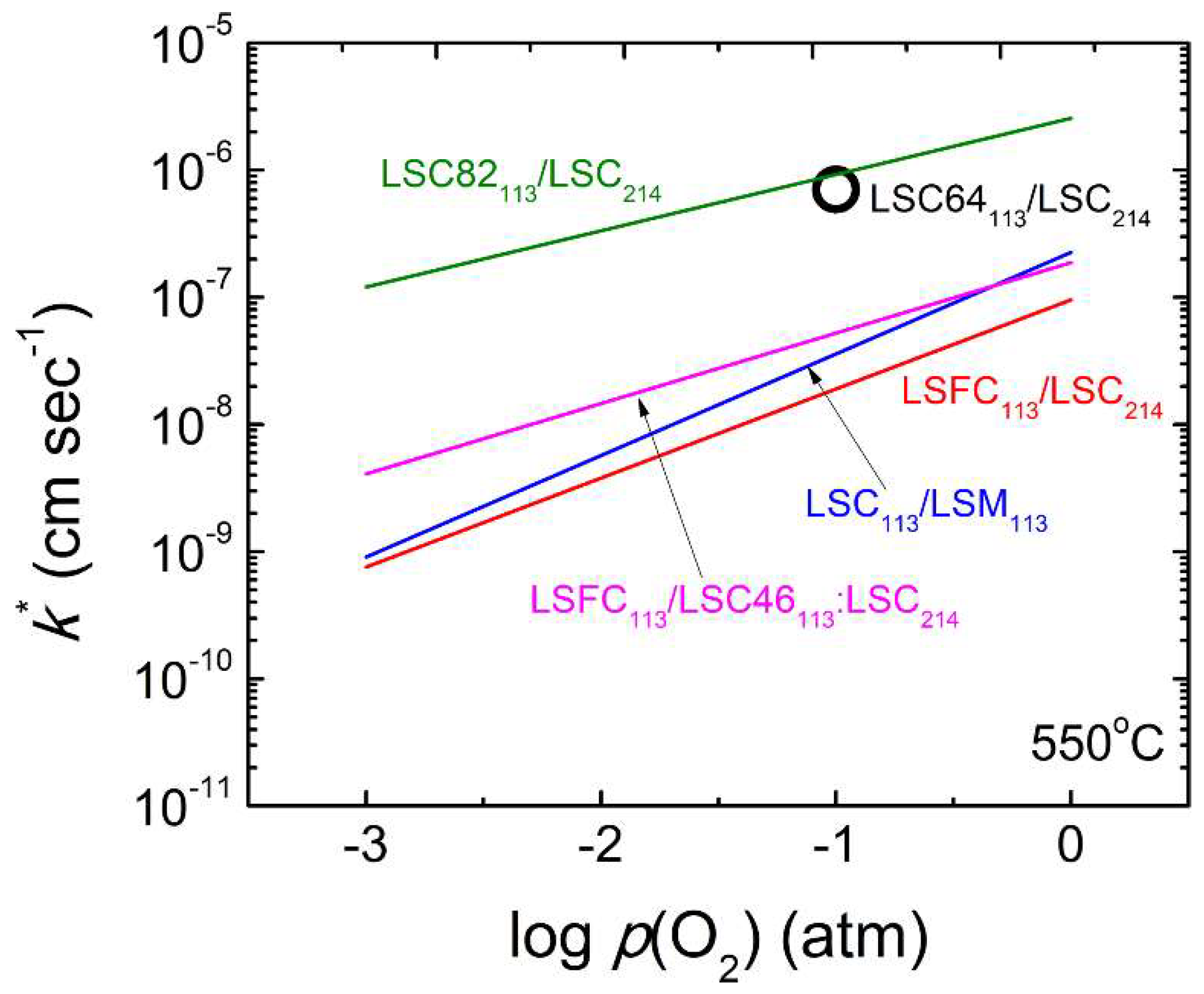
3.3. Oxide Heterostrcuture Interface
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adler, S.B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 2004, 104, 4791–4843. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.J. Materials for solid oxide fuel cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Shao, Z.P.; Haile, S.M. A high-performance cathode for the next generation of solid-oxide fuel cells. Nature 2004, 431, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Suzuki, J.; Sase, M.; Kaimai, A.; Yashiro, K.; Nigara, Y.; Mizusaki, J.; Kawamura, K.; Yugami, H. Determination of oxygen vacancy concentration in a thin film of La0.6Sr0.4CoO3−δ by an electrochemical method. J. Electrochem. Soc. 2002, 149, E252–E259. [Google Scholar] [CrossRef]
- Mizusaki, J.; Mima, Y.; Yamauchi, S.; Fueki, K.; Tagawa, H. Nonstoichiometry of the perovskite-type oxides La1−xSrxCoO3−δ. J. Solid State Chem. 1989, 80, 102–111. [Google Scholar] [CrossRef]
- De Souza, R.A.; Kilner, J.A. Oxygen transport in La1−xSrxMn1−yCoyO3±δ perovskites: Part II. Oxygen surface exchange. Solid State Ion. 1999, 126, 153–161. [Google Scholar] [CrossRef]
- De Souza, R.A.; Kilner, J.A. Oxygen transport in La1−xSrxMn1−yCoyO3±δ perovskites: Part I. Oxygen tracer diffusion. Solid State Ion. 1998, 106, 175–187. [Google Scholar] [CrossRef]
- Bassat, J.M.; Odier, P.; Villesuzanne, A.; Marin, C.; Pouchard, M. Anisotropic ionic transport properties in La2NiO4+δ single crystals. Solid State Ion. 2004, 167, 341–347. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Steil, M.C.; Dordor, P.; Mauvy, F.; Grenier, J.C. Oxygen transport properties of La2Ni1−xCuxO4+δ mixed conducting oxides. Solid State Sci. 2003, 5, 973–981. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H. Controlling oxygen mobility in Ruddlesden–Popper oxides. Materials 2017, 10, 368. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Dordor, P.; Mauvy, F.; Grenier, J.C.; Stevens, P. Oxygen diffusion and transport properties in non-stoichiometric Ln2−xNiO4+δ oxides. Solid State Ion. 2005, 176, 2717–2725. [Google Scholar] [CrossRef]
- Kilner, J.A.; Shaw, C.K.M. Mass transport in La2Ni1−xCoxO4+δ oxides with the K2NiF4 structure. Solid State Ion. 2002, 154–155, 523–527. [Google Scholar] [CrossRef]
- Ishigaki, T.; Yamauchi, S.; Kishio, K.; Mizusaki, J.; Fueki, K. Diffusion of oxide ion vacancies in perovskite-type oxides. J. Solid State Chem. 1988, 73, 179–187. [Google Scholar] [CrossRef]
- Ishigaki, T.; Yamauchi, S.; Mizusaki, J.; Fueki, K.; Tamura, H. Tracer diffusion-coefficient of oxide ions in LaCoO3 single-crystal. J. Solid State Chem. 1984, 54, 100–107. [Google Scholar] [CrossRef]
- Mizusaki, J. Nonstoichiometry, diffusion, and electrical-properties of perovskite-type oxide electrode materials. Solid State Ion. 1992, 52, 79–91. [Google Scholar] [CrossRef]
- Routbort, J.L.; Doshi, R.; Krumpelt, M. Oxygen tracer diffusion in La1−xSrxCoO3. Solid State Ion. 1996, 90, 21–27. [Google Scholar] [CrossRef]
- VanDoorn, R.H.E.; Fullarton, I.C.; De Souza, R.A.; Kilner, J.A.; Bouwmeester, H.J.M.; Burggraaf, A.J. Surface oxygen exchange of La0.3Sr0.7CoO3−δ. Solid State Ion. 1997, 96, 1–7. [Google Scholar] [CrossRef]
- Yildiz, B. “Stretching” the energy landscape of oxides-Effects on electrocatalysis and diffusion. MRS Bull. 2014, 39, 147–156. [Google Scholar] [CrossRef]
- Berenov, A.V.; Atkinson, A.; Kilner, J.A.; Bucher, E.; Sitte, W. Oxygen tracer diffusion and surface exchange kinetics in La0.6Sr0.4CoO3−δ. Solid State Ion. 2010, 181, 819–826. [Google Scholar] [CrossRef]
- Carter, S.; Selcuk, A.; Chater, R.J.; Kajda, J.; Kilner, J.A.; Steele, B.C.H. Oxygen transport in selected nonstoichiometric perovskite-structure oxides. Solid State Ion. 1992, 53, 597–605. [Google Scholar] [CrossRef]
- Lane, J.A.; Benson, S.J.; Waller, D.; Kilner, J.A. Oxygen transport in La0.6Sr0.4Co0.2Fe0.8O3−δ. Solid State Ion. 1999, 121, 201–208. [Google Scholar] [CrossRef]
- Wang, L.; Merkle, R.; Maier, J.; Acartürk, T.; Starke, U. Oxygen tracer diffusion in dense Ba0.5Sr0.5Co0.8Fe0.2O3−δ films. Appl. Phys. Lett. 2009, 94, 071908. [Google Scholar] [CrossRef]
- Munnings, C.N.; Skinner, S.J.; Amow, G.; Whitfield, P.S.; Davidson, I.J. Oxygen transport in the La2Ni1−xCoxO4+δ system. Solid State Ion. 2005, 176, 1895–1901. [Google Scholar] [CrossRef]
- Baumann, F.S.; Maier, J.; Fleig, J. The polarization resistance of mixed conducting SOFC cathodes: A comparative study using thin film model electrodes. Solid State Ion. 2008, 179, 1198–1204. [Google Scholar] [CrossRef]
- Crumlin, E.J.; Ahn, S.J.; Lee, D.; Mutoro, E.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Oxygen electrocatalysis on epitaxial La0.6Sr0.4CoO3−δ perovskite thin films for solid oxide fuel cells. J. Electrochem. Soc. 2012, 159, F219–F225. [Google Scholar] [CrossRef]
- LaO’, G.J.; Ahn, S.J.; Crumlin, E.; Orikasa, Y.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Catalytic activity enhancement for oxygen reduction on epitaxial perovskite thin films for solid-oxide fuel cells. Angew. Chem. 2010, 49, 5344–5347. [Google Scholar]
- Santiso, J.; Burriel, M. Deposition and characterisation of epitaxial oxide thin films for SOFCs. J. Solid State Electrochem. 2011, 15, 985–1006. [Google Scholar] [CrossRef]
- Lutchyn, R.M.; Sau, J.D.; Das Sarma, S. Majorana fermions and a topological phase transition in semiconductor-superconductor heterostructures. Phys. Rev. Lett. 2010, 105, 077001. [Google Scholar] [CrossRef]
- Smadici, Ş.; Abbamonte, P.; Bhattacharya, A.; Zhai, X.; Jiang, B.; Rusydi, A.; Eckstein, J.N.; Bader, S.D.; Zuo, J.M. Electronic reconstruction at SrMnO3-LaMnO3 superlattice interfaces. Phys. Rev. Lett. 2007, 99, 196404. [Google Scholar] [CrossRef]
- Chakhalian, J.; Freeland, J.W.; Srajer, G.; Strempfer, J.; Khaliullin, G.; Cezar, J.C.; Charlton, T.; Dalgliesh, R.; Bernhard, C.; Cristiani, G.; et al. Magnetism at the interface between ferromagnetic and superconducting oxides. Nat. Phys. 2006, 2, 244–248. [Google Scholar] [CrossRef]
- Sase, M.; Hermes, F.; Yashiro, K.; Sato, K.; Mizusaki, J.; Kawada, T.; Sakai, N.; Yokokawa, H. Enhancement of oxygen surface exchange at the hetero-interface of (La,Sr)CoO3/(La,Sr)2CoO4 with PLD-layered films. J. Electrochem. Soc. 2008, 155, B793–B797. [Google Scholar] [CrossRef]
- Chroneos, A.; Vovk, R.V.; Goulatis, I.L.; Goulatis, L.I. Oxygen transport in perovskite and related oxides: A brief review. J. Alloys Compd. 2010, 494, 190–195. [Google Scholar] [CrossRef]
- Kharton, V.V.; Viskup, A.P.; Kovalevsky, A.V.; Naumovich, E.N.; Marques, F.M.B. Ionic transport in oxygen-hyperstoichiometric phases with K2NiF4-type structure. Solid State Ion. 2001, 143, 337–353. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic fuel-cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Minervini, L.; Grimes, R.W.; Kilner, J.A.; Sickafus, K.E. Oxygen migration in La2NiO4+δ. J. Mater. Chem. 2000, 10, 2349–2354. [Google Scholar] [CrossRef]
- Opila, E.J.; Tuller, H.L.; Wuensch, B.J.; Maier, J. Oxygen tracer diffusion in La2−xSrxCuO4−δ single-crystals. J. Am. Ceram. Soc. 1993, 76, 2363–2369. [Google Scholar] [CrossRef]
- Horita, T.; Yamaji, K.; Sakai, N.; Xiong, Y.; Yokokawa, H.; Kawada, T.J.I. Application of novel SIMS technique for imaging the active sites of oxygen reduction at the SOFC cathode/electrolyte interfaces. Ionics 2002, 8, 108–117. [Google Scholar] [CrossRef]
- Horita, T.; Yamaji, K.; Sakai, N.; Yokokawa, H.; Weber, A.; Ivers-Tiffee, E. Oxygen reduction mechanism at porous La1−xSrxCoO3−δ cathodes/La0.8Sr0.2Ga0.8Mg0.2O2.8 electrolyte interface for solid oxide fuel cells. Electrochim. Acta 2001, 46, 1837–1845. [Google Scholar] [CrossRef]
- Kamata, H.; Hosaka, A.; Mizusaki, J.; Tagawa, H. High temperature electrocatalytic properties of the SOFC air electrode La0.8Sr0.2MnO3/YSZ. Solid State Ion. 1998, 106, 237–245. [Google Scholar] [CrossRef]
- Opitz, A.K.; Schintlmeister, A.; Hutter, H.; Fleig, J. Visualization of oxygen reduction sites at Pt electrodes on YSZ by means of 18O tracer incorporation: The width of the electrochemically active zone. Phys. Chem. Chem. Phys. 2010, 12, 12734–12745. [Google Scholar] [CrossRef]
- Østergård, M.J.L.; Mogensen, M. ac Impedance study of the oxygen reduction mechanism on La1−xSrxMnO3 in solid oxide fuel cells. Electrochim. Acta 1993, 38, 2015–2020. [Google Scholar] [CrossRef]
- Robertson, N.L.; Michaels, J.N. Oxygen exchange on platinum electrodes in zirconia cells: Location of electrochemical reaction sites. J. Electrochem. Soc. 1990, 137, 129–135. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.M.; Kruidhof, H.; Burggraaf, A.J. Importance of the surface exchange kinetics as rate-limiting step in oxygen permeation through mixed-conducting oxides. Solid State Ion. 1994, 72, 185–194. [Google Scholar] [CrossRef]
- Van der Haar, L.M.; den Otter, M.W.; Morskate, M.; Bouwmeester, H.J.M.; Verweij, H. Chemical diffusion and oxygen surface transfer of La1−xSrxCoO3−δ studied with electrical conductivity relaxation. J. Electrochem. Soc. 2002, 149, J41–J46. [Google Scholar] [CrossRef]
- Baumann, F.S.; Fleig, J.; Habermeier, H.U.; Maier, J. Impedance spectroscopic study on well-defined (La,Sr)(Co,Fe)O3−δ model electrodes. Solid State Ion. 2006, 177, 1071–1081. [Google Scholar] [CrossRef]
- Jamnik, J.; Maier, J. Generalised equivalent circuits for mass and charge transport: Chemical capacitance and its implications. Phys. Chem. Chem. Phys. 2001, 3, 1668–1678. [Google Scholar] [CrossRef]
- Yang, Y.L.; Chen, C.L.; Chen, S.Y.; Chu, C.W.; Jacobson, A.J. Impedance studies of oxygen exchange on dense thin film electrodes of La0.5Sr0.5CoO3−δ. J. Electrochem. Soc. 2000, 147, 4001–4007. [Google Scholar] [CrossRef]
- Endo, A.; Fukunaga, H.; Wen, C.; Yamada, K. Cathodic reaction mechanism of dense La0.6Sr0.4CoO3 and La0.81Sr0.09MnO3 electrodes for solid oxide fuel cells. Solid State Ion. 2000, 135, 353–358. [Google Scholar] [CrossRef]
- Yang, Y.L.; Jacobson, A.J.; Chen, C.L.; Luo, G.P.; Ross, K.D.; Chu, C.W. Oxygen exchange kinetics on a highly oriented La0.5Sr0.5CoO3−δ thin film prepared by pulsed-laser deposition. Appl. Phys. Lett. 2001, 79, 776–778. [Google Scholar] [CrossRef]
- Sase, M.; Ueno, D.; Yashiro, K.; Kaimai, A.; Kawada, T.; Mizusaki, J. Interfacial reaction and electrochemical properties of dense (La,Sr)CoO3−δ cathode on YSZ (100). J. Phys. Chem. Solids 2005, 66, 343–348. [Google Scholar] [CrossRef]
- Maier, J. Physical chemistry of ionic materials: Ions and electrons in solids. Wiley 2004. [Google Scholar]
- Fleig, J.; Maier, J. The polarization of mixed conducting SOFC cathodes: Effects of surface reaction coefficient, ionic conductivity and geometry. J. Eur. Ceram. Soc. 2004, 24, 1343–1347. [Google Scholar] [CrossRef]
- Cai, Z.; Kuru, Y.; Han, J.W.; Chen, Y.; Yildiz, B. Surface electronic structure transitions at high temperature on perovskite oxides: The case of strained La0.8Sr0.2CoO3 thin films. J. Am. Chem. Soc. 2011, 133, 17696–17704. [Google Scholar] [CrossRef]
- Donner, W.; Chen, C.; Liu, M.; Jacobson, A.J.; Lee, Y.L.; Gadre, M.; Morgan, D. Epitaxial strain-induced chemical ordering in La0.5Sr0.5CoO3−δ films on SrTiO3. Chem. Mater. 2011, 23, 984–988. [Google Scholar] [CrossRef]
- Garcia-Barriocanal, J.; Rivera-Calzada, A.; Varela, M.; Sefrioui, Z.; Iborra, E.; Leon, C.; Pennycook, S.J.; Santamaria, J. Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science 2008, 321, 676–680. [Google Scholar] [CrossRef]
- Schichtel, N.; Korte, C.; Hesse, D.; Janek, J. Elastic strain at interfaces and its influence on ionic conductivity in nanoscaled solid electrolyte thin films—Theoretical considerations and experimental studies. Phys. Chem. Chem. Phys. 2009, 11, 3043–3048. [Google Scholar] [CrossRef]
- Wang, Z.W.; Shu, D.J.; Wang, M.; Ming, N.B. Diffusion of oxygen vacancies on a strained rutile TiO2 (110) surface. Phys. Rev. B 2010, 82, 165309. [Google Scholar] [CrossRef]
- Kushima, A.; Yip, S.; Yildiz, B. Competing strain effects in reactivity of LaCoO3 with oxygen. Phys. Rev. B 2010, 82, 115435. [Google Scholar] [CrossRef]
- Burriel, M.; Garcia, G.; Santiso, J.; Kilner, J.A.; Chater, R.J.; Skinner, S.J. Anisotropic oxygen diffusion properties in epitaxial thin films of La2NiO4+δ. J. Mater. Chem. 2008, 18, 416–422. [Google Scholar] [CrossRef]
- Ji, H.I.; Hwang, J.; Yoon, K.J.; Son, J.W.; Kim, B.K.; Lee, H.W.; Lee, J.H. Enhanced oxygen diffusion in epitaxial lanthanum–strontium–cobaltite thin film cathodes for micro solid oxide fuel cells. Energy Environ. Sci. 2013, 6, 116–120. [Google Scholar] [CrossRef]
- Lee, D.; Grimaud, A.; Crumlin, E.J.; Mezghani, K.; Habib, M.A.; Feng, Z.; Hong, W.T.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Strain influence on the oxygen electrocatalysis of the (100)-oriented epitaxial La2NiO4+δ thin films at elevated temperatures. J. Phys. Chem. C 2013, 117, 18789–18795. [Google Scholar] [CrossRef]
- Kubicek, M.; Cai, Z.; Ma, W.; Yildiz, B.; Hutter, H.; Fleig, J. Tensile lattice strain accelerates oxygen surface exchange and diffusion in La1–xSrxCoO3−δ thin films. ACS Nano 2013, 7, 3276–3286. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.L.; Grimaud, A.; Hong, W.T.; Biegalski, M.D.; Morgan, D.; Shao-Horn, Y. Strontium influence on the oxygen electrocatalysis of La2−xSrxNiO4±δ (0.0 ≤ xSr ≤ 1.0) thin films. J. Mater. Chem. A 2014, 2, 6480–6487. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.L.; Wang, X.R.; Morgan, D.; Shao-Horn, Y. Enhancement of oxygen surface exchange on epitaxial La0.6Sr0.4Co0.2Fe0.8O3−δ thin films using advanced heterostructured oxide interface engineering. MRS Commun. 2016, 6, 204–209. [Google Scholar] [CrossRef]
- Sase, M.; Hermes, F.; Nakamura, T.; Yashiro, K.; Sato, K.; Mizusaki, J.; Kawada, T.; Sakai, N.; Yamaji, K.; Horita, T.; et al. Promotion of oxygen surface reaction at the hetero-interface of (La,Sr)CoO3/(La,Sr)2CoO4. ECS Trans. 2007, 7, 1055–1060. [Google Scholar]
- Yashiro, K.; Nakamura, T.; Sase, M.; Hermes, F.; Sato, K.; Kawada, T.; Mizusaki, J. Electrode performance at hetero-interface of perovskite-related oxides, (La,Sr)CoO3−δ/(La,Sr)2CoO4−δ. ECS Trans. 2007, 7, 1287–1292. [Google Scholar]
- Schlom, D.G.; Haeni, J.H.; Lettieri, J.; Theis, C.D.; Tian, W.; Jiang, J.C.; Pan, X.Q. Oxide nano-engineering using MBE. Mater. Sci. Eng. B 2001, 87, 282–291. [Google Scholar] [CrossRef]
- Elam, J.W.; Groner, M.D.; George, S.M. Viscous flow reactor with quartz crystal microbalance for thin film growth by atomic layer deposition. Rev. Sci. Instrum. 2002, 73, 2981–2987. [Google Scholar] [CrossRef]
- Lowndes, D.H.; Geohegan, D.B.; Puretzky, A.A.; Norton, D.P.; Rouleau, C.M. Synthesis of novel thin-film materials by pulsed laser deposition. Science 1996, 273, 898–903. [Google Scholar] [CrossRef]
- Alfonso, E.; Olaya, J.; Cubillos, G. Thin film growth through sputtering technique and its applications. In Crystallization—Science and Technology; IntechOpen: London, UK, 2012. [Google Scholar]
- Adler, S.B. Mechanism and kinetics of oxygen reduction on porous La1−xSrxCoO3−δ electrodes. Solid State Ion. 1998, 111, 125–134. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, S.Z.; Zhang, Y.H.; Yan, J.W.; Li, W.Z. Electrochemical reduction of oxygen on a strontium doped lanthanum manganite electrode. Solid State Ion. 1998, 110, 111–119. [Google Scholar] [CrossRef]
- Van Doorn, R.H.E.; Burggraaf, A.J. Structural aspects of the ionic conductivity of La1−xSrxCoO3−δ. Solid State Ion. 2000, 128, 65–78. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.L.; Grimaud, A.; Hong, W.T.; Biegalski, M.D.; Morgan, D.; Shao-Horn, Y. Enhanced oxygen surface exchange kinetics and stability on epitaxial La0.8Sr0.2CoO3−δ thin films by La0.8Sr0.2MnO3−δ decoration. J. Phys. Chem. C 2014, 118, 14326–14334. [Google Scholar] [CrossRef]
- Esquirol, A.; Brandon, N.P.; Kilner, J.A.; Mogensen, M. Electrochemical characterization of La0.6Sr0.4Co0.2Fe0.8O3 cathodes for intermediate-temperature SOFCs. J. Electrochem. Soc. 2004, 151, A1847–A1855. [Google Scholar] [CrossRef]
- Geary, T.C.; Lee, D.; Shao-Horn, Y.; Adler, S.B. Nonlinear impedance analysis of La0.4Sr0.6Co0.2Fe0.8O3−δ thin film oxygen electrodes. J. Electrochem. Soc. 2016, 163, F1107–F1114. [Google Scholar] [CrossRef]
- Jiang, S.P. A comparison of O2 reduction reactions on porous (La,Sr)MnO3 and (La,Sr)(Co,Fe)O3 electrodes. Solid State Ion. 2002, 146, 1–22. [Google Scholar] [CrossRef]
- Katsuki, M.; Wang, S.; Dokiya, M.; Hashimoto, T. High temperature properties of La0.6Sr0.4Co0.8Fe0.2O3−δ oxygen nonstoichiometry and chemical diffusion constant. Solid State Ion. 2003, 156, 453–461. [Google Scholar] [CrossRef]
- Kuhn, M.; Fukuda, Y.; Hashimoto, S.; Sato, K.; Yashiro, K.; Mizusaki, J. Oxygen nonstoichiometry and thermo-chemical stability of perovskite-type La0.6Sr0.4Co1−yFeyO3−δ (y = 0, 0.2, 0.4, 0.5, 0.6, 0.8, 1) materials. J. Electrochem. Soc. 2013, 160, F34–F42. [Google Scholar] [CrossRef]
- Lynch, M.E.; Yang, L.; Qin, W.T.; Choi, J.J.; Liu, M.F.; Blinn, K.; Liu, M.L. Enhancement of La0.6Sr0.4Co0.2Fe0.8O3−δ durability and surface electrocatalytic activity by La0.85Sr0.15MnO3±δ investigated using a new test electrode platform. Energy Environ. Sci. 2011, 4, 2249–2258. [Google Scholar] [CrossRef]
- Tai, L.W.; Nasrallah, M.M.; Anderson, H.U.; Sparlin, D.M.; Sehlin, S.R. Structure and electrical-properties of La1−xSrxCo1−yFeyO3 Part 1. The system La0.8Sr0.2Co1−yFeyO3. Solid State Ion. 1995, 76, 259–271. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Bae, J.M. Properties of La0.6Sr0.4Co0.2Fe0.8O3−x (LSCF) double layer cathodes on gadolinium-doped cerium oxide (CGO) electrolytes: II. Role of oxygen exchange and diffusion. Solid State Ion. 1998, 106, 255–261. [Google Scholar] [CrossRef]
- Ingram, B.J.; Eastman, J.A.; Chang, K.C.; Kim, S.K.; Fister, T.T.; Perret, E.; You, H.; Baldo, P.M.; Fuoss, P.H. In situ X-ray studies of oxygen surface exchange behavior in thin film La0.6Sr0.4Co0.2Fe0.8O3−δ. Appl. Phys. Lett. 2012, 101, 051603. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.L.; Hong, W.T.; Biegalski, M.D.; Morgan, D.; Shao-Horn, Y. Oxygen surface exchange kinetics and stability of (La,Sr)2CoO4±δ/La1−xSrxMO3−δ (M = Co and Fe) hetero-interfaces at intermediate temperatures. J. Mater. Chem. A 2015, 3, 2144–2157. [Google Scholar] [CrossRef]
- Cho, S.Y.; Chung, Y.C.; Ahn, K.; Lee, J.H.; Kim, B.K.; Kim, H. Oxygen transport in epitaxial La0.875Sr0.125CoO3−δ thin-film cathodes for solid oxide fuel cells: Roles of anisotropic strain. Scr. Mater. 2016, 115, 141–144. [Google Scholar] [CrossRef]
- Gan, L.Y.; Akande, S.O.; Schwingenschlogl, U. Anisotropic O vacancy formation and diffusion in LaMnO3. J. Mater. Chem. A 2014, 2, 19733–19737. [Google Scholar] [CrossRef]
- Kushima, A.; Yildiz, B. Role of lattice strain and defect chemistry on the oxygen vacancy migration at the (8.3% Y2O3-ZrO2) / SrTiO3 hetero-interface: A first principles study. ECS Trans. 2009, 25, 1599–1609. [Google Scholar]
- Mayeshiba, T.; Morgan, D. Strain effects on oxygen migration in perovskites. Phys. Chem. Chem. Phys. 2015, 17, 2715–2721. [Google Scholar] [CrossRef]
- Petrie, J.R.; Jeen, H.; Barron, S.C.; Meyer, T.L.; Lee, H.N. Enhancing perovskite electrocatalysis through strain tuning of the oxygen deficiency. J. Am. Chem. Soc. 2016, 138, 7252–7255. [Google Scholar] [CrossRef]
- Petrie, J.R.; Mitra, C.; Jeen, H.; Choi, W.S.; Meyer, T.L.; Reboredo, F.A.; Freeland, J.W.; Eres, G.; Lee, H.N. Strain control of oxygen vacancies in epitaxial strontium cobaltite films. Adv. Funct. Mater. 2016, 26, 1564–1570. [Google Scholar] [CrossRef]
- Xu, T.; Shimada, T.; Araki, Y.; Wang, J.; Kitamura, T. Defect-strain engineering for multiferroic and magnetoelectric properties in epitaxial (110) ferroelectric lead titanate. Phys. Rev. B 2015, 92, 104106. [Google Scholar] [CrossRef]
- Han, J.W.; Yildiz, B. Enhanced one dimensional mobility of oxygen on strained LaCoO3(001) surface. J. Mater. Chem. 2011, 21, 18983–18990. [Google Scholar] [CrossRef]
- Hong, W.T.; Gadre, M.; Lee, Y.L.; Biegalski, M.D.; Christen, H.M.; Morgan, D.; Shao-Horn, Y. Tuning the spin state in LaCoO3 thin films for enhanced high-temperature oxygen electrocatalysis. J. Phys. Chem. Lett. 2013, 4, 2493–2499. [Google Scholar] [CrossRef]
- Lee, D.; Jacobs, R.; Jee, Y.; Seo, A.; Sohn, C.; Ievlev, A.V.; Ovchinnikova, O.S.; Huang, K.; Morgan, D.; Lee, H.N. Stretching epitaxial La0.6Sr0.4CoO3−δ for fast oxygen reduction. J. Phys. Chem. C 2017, 121, 25651–25658. [Google Scholar] [CrossRef]
- Rupp, G.M.; Kubicek, M.; Opitz, A.K.; Fleig, J. In situ impedance analysis of oxygen exchange on growing La0.6Sr0.4CoO3−δ thin films. ACS Appl. Energy Mater. 2018, 1, 4522–4535. [Google Scholar] [CrossRef]
- Hou, F.; Cai, T.Y.; Ju, S.; Shen, M.R. Half-metallic ferromagnetism via the interface electronic reconstruction in LaAlO3/SrMnO3 nanosheet superlattices. ACS Nano 2012, 6, 8552–8562. [Google Scholar] [CrossRef]
- Rata, A.D.; Herklotz, A.; Nenkov, K.; Schultz, L.; Dörr, K. Strain-induced insulator state and giant gauge factor of La0.7Sr0.3CoO3 films. Phys. Rev. Lett. 2008, 100, 076401. [Google Scholar] [CrossRef]
- Lee, Y.L.; Kleis, J.; Rossmeisl, J.; Shao-Horn, Y.; Morgan, D. Prediction of solid oxide fuel cell cathode activity with first-principles descriptors. Energy Environ. Sci. 2011, 4, 3966–3970. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, Y.; Aruta, C.; Yang, N. Improving electronic conductivity and oxygen reduction activity in Sr-doped lanthanum cobaltite thin films: Cobalt valence state and electronic band structure effects. ACS Appl. Energy Mater. 2018, 1, 5308–5317. [Google Scholar] [CrossRef]
- Yamazoe, N.; Teraoka, Y. Oxidation catalysis of perovskites --- relationships to bulk structure and composition (valency, defect, etc.). Catal. Today 1990, 8, 175–199. [Google Scholar] [CrossRef]
- Fierro, J.L.G.; Tejuca, L.G. Non-stoichiometric surface behaviour of LaMO3 oxides as evidenced by XPS. Appl. Surf. Sci. 1987, 27, 453–457. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Chavan, S.V.; Singh, R.N. Preparation, properties, and reactivity of lanthanum strontium ferrite as an intermediate temperature SOFC cathode. J. Mater. Sci. 2013, 48, 6597–6604. [Google Scholar] [CrossRef]
- Simner, S.P.; Bonnett, J.F.; Canfield, N.L.; Meinhardt, K.D.; Shelton, J.P.; Sprenkle, V.L.; Stevenson, J.W. Development of lanthanum ferrite SOFC cathodes. J. Power Sources 2003, 113, 1–10. [Google Scholar] [CrossRef]
- Petitjean, M.; Caboche, G.; Siebert, E.; Dessemond, L.; Dufour, L.C. (La0.8Sr0.2)(Mn1−yFey)O3±δ oxides for IT-SOFC cathode materials?: Electrical and ionic transport properties. J. Eur. Ceram. Soc. 2005, 25, 2651–2654. [Google Scholar] [CrossRef]
- La O’, G.J.; Shao-Horn, Y. Oxygen surface exchange kinetics on Sr-substituted lanthanum manganite and ferrite thin-film microelectrodes. J. Electrochem. Soc. 2009, 156, B816–B824. [Google Scholar] [CrossRef]
- Wang, S.; Katsuki, M.; Dokiya, M.; Hashimoto, T. High temperature properties of La0.6Sr0.4Co0.8Fe0.2O3−δ phase structure and electrical conductivity. Solid State Ion. 2003, 159, 71–78. [Google Scholar] [CrossRef]
- Ralph, J.M.; Rossignol, C.; Kumar, R. Cathode materials for reduced-temperature SOFCs. J. Electrochem. Soc. 2003, 150, A1518–A1522. [Google Scholar] [CrossRef]
- Perry Murray, E.; Sever, M.J.; Barnett, S.A. Electrochemical performance of (La,Sr)(Co,Fe)O3–(Ce,Gd)O3 composite cathodes. Solid State Ion. 2002, 148, 27–34. [Google Scholar] [CrossRef]
- Adler, S.B.; Lane, J.A.; Steele, B.C.H. Electrode kinetics of porous mixed-conducting oxygen electrodes. J. Electrochem. Soc. 1996, 143, 3554–3564. [Google Scholar] [CrossRef]
- Chen, C.C.; Nasrallah, M.M.; Anderson, H.U.; Alim, M.A. Immittance response of La0.6Sr0.4Co0.2Fe0.8O3 based electrochemical cells. J. Electrochem. Soc. 1995, 142, 491–496. [Google Scholar] [CrossRef]
- Prestat, M.; Infortuna, A.; Korrodi, S.; Rey-Mermet, S.; Muralt, P.; Gauckler, L.J. Oxygen reduction at thin dense La0.52Sr0.48Co0.18Fe0.82O3–δ electrodes. J. Electroceram. 2007, 18, 111–120. [Google Scholar] [CrossRef]
- Angoua, B.F.; Slamovich, E.B. Single solution spray pyrolysis of La0.6Sr0.4Co0.2Fe0.8O3−δ–Ce0.8Gd0.2O1.9 (LSCF–CGO) thin film cathodes. Solid State Ion. 2012, 212, 10–17. [Google Scholar] [CrossRef]
- Develos-Bagarinao, K.; Kishimoto, H.; De Vero, J.; Yamaji, K.; Horita, T. Effect of La0.6Sr0.4Co0.2Fe0.8O3−δ microstructure on oxygen surface exchange kinetics. Solid State Ion. 2016, 288, 6–9. [Google Scholar] [CrossRef]
- Hopper, E.M.; Perret, E.; Ingram, B.J.; You, H.; Chang, K.C.; Baldo, P.M.; Fuoss, P.H.; Eastman, J.A. Oxygen exchange in La0.6Sr0.4Co0.2Fe0.8O3−δ thin-film heterostructures under applied electric potential. J. Phys. Chem. C 2015, 119, 19915–19921. [Google Scholar] [CrossRef]
- Plonczak, P.; Søgaard, M.; Bieberle-Hütter, A.; Hendriksen, P.V.; Gauckler, L.J. Electrochemical characterization of La0.58Sr0.4Co0.2Fe0.8O3−δ thin film electrodes prepared by pulsed laser deposition. J. Electrochem. Soc. 2012, 159, B471–B482. [Google Scholar] [CrossRef]
- Armstrong, E.N.; Duncan, K.L.; Oh, D.J.; Weaver, J.F.; Wachsman, E.D. Determination of surface exchange coefficients of LSM, LSCF, YSZ, GDC constituent materials in composite SOFC cathodes. J. Electrochem. Soc. 2011, 158, B492–B499. [Google Scholar] [CrossRef]
- Dumaisnil, K.; Carru, J.C.; Fasquelle, D.; Mascot, M.; Rolle, A.; Vannier, R.N. Promising performances for a La0.6Sr0.4Co0.8Fe0.2O3−δ cathode with a dense interfacial layer at the electrode-electrolyte interface. Ionics 2017, 23, 2125–2132. [Google Scholar] [CrossRef]
- Jang, I.; Kim, C.; Kim, S.; Yoon, H.; Paik, U. Fabrication of nanoparticle networked La0.6Sr0.4Co0.2Fe0.8O3−δ thin film layer between the cathode and electrolyte of solid oxide fuel cell by using a spin coating method. ECS Trans. 2017, 78, 741–745. [Google Scholar] [CrossRef]
- Jang, I.; Kim, S.; Kim, C.; Yoon, H.; Song, T. Enhancement of oxygen reduction reaction through coating a nano-web-structured La0.6Sr0.4Co0.2Fe0.8O3−δ thin-film as a cathode/electrolyte interfacial layer for lowering the operating temperature of solid oxide fuel cells. J. Power Sources 2018, 392, 123–128. [Google Scholar] [CrossRef]
- Ruddlesden, S.N.; Popper, P. The compound Sr3Ti2O7 and its structure. Acta Crystallogr. 1958, 11, 54–55. [Google Scholar] [CrossRef]
- Brown, I.D. Modeling the structures of La2NiO4. Z. Kristall. 1992, 199, 255–272. [Google Scholar] [CrossRef]
- Sase, M.; Yashiro, K.; Sato, K.; Mizusaki, J.; Kawada, T.; Sakai, N.; Yamaji, K.; Horita, T.; Yokokawa, H. Enhancement of oxygen exchange at the hetero interface of (La,Sr)CoO3/(La,Sr)2CoO4 in composite ceramics. Solid State Ion. 2008, 178, 1843–1852. [Google Scholar] [CrossRef]
- Lee, Y.L.; Lee, D.; Wang, X.R.; Lee, H.N.; Morgan, D.; Shao-Horn, Y. Kinetics of oxygen surface exchange on epitaxial Ruddlesden-Popper phases and correlations to first-principles descriptors. J. Phys. Chem. Lett. 2016, 7, 244–249. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Colsmann, G.; Reuter, B. Studies on La2−xSrxNiO4 (0 <x <1) system. J. Solid State Chem. 1977, 22, 145–149. [Google Scholar]
- Bassat, J.M.; Burriel, M.; Wahyudi, O.; Castaing, R.; Ceretti, M.; Veber, P.; Weill, I.; Villesuzanne, A.; Grenier, J.C.; Paulus, W.; et al. Anisotropic oxygen diffusion properties in Pr2NiO4+δ and Nd2NiO4+δ single crystals. J. Phys. Chem. C 2013, 117, 26466–26472. [Google Scholar] [CrossRef]
- Burriel, M.; Santiso, J.; Rossell, M.D.; Van Tendeloo, G.; Figueras, A.; Garcia, G. Enhancing total conductivity of La2NiO4+δ epitaxial thin films by reducing thickness. J. Phys. Chem. C. 2008, 112, 10982–10987. [Google Scholar] [CrossRef]
- Garcia, G.; Burriel, M.; Bonanos, N.; Santiso, J. Electrical conductivity and oxygen exchange kinetics of La2NiO4+δ thin films grown by chemical vapor deposition. J. Electrochem. Soc. 2008, 155, P28–P32. [Google Scholar] [CrossRef]
- Kharton, V.V.; Yaremchenko, A.A.; Shaula, A.L.; Patrakeev, M.V.; Naumovich, E.N.; Loginovich, D.I.; Frade, J.R.; Marques, F.M.B. Transport properties and stability of Ni-containing mixed conductors with perovskite- and K2NiF4-type structure. J. Solid State Chem. 2004, 177, 26–37. [Google Scholar] [CrossRef]
- Kim, G.T.; Wang, S.Y.; Jacobson, A.J.; Yuan, Z.; Chen, C.L. Impedance studies of dense polycrystalline thin films of La2NiO4+δ. J. Mater. Chem. 2007, 17, 1316–1320. [Google Scholar] [CrossRef]
- Burriel, M.; Téllez, H.; Chater, R.J.; Castaing, R.; Veber, P.; Zaghrioui, M.; Ishihara, T.; Kilner, J.A.; Bassat, J.M. Influence of crystal orientation and annealing on the oxygen diffusion and surface exchange of La2NiO4+δ. J. Phys. Chem. C. 2016, 120, 17927–17938. [Google Scholar] [CrossRef]
- Yamada, A.; Suzuki, Y.; Saka, K.; Uehara, M.; Mori, D.; Kanno, R.; Kiguchi, T.; Mauvy, F.; Grenier, J.C. Ruddlesden-Popper-type epitaxial film as oxygen electrode for solid-oxide fuel cells. Adv. Mater. 2008, 20, 4124. [Google Scholar] [CrossRef]
- Tsvetkov, N.; Lu, Q.; Chen, Y.; Yildiz, B. Accelerated oxygen exchange kinetics on Nd2NiO4+δ thin films with tensile strain along c-axis. ACS Nano 2015, 9, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Wang, S.; Jacobson, A.J.; Chen, C.L. Measurement of oxygen transport kinetics in epitaxial LaNiO4+δ thin films by electrical conductivity relaxation. Solid State Ion. 2006, 177, 1461–1467. [Google Scholar] [CrossRef]
- Xie, W.; Lee, Y.L.; Shao-Horn, Y.; Morgan, D. Oxygen point defect chemistry in Ruddlesden–Popper oxides (La1–xSrx)2MO4±δ (M = Co, Ni, Cu). J. Phys. Chem. Lett. 2016, 7, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Manthiram, A. Crystal-chemistry and superconductivity of the copper oxides. J. Solid State Chem. 1990, 88, 115–139. [Google Scholar] [CrossRef]
- Gozar, A.; Logvenov, G.; Kourkoutis, L.F.; Bollinger, A.T.; Giannuzzi, L.A.; Muller, D.A.; Bozovic, I. High-temperature interface superconductivity between metallic and insulating copper oxides. Nature 2008, 455, 782. [Google Scholar] [CrossRef]
- Hoppler, J.; Stahn, J.; Niedermayer, C.; Malik, V.K.; Bouyanfif, H.; Drew, A.J.; Rossle, M.; Buzdin, A.; Cristiani, G.; Habermeier, H.U.; et al. Giant superconductivity-induced modulation of the ferromagnetic magnetization in a cuprate-manganite superlattice. Nat. Mater. 2009, 8, 315–319. [Google Scholar] [CrossRef]
- Opila, E.J.; Tuller, H.L. Thermogravimetric analysis and defect models of the oxygen nonstoichiometry in La2–xSrxCuO4–y. J. Am. Ceram. Soc. 1994, 77, 2727–2737. [Google Scholar] [CrossRef]
- Meyer, T.L.; Jacobs, R.; Lee, D.; Jiang, L.; Freeland, J.W.; Sohn, C.; Egami, T.; Morgan, D.; Lee, H.N. Strain control of oxygen kinetics in the Ruddlesden-Popper oxide La1.85Sr0.15CuO4. Nat. Commun. 2018, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, K.; Nakamura, T.; Sase, M.; Hermes, F.; Sato, K.; Kawada, T.; Mizusaki, J. Composite cathode of perovskite-related oxides, (La,Sr)CoO3−δ/(La,Sr)2CoO4–δ, for solid oxide fuel cells. Electrochem. Solid State Lett. 2009, 12, B135–B137. [Google Scholar] [CrossRef]
- Chen, Y.; Téllez, H.; Burriel, M.; Yang, F.; Tsvetkov, N.; Cai, Z.; McComb, D.W.; Kilner, J.A.; Yildiz, B. Segregated chemistry and structure on (001) and (100) Surfaces of (La1–xSrx)2CoO4 override the crystal anisotropy in oxygen exchange kinetics. Chem. Mater. 2015, 27, 5436–5450. [Google Scholar] [CrossRef]
- Chen, J.; Liang, F.L.; Chi, B.; Pu, J.; Jiang, S.P.; Jian, L. Palladium and ceria infiltrated La0.8Sr0.2Co0.5Fe0.5O3–δ cathodes of solid oxide fuel cells. J. Power Sources 2009, 194, 275–280. [Google Scholar] [CrossRef]
- Han, J.W.; Yildiz, B. Mechanism for enhanced oxygen reduction kinetics at the (La,Sr)CoO3–δ/(La,Sr)2CoO4+δ hetero-interface. Energy Environ. Sci. 2012, 5, 8598–8607. [Google Scholar] [CrossRef]
- Liu, M.F.; Ding, D.; Blinn, K.; Li, X.X.; Nie, L.F.; Liu, M. Enhanced performance of LSCF cathode through surface modification. Int. J. Hydrogen Energy 2012, 37, 8613–8620. [Google Scholar] [CrossRef]
- Crumlin, E.J.; Mutoro, E.; Ahn, S.J.; la O’, G.J.; Leonard, D.N.; Borisevich, A.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Oxygen reduction kinetics enhancement on a heterostructured oxide surface for solid oxide fuel cells. J. Phys. Chem. Lett. 2010, 1, 3149–3155. [Google Scholar] [CrossRef]
- Atkinson, A.; Ramos, T. Chemically-induced stresses in ceramic oxygen ion-conducting membranes. Solid State Ion. 2000, 129, 259–269. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, X.; Zhang, W.; Zheng, Y.; Li, Y.; Yu, B.; Wang, J.; Chen, J. Measurement of oxygen reduction/evolution kinetics enhanced (La,Sr)CoO3/(La,Sr)2CoO4 hetero-structure oxygen electrode in operating temperature for SOCs. Int. J. Hydrog. Energy 2018. [Google Scholar] [CrossRef]
- Feng, Z.; Yacoby, Y.; Hong, W.T.; Zhou, H.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Revealing the atomic structure and strontium distribution in nanometer-thick La0.8Sr0.2CoO3–δ grown on (001)-oriented SrTiO3. Energy Environ. Sci. 2014, 7, 1166–1174. [Google Scholar] [CrossRef]
- Feng, Z.; Crumlin, E.J.; Hong, W.T.; Lee, D.; Mutoro, E.; Biegalski, M.D.; Zhou, H.; Bluhm, H.; Christen, H.M.; Shao-Horn, Y. In situ studies of the temperature-dependent surface structure and chemistry of single-crystalline (001)-oriented La0.8Sr0.2CoO3−δ perovskite thin films. J. Phys. Chem. Lett. 2013, 4, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.Y.; Wang, S.Z.; Liu, Z.; Yang, L.; Liu, M.L. Improving La0.6Sr0.4Co0.2Fe0.8O3−δ cathode performance by infiltration of a Sm0.5Sr0.5CoO3− δ coating. Solid State Ion. 2009, 180, 1285–1289. [Google Scholar] [CrossRef]
- Nie, L.F.; Liu, M.F.; Zhang, Y.J.; Liu, M.L. La0.6Sr0.4Co0.2Fe0.8O3−δ cathodes infiltrated with samarium-doped cerium oxide for solid oxide fuel cells. J. Power Sources 2010, 195, 4704–4708. [Google Scholar] [CrossRef]
- Chen, H.; Guo, Z.; Zhang, L.A.; Li, Y.; Li, F.; Zhang, Y.; Chen, Y.; Wang, X.; Yu, B.; Shi, J.M.; et al. Improving the electrocatalytic activity and durability of the La0.6Sr0.4Co0.2Fe0.8O3−δ cathode by surface modification. ACS Appl. Mater. Interfaces 2018, 10, 39785–39793. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Jung, W.; Ahn, S.-J.; Lee, D. Controlling the Oxygen Electrocatalysis on Perovskite and Layered Oxide Thin Films for Solid Oxide Fuel Cell Cathodes. Appl. Sci. 2019, 9, 1030. https://doi.org/10.3390/app9051030
Yang G, Jung W, Ahn S-J, Lee D. Controlling the Oxygen Electrocatalysis on Perovskite and Layered Oxide Thin Films for Solid Oxide Fuel Cell Cathodes. Applied Sciences. 2019; 9(5):1030. https://doi.org/10.3390/app9051030
Chicago/Turabian StyleYang, Gene, Wonsang Jung, Sung-Jin Ahn, and Dongkyu Lee. 2019. "Controlling the Oxygen Electrocatalysis on Perovskite and Layered Oxide Thin Films for Solid Oxide Fuel Cell Cathodes" Applied Sciences 9, no. 5: 1030. https://doi.org/10.3390/app9051030
APA StyleYang, G., Jung, W., Ahn, S.-J., & Lee, D. (2019). Controlling the Oxygen Electrocatalysis on Perovskite and Layered Oxide Thin Films for Solid Oxide Fuel Cell Cathodes. Applied Sciences, 9(5), 1030. https://doi.org/10.3390/app9051030






