In Vitro Antioxidant Activity and In Vivo Topical Efficacy of Lipid Nanoparticles Co-Loading Idebenone and Tocopheryl Acetate
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lipid Nanoparticles (SLN and NLC)
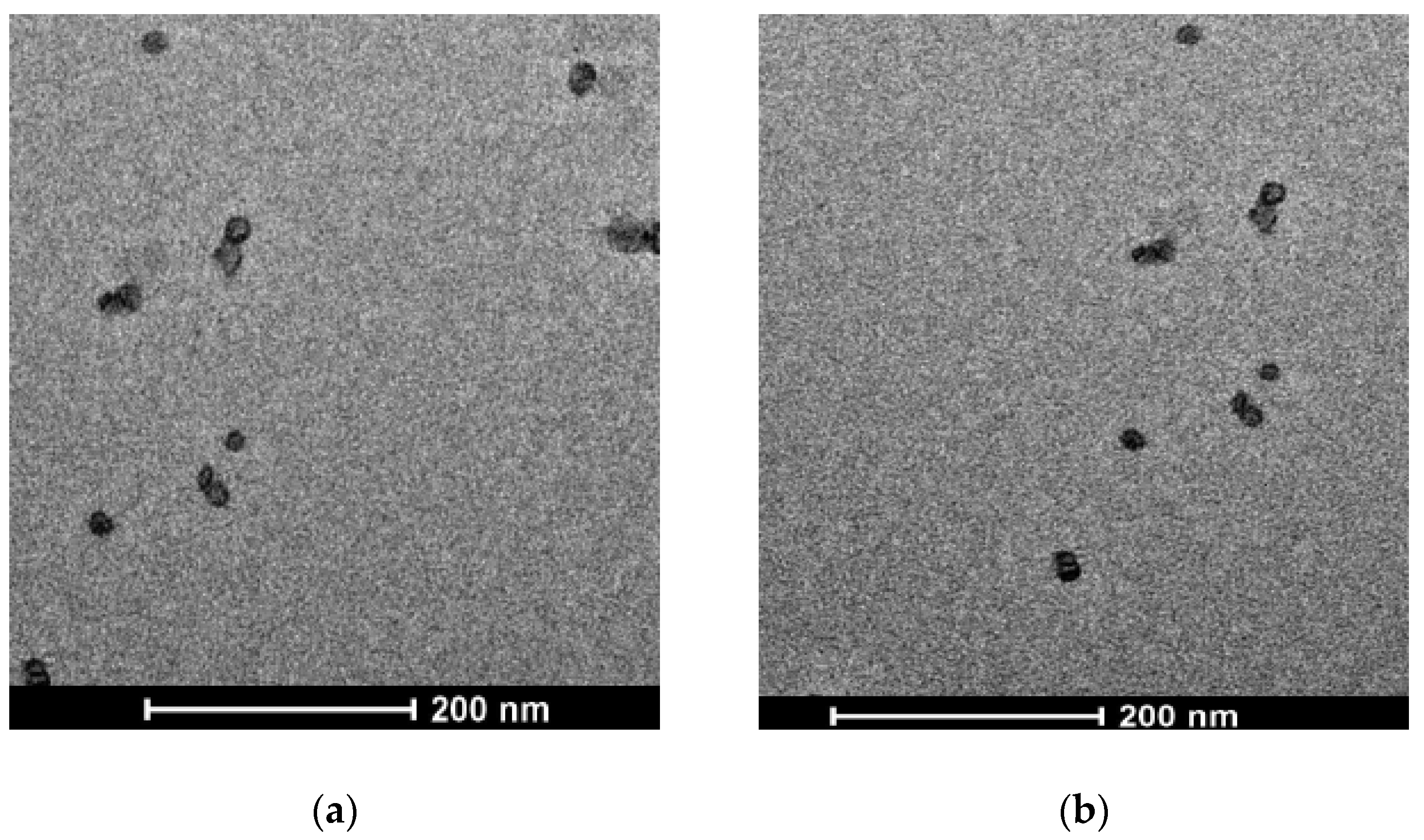
2.3. Transmission Electron Microscopy (TEM)
2.4. Photon Correlation Spectroscopy (PCS)
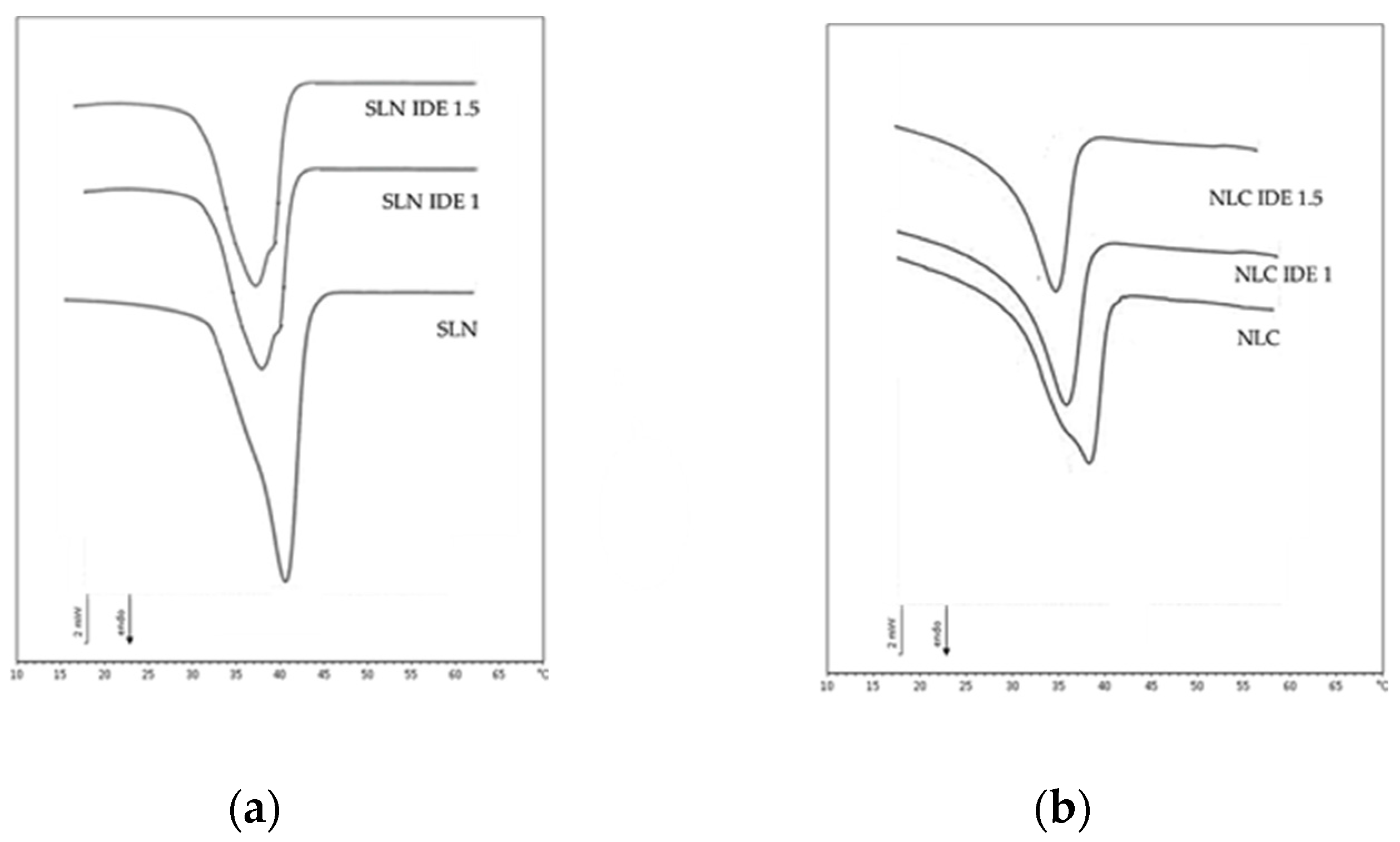
2.5. Differential Scanning Calorimetry (DSC)
2.6. Stability Tests
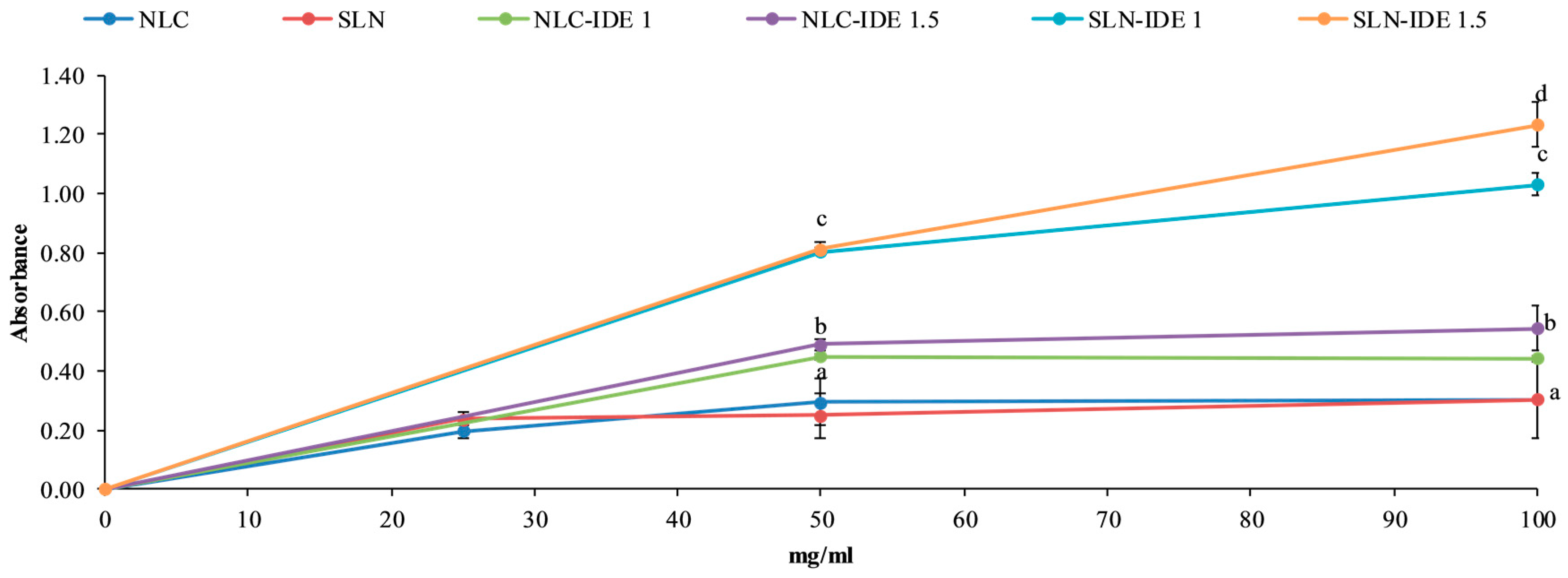
2.7. Reducing Power
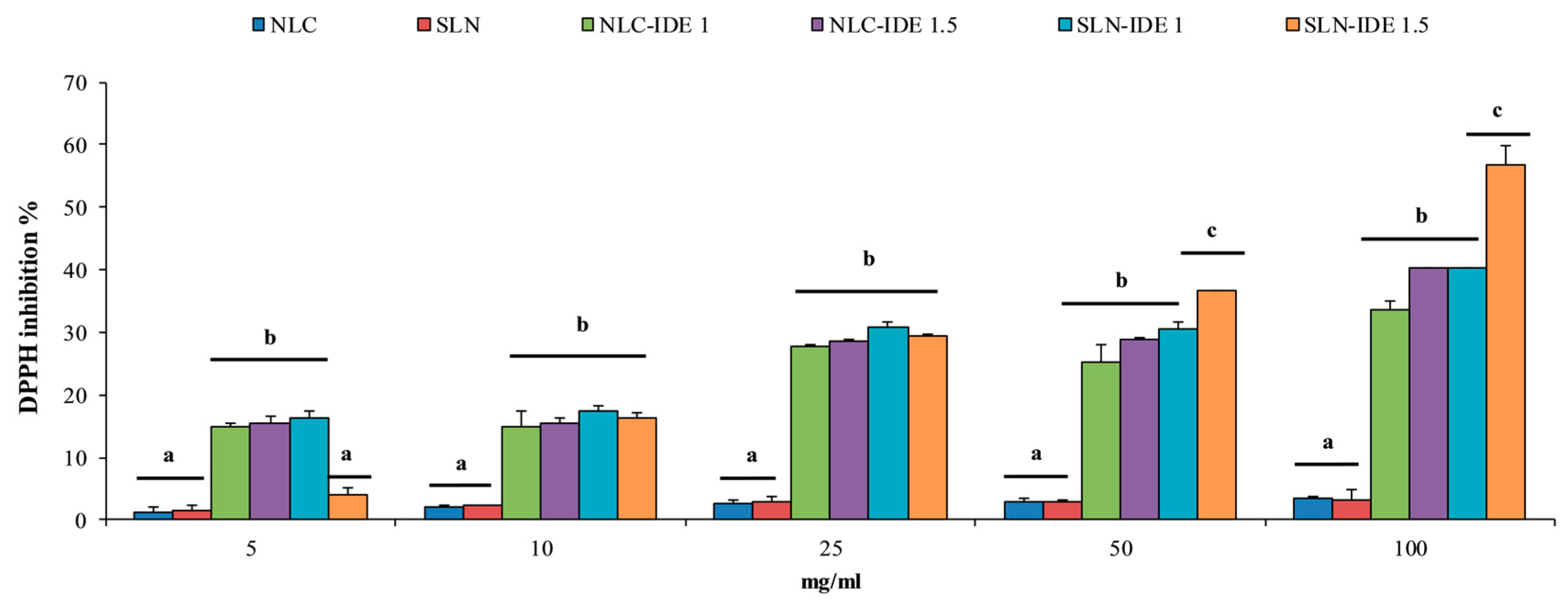
2.8. DPPH Radical Scavenging Activity
2.9. Cell Culture
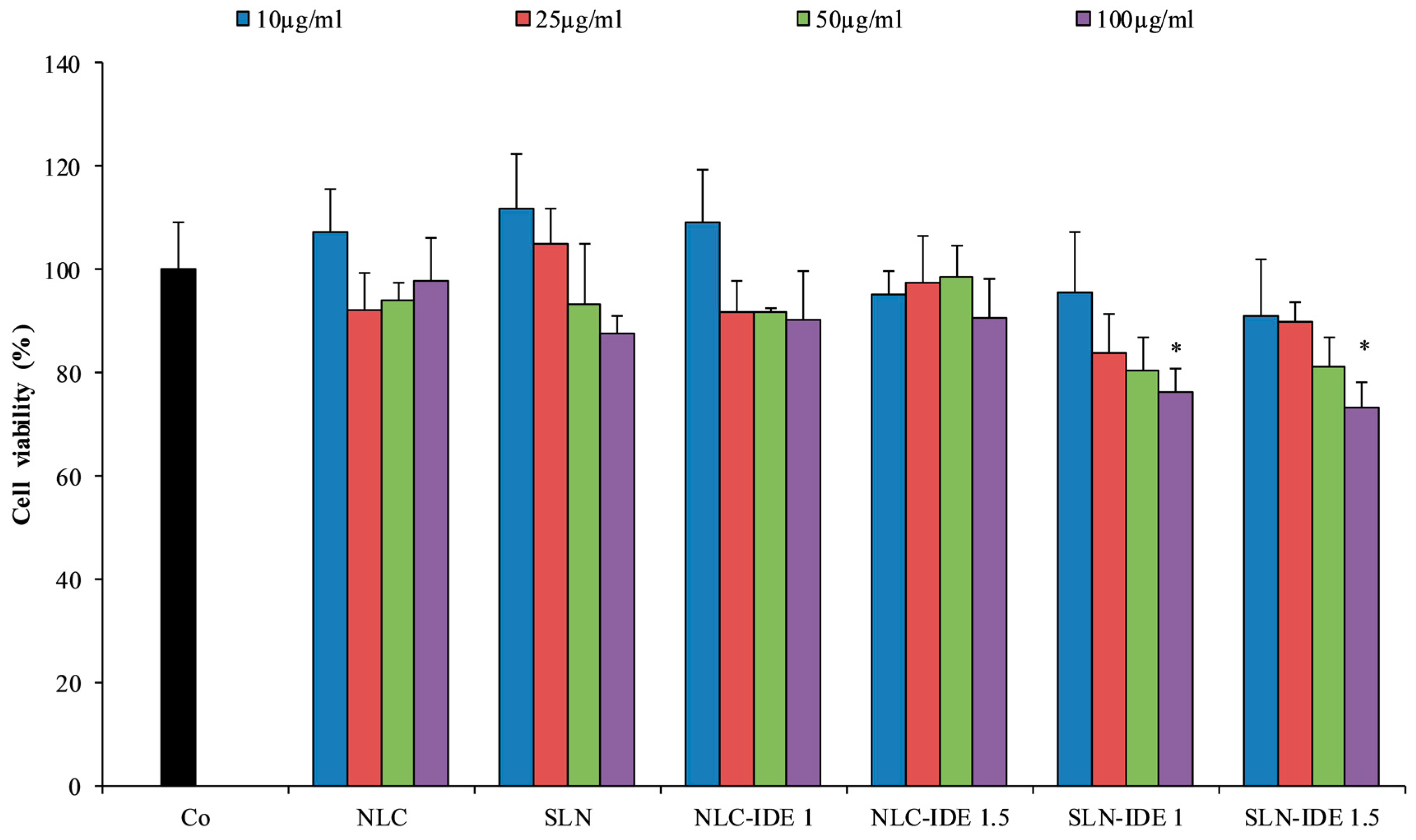
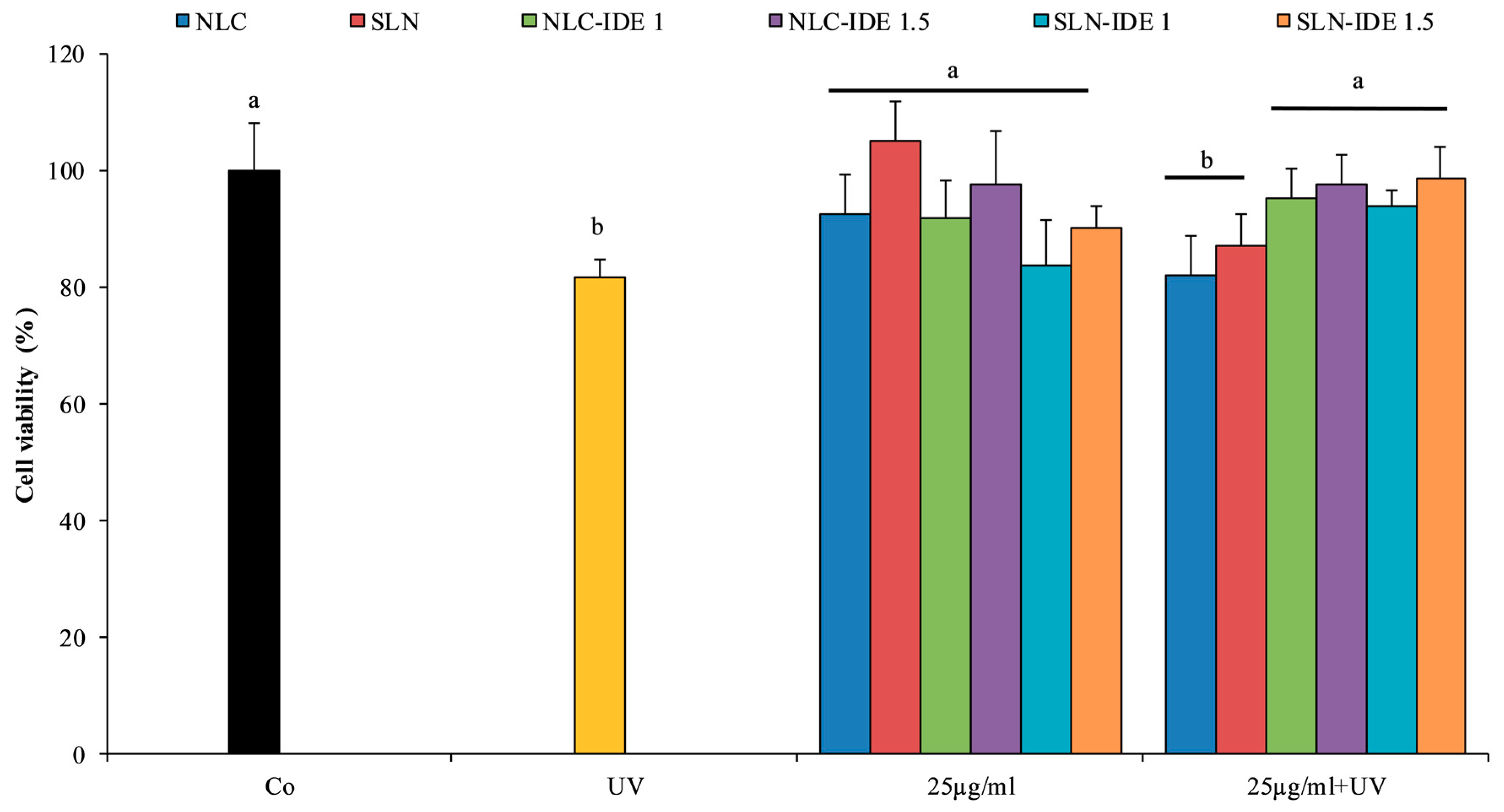
2.10. Evaluation of Cytotoxicity and Photo-Protective Effect in Fibroblast Cell Line HS-68
2.11. Gel Preparation
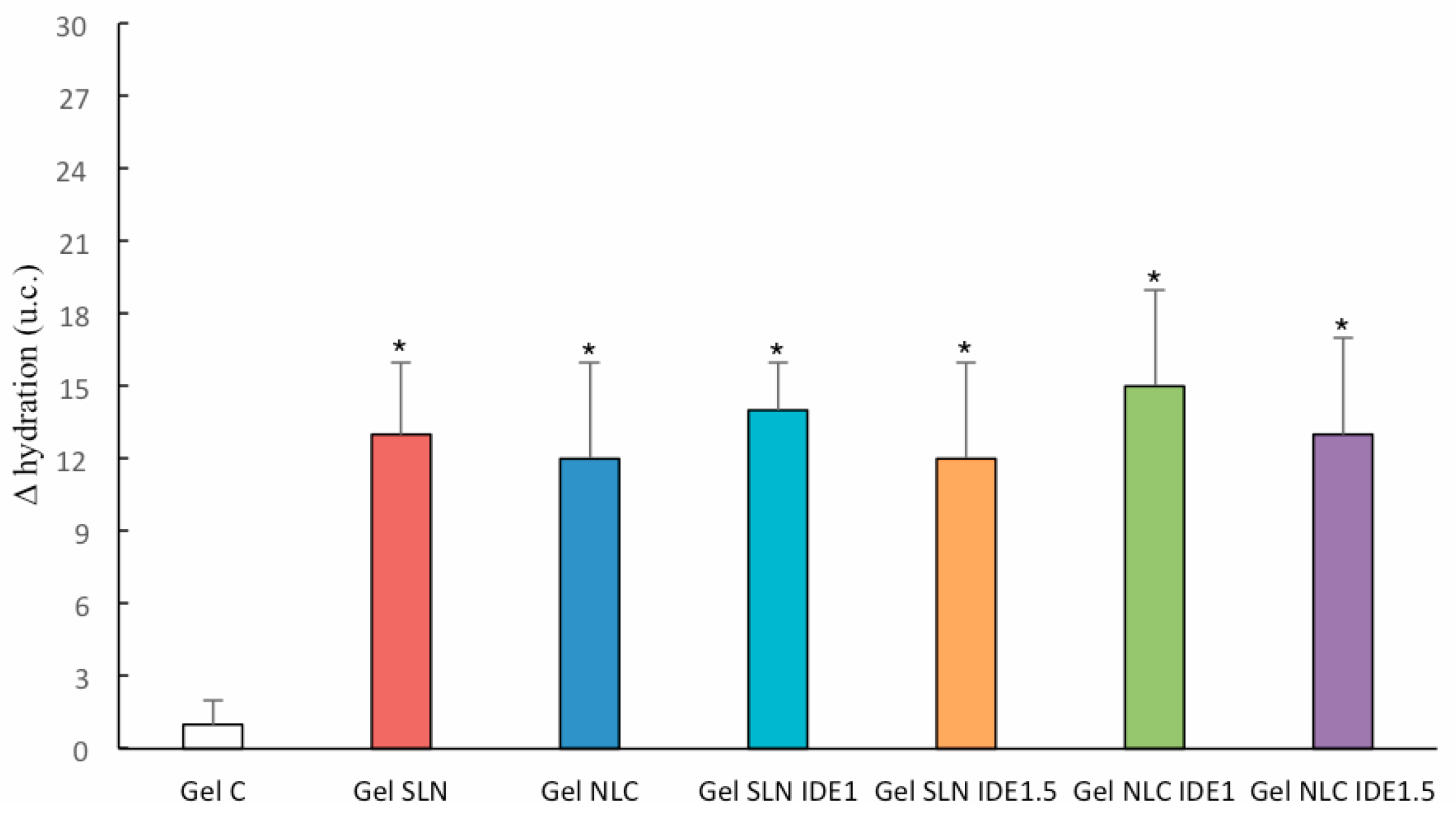
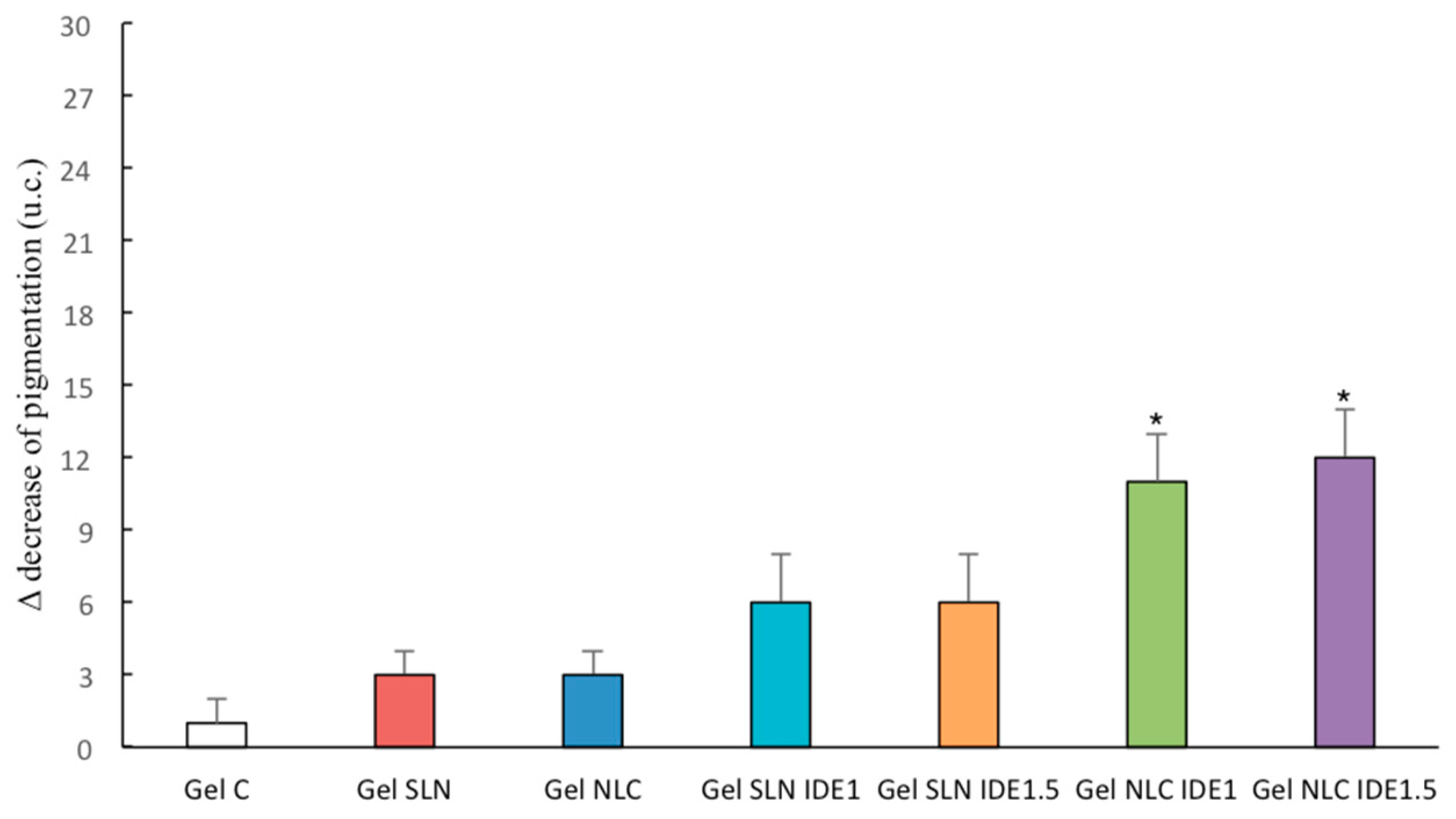
2.12. In Vivo Evaluation of Gel Formulations
2.13. Statistical Analyses
3. Results and Discussion
3.1. Lipid Nanoparticle Characterization
3.2. Reducing Power
3.3. Free Radical Scavenging Activity
3.4. Evaluation of Cytotoxicity and Photo-Protective Effect in Fibroblast Cell Line HS-68
3.5. In Vivo Topical Effects
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Nair, R.; Arun Kumar, K.S.; Vishnu Priya, K.; Sevukarajan, M. Recent advances in solid lipid nanoparticle based drug delivery systems. J. Biomed. Sci. Res. 2011, 3, 368–384. [Google Scholar]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L. Nanocarriers for skin delivery of cosmetic antioxidants. J. Pharm. Pharmacogn. Res. 2014, 2, 73–92. [Google Scholar]
- Dasgupta, S.; Ghosh, SK.; Ray, S.; Mazumder, B. Solid lipid nanoparticles (SLNs) gels for topical delivery of aceclofenac in vitro and in vivo evaluation. Curr. Drug. Deliv. 2013, 10, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Khallaf, R.A.; Salem, H.F.; Abdelbary, A. 5-Fluorouracil shell-enriched solid lipid nanoparticles (SLN) for effective skin carcinoma treatment. Drug Deliv. 2016, 23, 3452–3460. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.R.; Khude, P.A.; Dua, K. Development and evaluation of solid lipid nanoparticles of mometasone furoate for topical delivery. Int. J. Pharm. Investig. 2014, 4, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Pasquinucci, L.; Zappalà, A.; Chiechio, S.; Turnaturi, R.; Parenti, C. Rosemary Essential Oil-Loaded Lipid Nanoparticles: In Vivo Topical Activity from Gel Vehicles. Pharmaceutics 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Parenti, C.; Turnaturi, R.; Pasquinucci, L. Resveratrol-Loaded Lipid Nanocarriers: Correlation between In Vitro Occlusion Factor and In Vivo Skin Hydrating Effect. Pharmaceutics 2017, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Mellace, S.; Cassano, R. Solid lipid nanoparticles for antifungal drugs delivery for topical applications. Ther. Deliv. 2016, 7, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Tupal, A.; Sabzichi, M.; Ramezani, F.; Kouhsoltani, M.; Hamishehkar, H. Dermal delivery of doxorubicin-loaded solid lipid nanoparticles for the treatment of skin cancer. J. Microencapsul. 2016, 33, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L. Lipid-based nanoparticles as carriers for dermal delivery of antioxidants. Curr. Drug Metab. 2017, 18, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Turnaturi, R.; Parenti, C.; Pasquinucci, L. Idebenone: Novel strategies to improve its systemic and local efficacy. Nanomaterials 2018, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Sinico, C.; Castangia, I.; Carbone, C.; Puglisi, G. Idebenone-loaded solid lipid nanoparticles for drug delivery to the skin: In vitro evaluation. Int. J. Pharm. 2012, 434, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Ekanayake-Mudiyanselage, S. Vitamin E in human skin: Organ-specific physiology and considerations for its use in dermatology. Mol. Aspects Med. 2007, 2007 28, 646–667. [Google Scholar] [CrossRef]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Campisi, A.; Sarpietro, M.G.; Carbone, C.; Acquaviva, R.; Raciti, G.; Puglisi, G. In vitro evaluation of idebenone-loaded solid lipid nanoparticles for drug delivery to the brain. Drug Dev. Ind. Pharm. 2011, 37, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Trapani, A.; Latrofa, A.; Puglisi, G. In vitro evaluation on a model of blood brain barrier of idebenone-loaded solid lipid nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Radomska-Soukharev, A. Stability of lipid excipients in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Sarpietro, M.G.; Accolla, M.L.; Puglisi, G.; Castelli, F.; Montenegro, L. Idebenone loaded solid lipid nanoparticles: Calorimetric studies on surfactant and drug loading effects. Int. J. Pharm. 2014, 471, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ksouri, R.; Medini, F.; Guyot, S.; Abdelly, C.; Magné, C. Antioxidant activity and phenolic composition of the medicinal and edible halophyte Mesembryanthemum edule L. Ind. Crop. Prod. 2011, 34, 1066–1071. [Google Scholar] [CrossRef]
- Manuguerra, S.; Caccamo, L.; Mancuso, M.; Arena, R.; Rappazzo, A.C.; Genovese, L.; Santulli, A.; Messina, C.M.; Maricchiolo, G. The antioxidant power of horseradish, Armoracia rusticana, underlies antimicrobial and antiradical effects, exerted in vitro. Natl. Prod. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Tomaino, A.; Trombetta, D.; Pellegrino, M.L.; Tita, B.; Messina, C.; Bonina, F.P.; Rocco, C.; Nicolosi, G.; Castelli, F. In vitro antioxidant and photoprotective properties and interaction with model membranes of three new quercetin esters. Eur. J. Pharm. Biopharm. 2003, 56, 167–174. [Google Scholar] [CrossRef]
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55. [Google Scholar] [CrossRef]
- Montenegro, L.; Panico, A.M.; Santagati, L.M.; Siciliano, E.A.; Intagliata, S.; Modica, M.N. Solid lipid nanoparticles loading idebenone ester with pyroglutamic acid: In vitro antioxidant activity and in vivo topical efficacy. Nanomaterials 2019, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Montenegro, L.; Modica, M.N.; Salerno, L.; Panico, A.M.; Crascì, L.; Puglisi, G.; Romeo, G. In vitro antioxidant activity of idebenone derivative-loaded solid lipid nanoparticles. Molecules 2017, 22, 887. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Sarpietro, M.G.; Ottimo, S.; Puglisi, G.; Castelli, F. Differential scanning calorimetry studies on sunscreen loaded solid lipid nanoparticles prepared by the phase inversion temperature method. Int. J. Pharm. 2011, 415, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Lim, S.J.; Kim, C.K. Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials 2007, 28, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Castelli, F.; Sarpietro, M.G. Differential scanning calorimetry analyses of idebenone-loaded solid lipid nanoparticles interactions with a model of bio-membrane: A comparison with in vitro skin permeation data. Pharmaceuticals 2018, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Ottimo, S.; Puglisi, G.; Castelli, F.; Sarpietro, M.G. Idebenone loaded solid lipid nanoparticles interact with biomembrane models: Calorimetric evidence. Mol. Pharm. 2012, 9, 2534–2541. [Google Scholar] [CrossRef] [PubMed]
- zur Mühlen, A.; Schwarz, C.; Mehnert, W. Solid lipid nanoparticles (SLN) for controlled drug delivery—Drug release and release mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Chung, Y.C.; Chang, C.T.; Chao, W.W.; Lin, C.F.; Chou, S.T. Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 2002, 50, 2454–2458. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Weyenberg, W.; Filev, P.; Van den Plas, D.; Vandervoort, J.; De Smet, K.; Sollie, P.; Ludwig, A. Cytotoxicity of submicron emulsions and solid lipid nanoparticles for dermal application. Int. J. Pharm. 2007, 337, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Ridolfi, D.M.; Marcato, P.D.; Machado, D.; Silva, R.A.; Justo, G.Z.; Duran, N. In vitro cytotoxicity assays of solid lipid nanoparticles in epithelial and dermal cells. J. Phys. Conf. Ser. 2012, 304, 012032. [Google Scholar] [CrossRef]
- Wissing, S.A.; Müller, R.H. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity-in vivo study. Eur. J. Pharm. Biopharm. 2003, 56, 67–72. [Google Scholar] [CrossRef]
- Krol, E.S.; Kramer-Stickland, K.A.; Liebler, D.C. Photoprotective actions of topically applied vitamin E. Drug Metab. Rev. 2000, 32, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, S.S.; Bhatia, N.M.; Pokharkar, V.B.; Thorat, J.D.; Bhatia, M.S. Topical delivery of Idebenone using nanostructured lipid carriers: Evaluations of sun-protection and anti-oxidant effects. J. Pharm. Investig. 2013, 43, 287–303. [Google Scholar] [CrossRef]
| Code | Ingredients (% w/w) * | ||||
|---|---|---|---|---|---|
| Oleth-20 | GO | CP | VitE | IDE | |
| SLN | 9.00 | 5.00 | 7.00 | - | - |
| SLN IDE1 | 9.00 | 5.00 | 7.00 | - | 1.00 |
| SLN IDE1.5 | 9.00 | 5.00 | 7.00 | - | 1.50 |
| NLC | 9.00 | 5.00 | 6.00 | 1.00 | - |
| NLC IDE1 | 9.00 | 5.00 | 6.00 | 1.00 | 1.00 |
| NLC IDE1.5 | 9.00 | 5.00 | 6.00 | 1.00 | 1.50 |
| Code | Size ± SD (nm) | PDI ± SD | ζ ± SD (mV) | PIT (°C) |
|---|---|---|---|---|
| SLN | 36.40 ± 0.30 | 0.260 ± 0.010 | −1.56 ± 0.48 | 78 |
| SLN IDE1 | 23.55 ± 1.08 | 0.266 ± 0.023 | −2.02 ± 0.54 | 69 |
| SLN IDE 1.5 | 42.33 ± 0.29 | 0.230 ±0.031 | −2.01 ± 0.33 | 77 |
| NLC | 28.78 ± 0.90 | 0.235 ± 0.030 | −2.00 ± 0.24 | 85 |
| NLC IDE1 | 26.76 ± 0.33 | 0.178 ± 0.041 | −1.99 ± 0.33 | 78 |
| NLC IDE1.5 | 27.33 ± 0.55 | 0.198 ± 0.023 | −2.32 ± 0.63 | 71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro, L.; Messina, C.M.; Manuguerra, S.; Santagati, L.M.; Pasquinucci, L.; Turnaturi, R.; Parenti, C.; Arena, R.; Santulli, A. In Vitro Antioxidant Activity and In Vivo Topical Efficacy of Lipid Nanoparticles Co-Loading Idebenone and Tocopheryl Acetate. Appl. Sci. 2019, 9, 845. https://doi.org/10.3390/app9050845
Montenegro L, Messina CM, Manuguerra S, Santagati LM, Pasquinucci L, Turnaturi R, Parenti C, Arena R, Santulli A. In Vitro Antioxidant Activity and In Vivo Topical Efficacy of Lipid Nanoparticles Co-Loading Idebenone and Tocopheryl Acetate. Applied Sciences. 2019; 9(5):845. https://doi.org/10.3390/app9050845
Chicago/Turabian StyleMontenegro, Lucia, Concetta Maria Messina, Simona Manuguerra, Ludovica Maria Santagati, Lorella Pasquinucci, Rita Turnaturi, Carmela Parenti, Rosaria Arena, and Andrea Santulli. 2019. "In Vitro Antioxidant Activity and In Vivo Topical Efficacy of Lipid Nanoparticles Co-Loading Idebenone and Tocopheryl Acetate" Applied Sciences 9, no. 5: 845. https://doi.org/10.3390/app9050845






