Effects of Mixing Conditions on Floc Properties in Magnesium Hydroxide Continuous Coagulation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Test Water and Coagulant
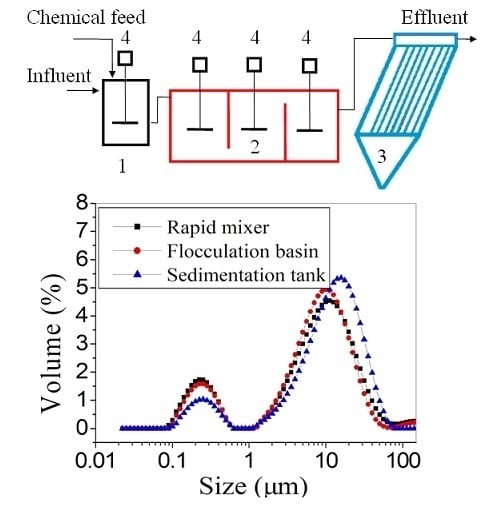
2.2. Apparatus and Procedures
2.3. Floc Size Distribution and Properties Analysis
3. Results and Discussion
3.1. Coagulation Behaviors under Different Rapid Mixing Speed
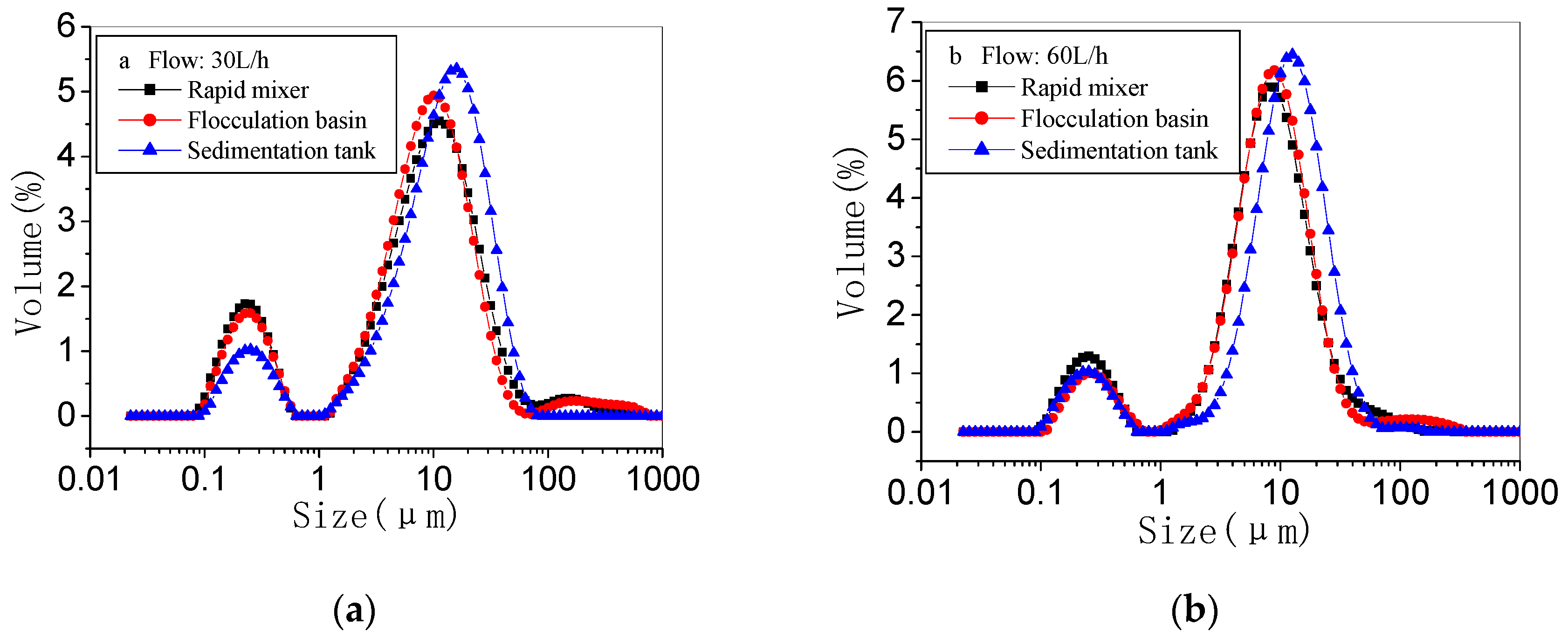
3.1.1. Floc Size Distribution in Three Processes
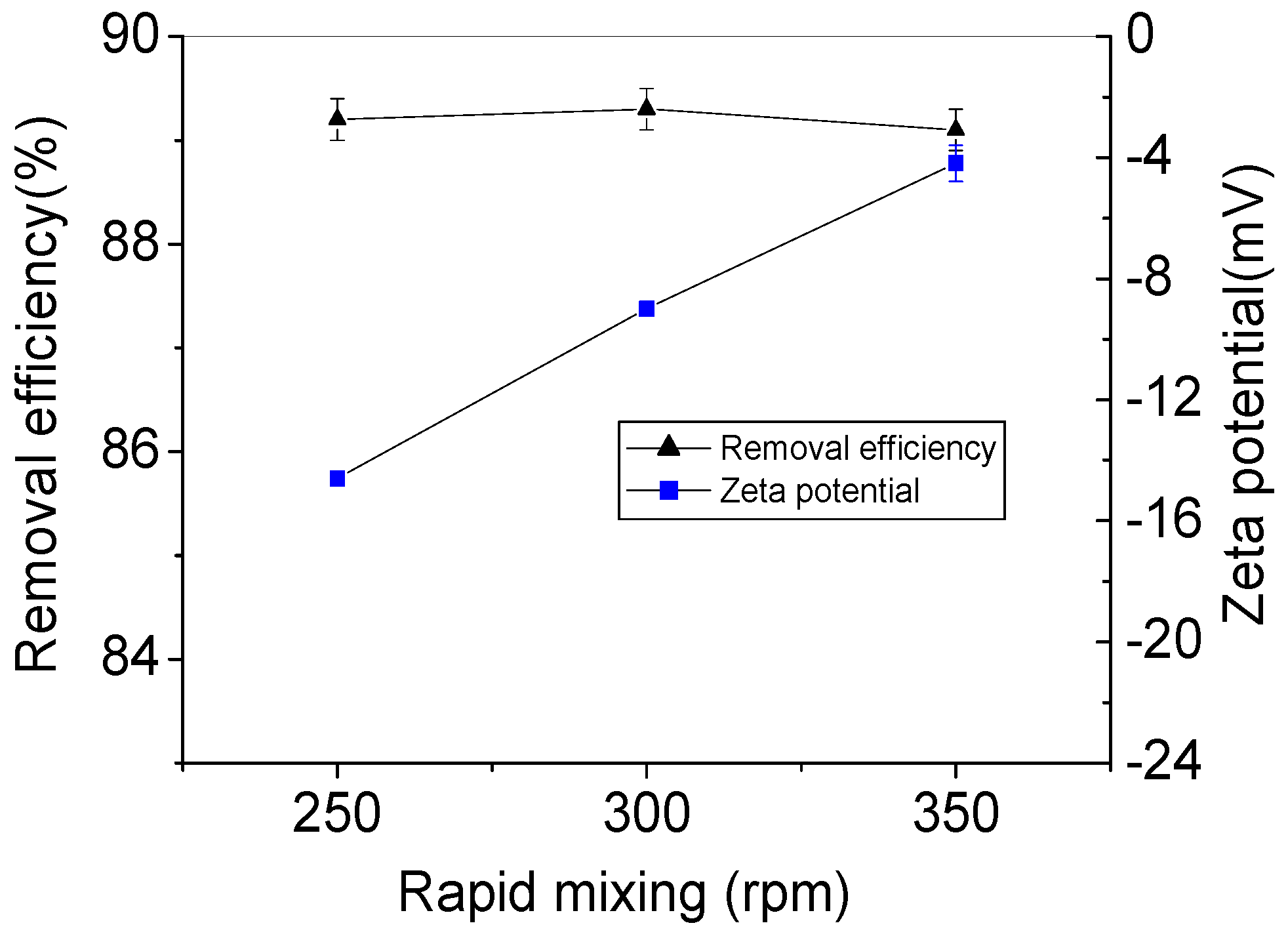
3.1.2. Removal Efficiency and Zeta Potential
3.2. Effect of Flow on Coagulation Performance
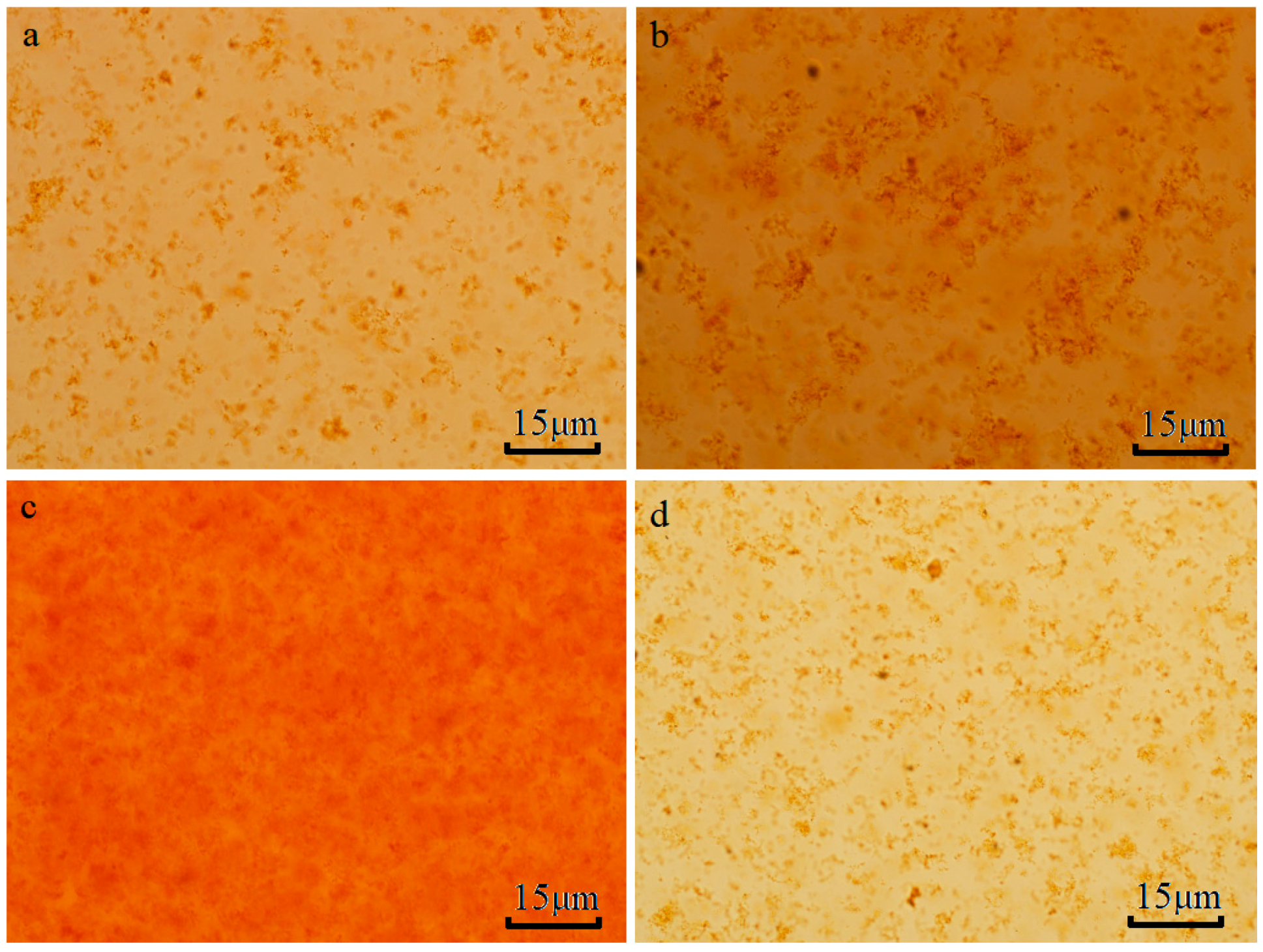
3.3. Images Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Wu, T.; Li, Y.; Sun, D.; Zhang, G.; Wang, Y.; Wang, G.; Zhang, M. Removal of petroleum sulfonate from aqueous solutions using freshly generated magnesium hydroxide. J. Hazard. Mater. 2012, 82–88, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.Y.; Yue, Q.Y.; Wang, Y.; Zhou, W.Z. Color removal from dye-containing wastewater by magnesium chloride. J. Environ. Manag. 2007, 82, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, G.M.; BinAhmed, S.W.; Al-Hindi, M.; Azizi, F. Coagulation of highly turbid suspensions using magnesium hydroxide: Effects of slow mixing conditions. Environ. Sci. Pollut. Res. 2014, 21, 10502–10513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Luan, Z.; Wei, N.; Li, J.; Liu, C. The color removal of dye wastewater by magnesium chloride/red mud (MRM) from aqueous solution. J. Hazard. Mater. 2009, 170, 690–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.H.; Teng, T.T.; Mohd Omar, A.K. Removal of dyes and industrial dye wastes by magnesium chloride. Water. Res. 2000, 34, 597–601. [Google Scholar] [CrossRef]
- Bouyakoub, A.Z.; Lartiges, B.S.; Ouhib, R.; Kacha, S.; El Samrani, A.G.; Ghanbaja, J.; Barres, O. MnCl2 and MgCl2 for the removal of reactive dye Levafix Brilliant Blue EBRA from synthetic textile wastewaters: An adsorption/aggregation mechanism. J. Hazard. Mater. 2011, 187, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, H.; Yan, H.; Wu, H.; Yang, H.; Wu, Q.; Li, H.; Li, A.; Cheng, R. Evaluation of a novel chitosan-based flocculant with high flocculation performance, low toxicity and good floc properties. J. Hazard. Mater. 2014, 276, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhu, Z.; Wang, D.S. Characterization of floc size, strength and structure under various coagulation mechanisms. Powder. Technol. 2006, 168, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Yi, P.; Pan, X.R.; Zhang, B.J.; Lee, C. Comparative study of the effects of experimental variables on growth rates of aluminum and iron hydroxide flocs during coagulation and their structural characteristics. Desalination 2010, 250, 902–907. [Google Scholar] [CrossRef]
- McCurdy, K.; Carlson, K.; Gregory, D. Floc morphology and cyclic shearing recovery: Comparison ofalum and polyaluminum chloride coagulants. Water Res. 2004, 38, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Z.; Gregory, J.; Campos, L.; Li, G. The role of mixing conditions on floc growth, breakage and re-growth. Chem. Eng. J. 2011, 171, 425–430. [Google Scholar] [CrossRef]
- Liu, M.; Lu, J.; Wei, L.; Wang, K.; Zhao, J. Magnesium hydroxide coagulation performance and floc properties in treating high pH reactive orange wastewater. Water Sci. Technol. 2015, 71, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shi, H.; Liu, M.; Lu, J.; Li, W. Coagulation-adsorption of reactive orange from aqueous solution by freshly formed magnesium hydroxide: Mixing time and mechanistic study. Water Sci. Technol. 2017, 71, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, A.; Wei, L.; Ge, W.; Chi, Y.; Lai, Y. Effect of kaolin on floc properties for reactive orange removal in continuous coagulation process. Water Sci. Technol. 2018, 78, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.Y.; Gao, B.Y.; Yue, Q.Y.; Wang, Y. Effect of shear force and solution pH on flocs breakage and re-growth formed by nano-Al13 polymer. Water Res. 2010, 44, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- De Feo, G.; Galasso, M.; Landi, R.; Donnarumma, A.; De Gisi, S. A comparison of the efficacy of organic and mixed-organic polymers with polyaluminium chloride in chemically assisted primary sedimentation (CAPS). Environ. Technol. 2013, 34, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Semerjian, L.; Ayoub, G.M. High-pH—magnesium coagulation—flocculation in wastewatertreatment. Adv. Environ. Res. 2003, 7, 389–403. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, F.; Wu, W.; Bu, R.; Li, W.; Yang, F. Reactive orange 5 removal from aqueous solution using hydroxyl ammonium ionic liquids/layered double hydroxides intercalation composites. Chem. Eng. J. 2016, 285, 198–206. [Google Scholar] [CrossRef]
- Sharp, E.L.; Jarvis, P.; Parsons, S.A.; Jefferson, B. The impact of zeta potential on the physical properties of ferric-NOM flocs. Environ. Sci. Technol. 2006, 40, 3934–3940. [Google Scholar] [CrossRef] [PubMed]
- Leentvaar, J.; Rebhun, M. Effect of magnesium and calcium precipitation on coagulation—Flocculation with lime. Water Res. 1982, 16, 655–662. [Google Scholar] [CrossRef]


| Name | Molecular Structure | λmax (nm) |
|---|---|---|
| Reactive orange (K-GN) | 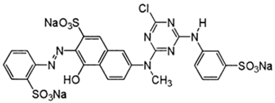 | 476 |
| Flux | Rapid Mixer | Flocculation Basin | Sedimentation Tank | ||
|---|---|---|---|---|---|
| 30 L/h | speed | time | speed | time | time |
| 250 rpm | 2 min | 80 rpm | 24 min | 60 min | |
| 300 rpm | |||||
| 350 rpm | |||||
| 60 L/h | 300 rpm | 1 min | 80 rpm | 12 min | 30 min |
| Rapid Mixing (rpm) | Average Floc Size (μm) | ||
|---|---|---|---|
| Rapid Mixer | Flocculation Basin | Sedimentation Tank | |
| 250 | 8.39 | 7.96 | 14.41 |
| 300 | 8.06 | 7.89 | 11.21 |
| 350 | 8.04 | 7.95 | 10.58 |
| Flow (L/h) | Average Size (μm) | ||
|---|---|---|---|
| Rapid Mixer | Flocculation Basin | Sedimentation Tank | |
| 30 | 8.06 | 7.89 | 11.21 |
| 60 | 7.25 | 7.52 | 10.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Zhao, J.; Wei, L.; Li, W.; Chi, Y. Effects of Mixing Conditions on Floc Properties in Magnesium Hydroxide Continuous Coagulation Process. Appl. Sci. 2019, 9, 973. https://doi.org/10.3390/app9050973
Ding Y, Zhao J, Wei L, Li W, Chi Y. Effects of Mixing Conditions on Floc Properties in Magnesium Hydroxide Continuous Coagulation Process. Applied Sciences. 2019; 9(5):973. https://doi.org/10.3390/app9050973
Chicago/Turabian StyleDing, Yanmei, Jianhai Zhao, Lei Wei, Wenpu Li, and Yongzhi Chi. 2019. "Effects of Mixing Conditions on Floc Properties in Magnesium Hydroxide Continuous Coagulation Process" Applied Sciences 9, no. 5: 973. https://doi.org/10.3390/app9050973