Review of Coal-Fired Electrification and Magnetic Separation Desulfurization Technology
Abstract
:1. Introduction
2. The Form of Sulfur in Coal
3. Comparison of Coal-Fired Desulfurization Methods
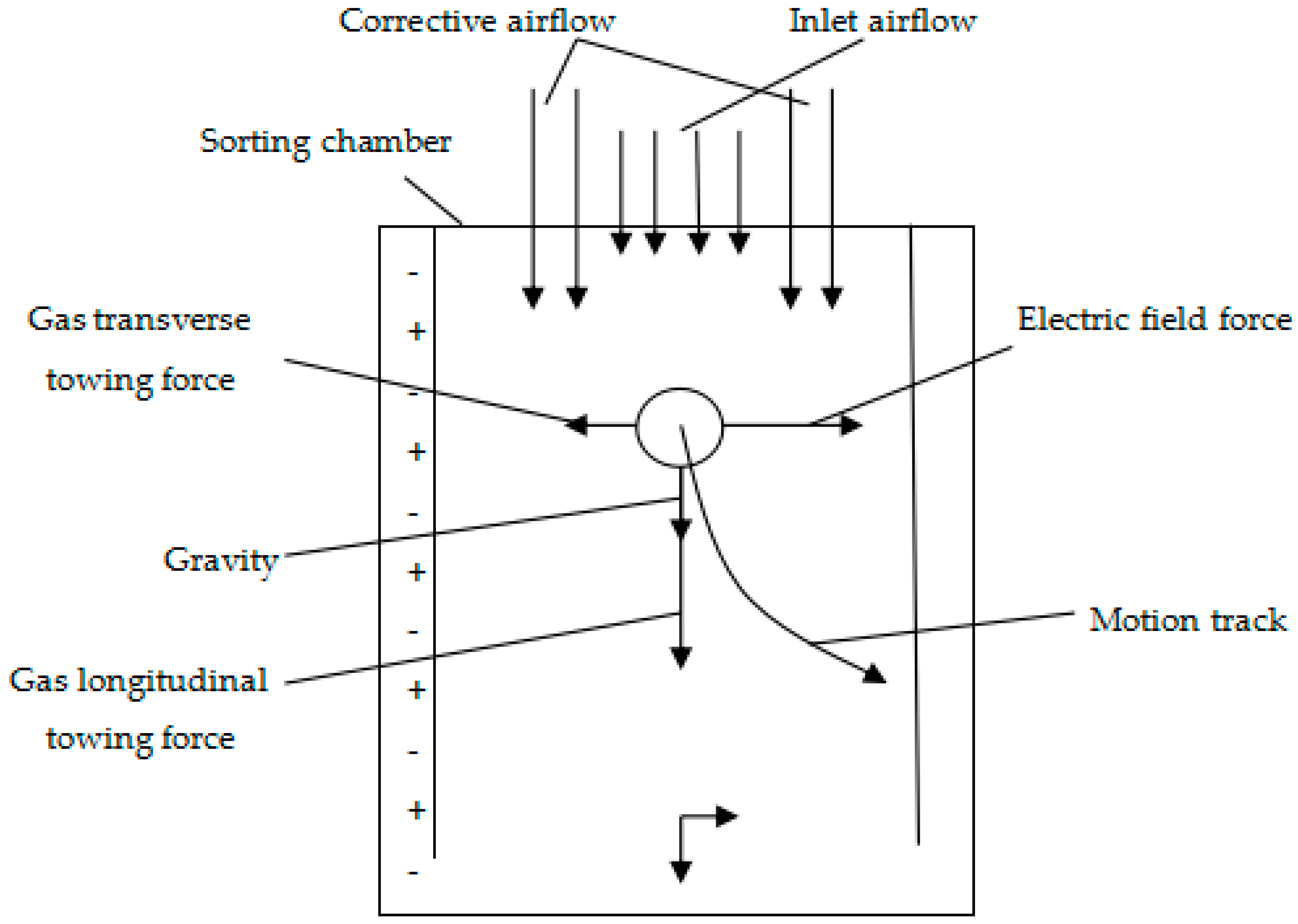
4. Friction Electrostatic Separation Technology
4.1. The Basic Principle of Friction Electrostatic Separation Technology
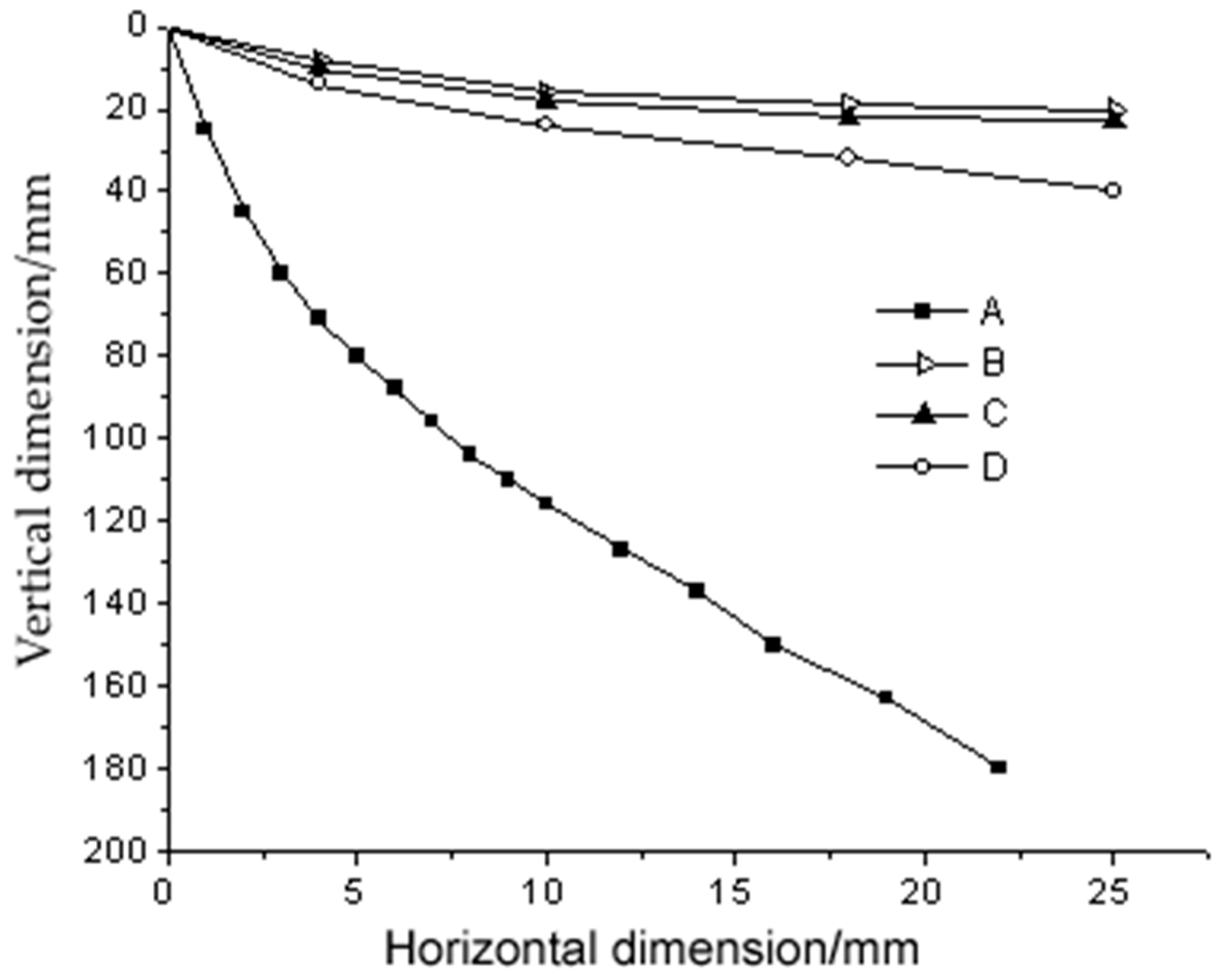
4.2. The Development of Friction Electrostatic Separation Technology
5. High Gradient Magnetic Separation Technology
5.1. The Basic Principle of High Gradient Magnetic Separation
5.2. The Development of Desulfurization by High Gradient Magnetic Separation
5.3. The Development of High Gradient Magnetic Separators
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The National Development and Reform Commission, the State Energy Administration. The 13th Five-Year Plan for the Development of Coal Industry. Development and Transformation of Energy. 2016. Available online: http://www.ndrc.gov.cn/fzgggz/fzgh/ghwb/gjjgh/201706/t20170605_850004.html (accessed on 15 January 2019).
- Wang, J.; Chen, X.; Ning, M. Environmental Challenges and Strategic Countermeasures for Medium and Long Term Energy Development in China. Chin. Eng. Sci. 2011, 13, 2419–2424. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection. Annual Report of Environment Statistics of China (2001–2015). Available online: http://www.cnemc.cn/jcbg/zghjtjnb/ (accessed on 14 March 2019).
- CNTV. Is It Possible for China to Have No Coal? Available online: http://jingji.cntv.cn/2015/01/12/ARTI1421026493565786.shtml (accessed on 14 March 2019).
- Jiao, H.; Ding, L.; Chen, Q. Development and consideration of high gradient magnetic separation desulfurization technology for fine coal. China Min. Ind. 2007, 16, 79–81. [Google Scholar] [CrossRef]
- Jiao, D.; Hu, T.; Jin, H.; Wang, Z.; Zhang, Y. Desulfurization technology and prospect of high sulfur coal. Energy Eng. 2010, 4, 55–58. [Google Scholar] [CrossRef]
- Amir, A.; Corcoran, W.H. Corcoran. Sulfur compounds in coal. Ind. Eng. Chem. Prod. Res. Dev. 1977, 16, 168–170. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, Z.; Liu, J.; Li, W.; Yuan, L. Application of microorganisms in coal desulfurization. Coal Prep. Technol. 2003, 6, 19–25. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, Q.; Zhang, P.; Zhang, C. Research status of coal desulfurization. Guangxi Light Ind. 2007, 5, 84–85. [Google Scholar] [CrossRef]
- Zuo, W.; Luo, Z.; Wu, W.; Chen, S.; Guo, J.; Liu, X.; Ji, Y. Dry Separation Technology of High sulfur Coal. China Univ. Min. Technol. 2009, 6, 17–21. [Google Scholar] [CrossRef]
- Zhu, F.; Meng, J.; Zhu, S. Application of magnetic separation technology in coal desulfurization. Coal Process. Compr. Util. 2004, 1. [Google Scholar] [CrossRef]
- Ju, C.; Zhang, W.; Li, F. Research progress of coal desulfurization technology at home and abroad. Heilongjiang Sci. 2011, 2, 40–44. [Google Scholar]
- Huang, W.; Zhao, M. Biological desulfurization of coal. Coal Process. Compr. Util. 2014, 5, 70–72. [Google Scholar] [CrossRef]
- Jiao, H.; Cui, J.; Chen, Q. Review on dry desulphurization and ash reduction technology of pulverized coal before combustion. Coal Qual. Technol. 2007, 2, 43–45, 47. [Google Scholar] [CrossRef]
- Yu, H.; Dai, H.; Chen, X.; Yang, W.; He, D.; Jiang, H. Research and development of friction electroseparation abroad. Miner. Prot. Util. 2015, 4, 67–72. [Google Scholar] [CrossRef]
- Xin, D.Z.; Chen, S.L.; Wang, Q.F. Technology of load—Sensitivity used in the hydraulic system of an all—Hydraulic core rig. J. Coal Sci. Eng. 2009, 3, 318–323. [Google Scholar] [CrossRef]
- Tao, D.; Fan, M.; Jiang, X. Dry coal fly ash cleaning using rotary triboelectrostatic separator. Min. Sci. Technol. 2009, 19, 642–647. [Google Scholar] [CrossRef]
- Tao, D.; Al-Hwaiti, M. Beneficiation study of Eshidiya phosphorites using a rotary triboelectrostatic separator. Min. Sci. Technol. 2010, 20, 357–364. [Google Scholar] [CrossRef]
- Inculet, I.I.; Bergougnou, M.A.; Brown, J.D. Electrostatic separation of particles below 40 μm in a dilute phase continuous Loop. IEEE Trans. Ind. Appl. 1977, 13, 370–373. [Google Scholar] [CrossRef]
- Kiewiet, C.W.; Bergougnou, M.A.; Brown, J.D.; Inculet, I.I. Electrostatic separation of fine particles in vibrated fluidized beds. IEEE Trans. Ind. Appl. 1978, 14, 526–530. [Google Scholar] [CrossRef]
- Beeckmans, J.M.; Inculet, I.I.; Dumas, G. Enhancement in segregation of a mixed powder in a fluidized bed in the presence of an electrostatic field. Powder Technol. 1979, 24, 267–269. [Google Scholar] [CrossRef]
- Alfano, G.; Liu, Y.; Yang, Y. Progress of triboelectric separation of minerals. Ore Dress. Foreign Metals 1989, 2, 1–9. [Google Scholar]
- Singewald, A.; Fricke, G. Process for Electrostatic Separation of Pyrite from Crude Coal. U.S. Patent 3,941,685, 2 March 1976. [Google Scholar]
- Huang, K. Separation of Unburned Carbon from Fly Ash. Foreign Miner. Process. KuaiBao 1997, 19, 6–8. [Google Scholar]
- Tao, D. Electrostatic Particle Separation Device. Patent 200420079562.1, 14 December 2005. [Google Scholar]
- Tao, D.; Sobhy, A.; Li, Q.; Zhao, Y. Dry cleaning of pulverized coal using a novel rotary triboelectrostatic separator (RTS). Int. J. Coal Prep. Util. 2011, 31, 187–202. [Google Scholar] [CrossRef]
- Brown, D.K. Electrostatic Pyrite Ash and Toxic Mineral Separator. U.S. Patent 5637122, 10 June 1997. [Google Scholar]
- Zhang, X.; Duan, C.; Yu, F.; Gao, M.; He, J. Study on Electrical Properties and friction electrification of pulverized Coal. J. China Univ. Min. Technol. 2005, 34, 694–697. [Google Scholar] [CrossRef]
- Ma, R.; Shi, C.; Zhang, X. Separation of coal from single mineral in triboelectric separation. J. China Univ. Min. Technol. 2010, 39, 270–274. [Google Scholar]
- Li, H.; Zhang, X.; Chen, Y.; Chen, M.; Chen, F. Numerical simulation of gas-solid two-phase flow field in fly ash electric separation and decarburization friction device. Coal Technol. 2012, 31, 140–142. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Chen, Y.; Chen, M.; Chen, F. Study on gas flow and single particle motion characteristics of electric friction separator. Coal Prep. Technol. 2012, 3, 2219–2240. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Chen, Y. Characteristics of collision of particles in frictional electroelector. J. China Univ. Min. Technol. 2012, 41, 607–612. [Google Scholar]
- Huang, G.; Luo, S.; Zhou, Y.; Ma, Y.; Yi, Y. Triboelectric separation of pulverized coal. Coal Technol. 2015, 34, 315–317. [Google Scholar] [CrossRef]
- Zhang, W.C.; Tao, Y.; Song, A.; Xian, Y.S.; Man, Z.P.; Xue, Y.W. Experimental study on dust reduction by rotating friction electric separation of micro-pulverized coal. Coal Sci. Technol. 2017, 45, 196–200. [Google Scholar] [CrossRef]
- Chen, B.; Yang, G.; Gao, J. Modeling and simulation of gas-liquid hydraulic impactor based on AMESim. J. Shanghai Univ. Eng. Technol. 2011, 25, 292–295. [Google Scholar] [CrossRef]
- Wang, C. Mechanism and Research status of Coal Desulfurization by Magnetic Separation. Qual. Saf. 2014, 26, 219, 221. [Google Scholar] [CrossRef]
- Kelland, D.R.; Lai-Fook, M.; Maxwell, E.; Takayasu, M.; Jacobs, I.S.; McConnel, M.D. HGMS coal desulfurization with microwave magnetization enhancement. IEEE Trans. Magn. 1988, 24, 2434–2436. [Google Scholar] [CrossRef]
- Hise, E.C.; Holman, A.S.; Friedlaender, F.J. Development of high-gradient and open-gradient magnet separation of dry fine coal. IEEE Trans. Magn. 1981, MAG-17, 3314–3316. [Google Scholar] [CrossRef]
- Fine, H.A.; Lowry, M.; Power, L.F.; Geiger, G.H. A proposed process for the finely divided coal by flash roasting and magnetic separation. IEEE Trans. Magn. 1976, MAG-12, 523–527. [Google Scholar] [CrossRef]
- Tang, Y.; Yam, D.; Zheng, J.; Guo, M.; Rong, X.; Ni, Y. Magnetic properties and mechanism of pyrite in coal. Sci. Bull. 1995, 40, 1483–1486. [Google Scholar]
- Zheng, J. Fine Coal Desulphurization Study on Magnetic Susceptibility of Coal and Associated Minerals and Desulfurization of High Gradient Magnetic Separation Coal. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 1993. [Google Scholar]
- Wu, S.; Zhang, H.; Zhang, S. Experimental study on Desulfurization of dry pulverized Coal by Magnetic Separation. Environ. Sci. 1989, 11, 25–28. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, S. Experimental study on desulfurization and deashing of coal-fired high gradient magnetic separation. Coal Sci. Technol. 2005, 33, 61–68. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y. Experimental study on Desulfurization by High gradient Magnetic Separation. Shandong Electr. Power Technol. 2004, 3, 7–11. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, Y.; Zhou, C.; Duan, C. Fine Coal Desulfurization by Magnetic Separation and the Behavior of Sulfur Component Response in Microwave Energy Pretreatment. Energy Fuels 2015, 29, 1243–1248. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, X.; Zhao, Y.; Cai, L. Desulfurization of microwave pretreated fine coal by magnetic separation. Part. Sci. Technol. 2018, 36, 600–608. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, G.; Sun, Z.; Yan, G.; Yao, H. Fine coal desulfurization and modeling based on high-gradient magnetic separation by microwave energy. Fuel 2018, 217, 434–443. [Google Scholar] [CrossRef]
- Yan, Y.; Deng, S.; Zhou, W.; Lin, M. Research and application of magnetic separation technology for coal desulfurization. Environ. Prot. Coal Mines 1999, 13, 17. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Romas, J.K.; Josephson, L. Magnetic separation in biotechnology. Trends Biotechnol. 1983, 11, l44–148. [Google Scholar] [CrossRef]
- Sun, Z. Development of permanent magnets and some magnetic separators. Metal Mine 2006, 8, 52–59. [Google Scholar]
- Li, X.; Xu, X.; Zhou, Y.; Wang, M. The role of CRMM High gradient Magnetic Separator in Kaolin Refining. Miner. Prot. Util. 2005, 6, 25–27. [Google Scholar] [CrossRef]
- Tang, Y. Development and Application of Wet Vertical Ring High Gradient Magnetic Separator. In Proceedings of the Fourth National Academic Conference of Mineral Processing Equipment, Kunming, China, 1 September 2001. [Google Scholar]
- Tang, Y. Development of SSS-II wet double frequency double vertical ring high gradient magnetic separator. Metal Mines 2004, 333, 37–39. [Google Scholar] [CrossRef]
- Xiong, D.; Liu, J. New Progress of Slon pulsation and Vibration High gradient Magnetic Separator. Metal Mine 2006, 361, 4–7. [Google Scholar] [CrossRef]
- Yi, D. Application of Vertical Ring pulsating High gradient Magnetic Separator in Meishan Iron Mine. Des. Constr. Metall. Mines 1999, 2, 35–39. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J. Development of a new Slon- 1000 dry vibration high gradient magnetic separator. Nonmet. Ores 2006, 29, 32–34. [Google Scholar] [CrossRef]
- Luo, Z. Summary of Papers of the Fifteenth International Concentration Conference (Gravity and Magnetic Separation). Yunnan Metallurgy 1987, 4, 20–24, 32. [Google Scholar]
- Wu, S.; Wu, Z.; Shen, H.; Wei, J.; Zhao, G.; Wu, F.; Yu, H. Current Situation and Development Direction of Magnetic Separation Equipment in China. In Proceedings of the National New Beneficiation Technology and Its Development Direction of Academic Research and Technology Exchange, Hainan, China, 1 October 2004. [Google Scholar]
- Feng, D.; Sun, Z. Application of NdFeB Permanent Magnet in Mineral Processing. Magn. Mater. Devices 1994, 25, 51–55. [Google Scholar]
- Feng, D.; Sun, Z. The present situation and Progress of permanent Magnet Separator. Ore Dress. Foreign Metals 1992, 12, 1–5. [Google Scholar]
- Li, X.; Zhou, Y.; Cao, C. Development and Application of CRIMM double-box reciprocating permanent Magnet High gradient Magnetic Separator. Metal Mine 2008, 1, 47–48. [Google Scholar] [CrossRef]
- Cheng, B. Identification of two subjects in Baotou rare Earth Research Institute. Rare Earth 2006, 2, 56. [Google Scholar] [CrossRef]
- Harris, P. Pulling power of Eriez Magnetics. Ind. Miner. 2002, 416, 60–62. [Google Scholar]
- Sun, X. Review of superconducting magnetic separation. In Proceedings of the 9th Conference National Annual Review of Mineral Collected Non-Ferrous Metals, Xiamen, Fujian, 1 November 2001. [Google Scholar]
- Kai, Y. The High Gradient Magnetic Separation Desulfurization Experiment Study of Pulverized Coal Combustion Based on Enhanced Acid. Master’s Thesis, Henan University of Technology, Zhengzhou, China, 2017. [Google Scholar]

| Method Name | Fundamental | Removal of Pyrite/% | Removal of Organic Sulfur/% | |
|---|---|---|---|---|
| Physical desulphurization method | Gravity method | Coal and pyrite have density difference | 40–70 | 0 |
| Flotation method | Coal is hydrophobic, pyrite is hydrophilic | 53 | 0 | |
| Magnetic separation method | Pyrite has weak magnetism | 60–80 | 0 | |
| Friction electrical separation method | Coal and pyrite are electrically different | 50–80 | 0 | |
| Chemical desulfurization method | BHC (benzenehexachloride) method | Alkali aqueous solution method | 50–84 | 0 |
| Microwave method | Microwave energy | 10–40 | 10–30 | |
| High energy radiation method | Radiation forming free radical and oxidation | 30–80 | 10–70 | |
| Chlorine decomposition method | Cl2 decomposition | >90 | 70 | |
| Biochemical method | Biological oxidation–reduction | 60–90 | 50–60 |
| Component | Specific Magnetic Susceptibility ×10−9/kg·m−3 |
|---|---|
| Pyrite | 3.4 |
| Pyrrhotite | Strong magnetism |
| Limonite | 700 |
| Siderite | 4000 |
| Iron silicate | 1260 |
| Calcite | 12.0 |
| Clay | 250–490 |
| Shale | 490–570 |
| Sandstone | 180–250 |
| Coal | −5.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Cao, M.; Ma, C. Review of Coal-Fired Electrification and Magnetic Separation Desulfurization Technology. Appl. Sci. 2019, 9, 1158. https://doi.org/10.3390/app9061158
Chen Y, Cao M, Ma C. Review of Coal-Fired Electrification and Magnetic Separation Desulfurization Technology. Applied Sciences. 2019; 9(6):1158. https://doi.org/10.3390/app9061158
Chicago/Turabian StyleChen, Yan, Min Cao, and Chunyan Ma. 2019. "Review of Coal-Fired Electrification and Magnetic Separation Desulfurization Technology" Applied Sciences 9, no. 6: 1158. https://doi.org/10.3390/app9061158




