Electrochemical Removal of Chromium (VI) from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Kinetics Model
3. Results and Discussions
3.1. Electro-Reduction of Chromium (VI)
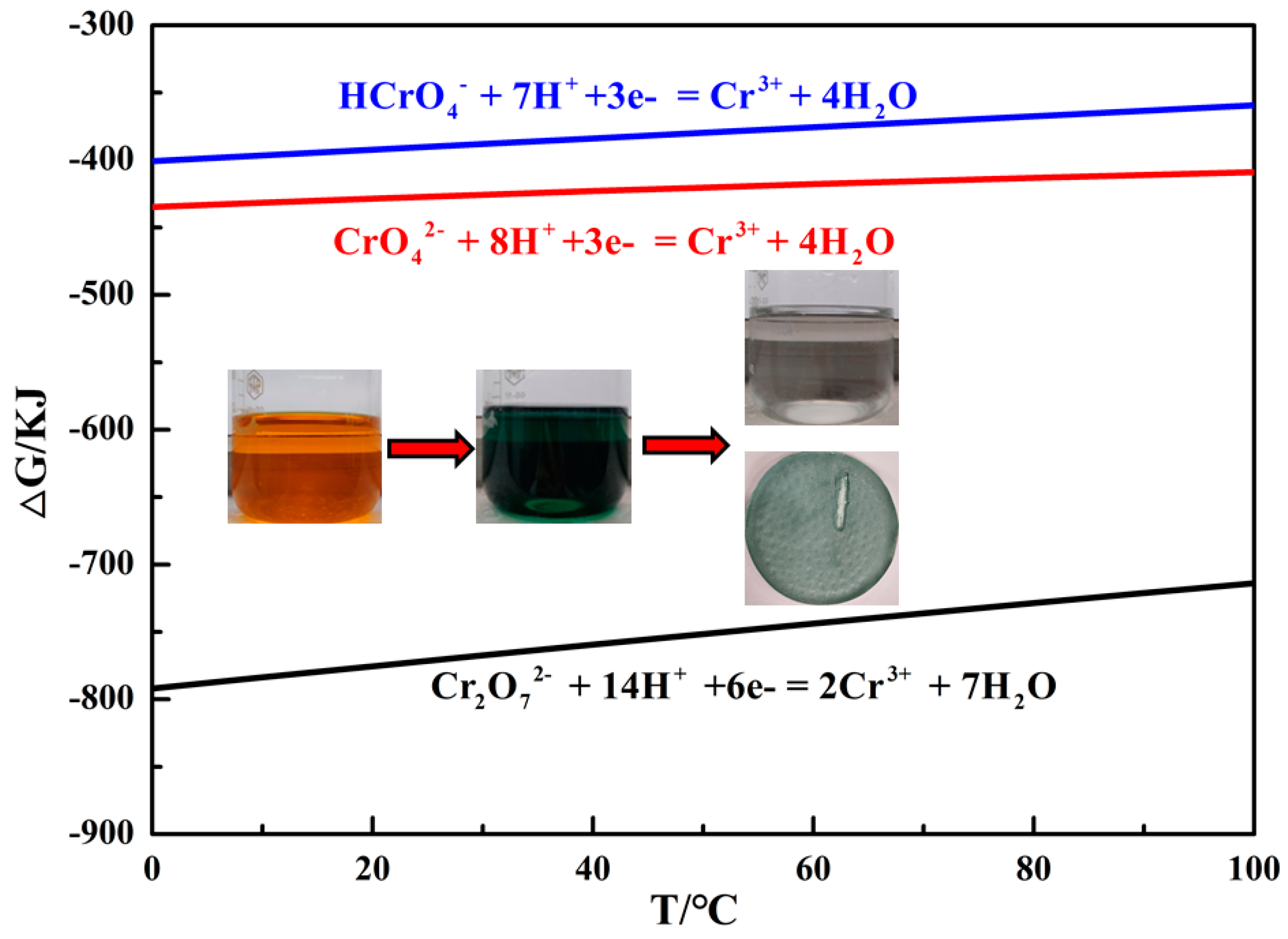
3.1.1. Reaction Mechanism
3.1.2. Effect of Concentration of H2SO4
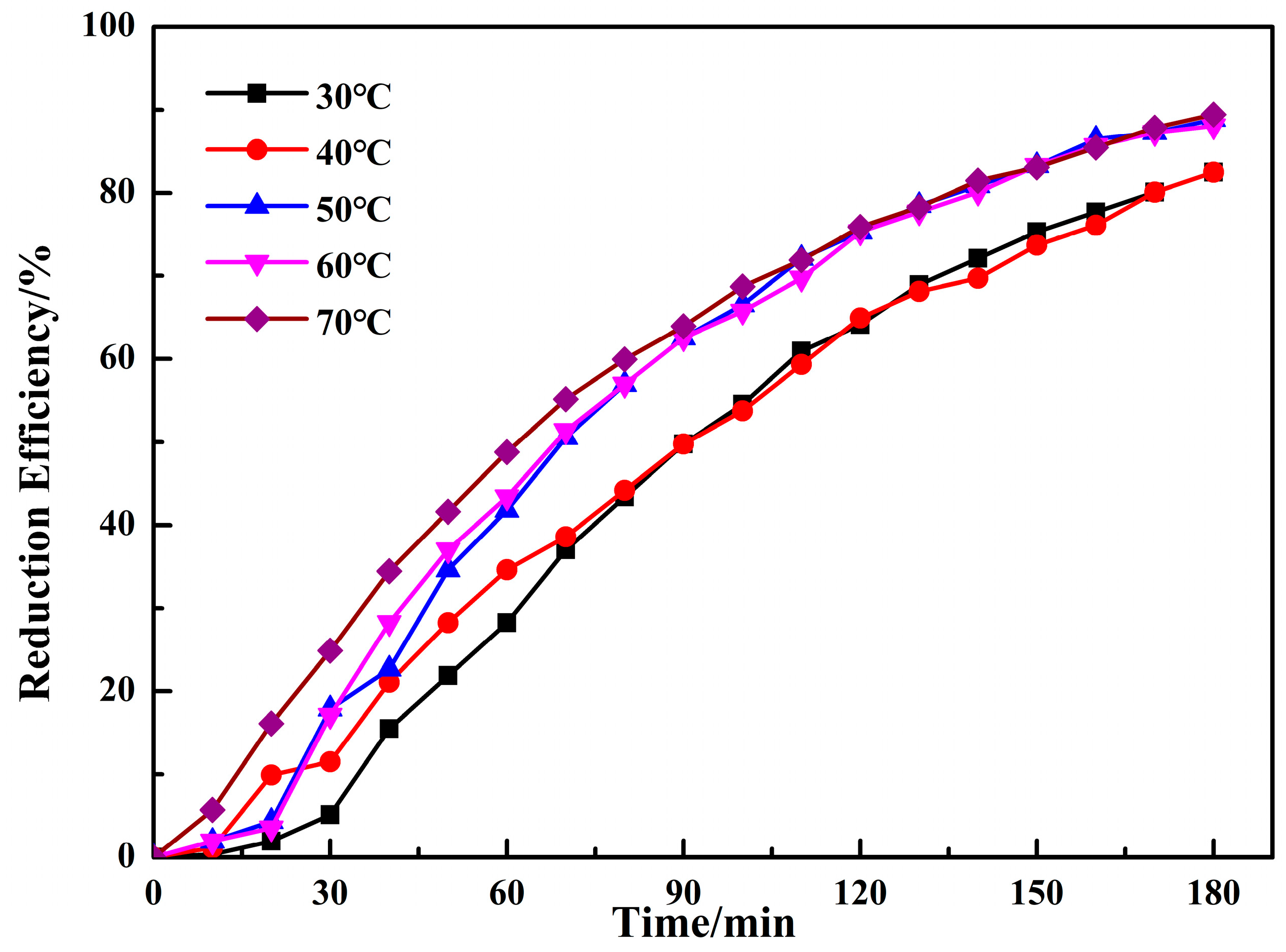
3.1.3. Effect of Reaction Temperature
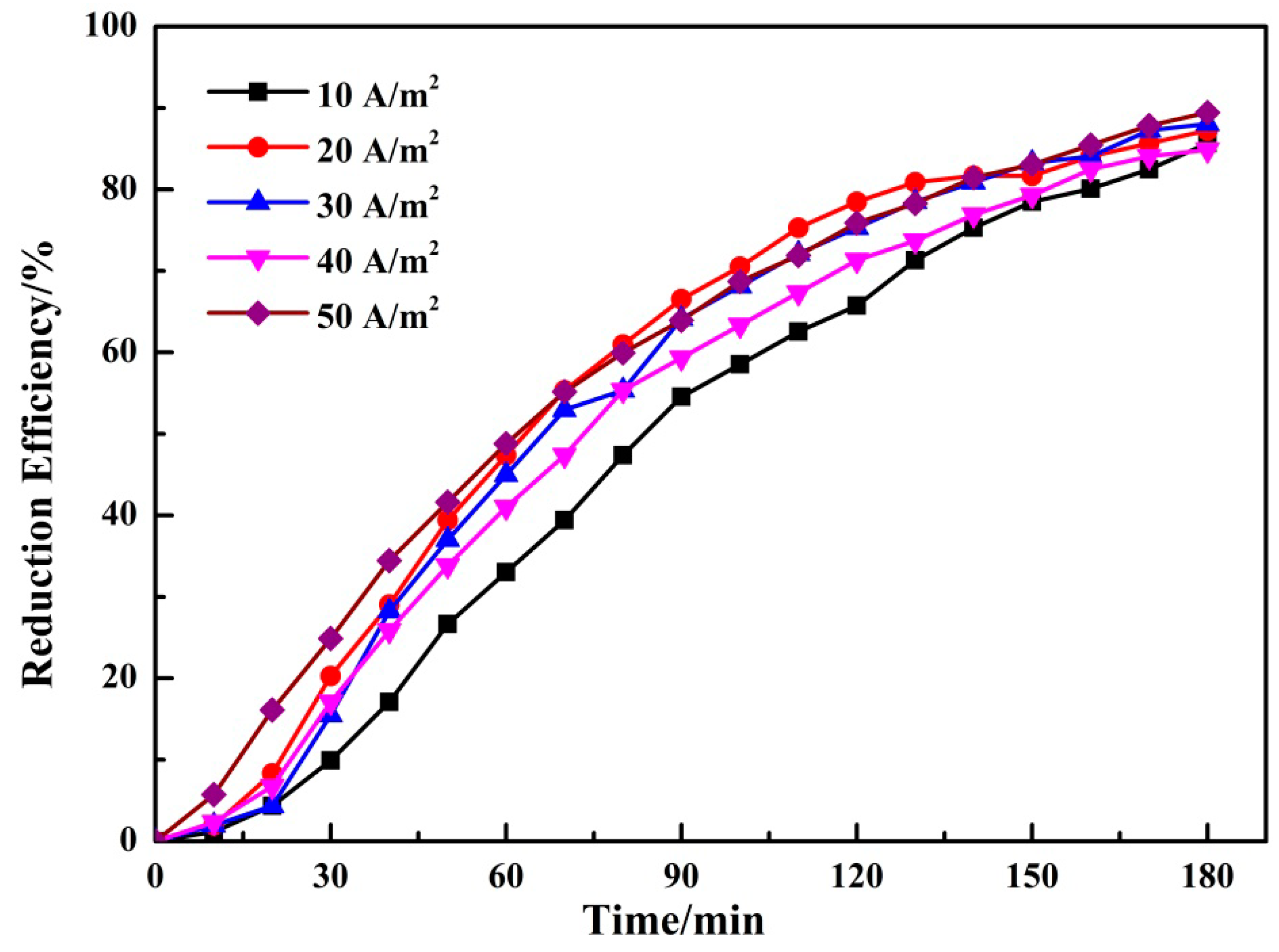
3.1.4. Effect of Current Intensity
3.2. Kinetic Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zou, H.; Hu, E.; Yang, S.; Gong, L.; He, F. Chromium(VI) removal by mechanochemically sulfidated zero valent iron and its effect on dechlorination of trichloroethene as a co-contaminant. Sci. Total Environ. 2019, 650, 419–426. [Google Scholar] [CrossRef]
- Wang, P.; Yin, N.; Cai, X.; Du, H.; Li, Z.; Sun, G.; Cui, Y. Variability of chromium bioaccessibility and speciation in vegetables: The influence of in vitro methods, gut microbiota and vegetable species. Food Chem. 2019, 277, 347–352. [Google Scholar] [CrossRef]
- Su, M.; Fang, Y.; Li, B.; Yin, W.; Gu, J.; Liang, H.; Li, P.; Wu, J. Enhanced hexavalent chromium removal by activated carbon modified with micro-sized goethite using a facile impregnation method. Sci. Total Environ. 2019, 647, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.-Y.; Tseng, Y.-H.; Tsang, D.C.W.; Hu, A.; Yu, C.-P. Wetland plant microbial fuel cells for remediation of hexavalent chromium contaminated soils and electricity production. J. Hazard. Mater. 2019, 365, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, X.; Han, R.; Zang, S.; Yang, G. Cr(VI) removal via anion exchange on a silver-triazolate MOF. J. Hazard. Mater. 2017, 321, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, A.; Hubicki, Z.; Podkoscielny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Jin, W.; Du, H.; Yan, K.; Zheng, S.; Zhang, Y. Improved electrochemical Cr(VI) detoxification by integrating the direct and indirect pathways. J. Electroanal. Chem. 2016, 775, 325–328. [Google Scholar] [CrossRef]
- Peng, H.; Leng, Y.; Cheng, Q.; Shang, Q.; Shu, J.; Guo, J. Efficient Removal of Hexavalent chromium from Wastewater with Electro-reduction. Processes 2019, 7, 41. [Google Scholar] [CrossRef]
- Golder, A.K.; Chanda, A.K.; Samanta, A.N.; Ray, S. Removal of hexavalent chromium by electrochemical reduction–precipitation: Investigation of process performance and reaction stoichiometry. Sep. Purif. Technol. 2011, 76, 345–350. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Li, B.; Liu, Z.; Tao, C. High-efficient recovery of chromium (VI) with lead sulfate. J. Taiwan Inst. Chem. Eng. 2018, 85, 149–154. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.-R.; Liu, Y.-L.; Tang, Q. Removal of Cr ions from aqueous solution using batch electrocoagulation: Cr removal mechanism and utilization rate of in situ generated metal ions. Process Saf. Environ. Prot. 2016, 104, 436–443. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H.; Zhang, G.; Meng, F.; Li, L.; Wu, S. Removal of hexavalent chromium from aqueous solution by different surface-modified biochars: Acid washing, nanoscale zero-valent iron and ferric iron loading. Bioresour. Technol. 2018, 261, 142–150. [Google Scholar] [CrossRef]
- Yin, W.; Li, Y.; Wu, J.; Chen, G.; Jiang, G.; Li, P.; Gu, J.; Liang, H.; Liu, C. Enhanced Cr(VI) removal from groundwater by Fe0-H2O system with bio-amended iron corrosion. J. Hazard. Mater. 2017, 332, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Marques Neto, J.D.O.; Bellato, C.R.; Silva, D.D.C. Iron oxide/carbon nanotubes/chitosan magnetic composite film for chromium species removal. Chemosphere 2019, 218, 391–401. [Google Scholar] [CrossRef]
- Tangtubtim, S.; Saikrasun, S. Adsorption behavior of polyethyleneimine-carbamate linked pineapple leaf fiber for Cr(VI) removal. Appl. Surf. Sci. 2019, 467–468, 596–607. [Google Scholar] [CrossRef]
- Adio, S.O.; Asif, M.; Mohammed, A.-R.I.; Baig, N.; Al-Arfaj, A.A.; Saleh, T.A. Poly (amidoxime) modified magnetic activated carbon for chromium and thallium adsorption: Statistical analysis and regeneration. Process Saf. Environ. Prot. 2019, 121, 254–262. [Google Scholar] [CrossRef]
- Campos, A.F.C.; de Oliveira, H.A.L.; da Silva, F.N.; da Silva, F.G.; Coppola, P.; Aquino, R.; Mezzi, A.; Depeyrot, J. Core-Shell Bimagnetic Nanoadsorbents for Hexavalent Chromium Removal from Aqueous Solutions. J. Hazard. Mater. 2019, 362, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Nhat-Thien, N.; Lee, S.-Y.; Chen, S.-S.; Nguyen-Cong, N.; Chang, C.-T.; Hsiao, S.-S.; le Thuy, T.; Kao, C.-Y.; Lin, M.-F.; Wang, L. Preparation of Zn-Doped Biochar from Sewage Sludge for Chromium Ion Removal. J. Nanosci. Nanotechnol. 2018, 18, 5520–5527. [Google Scholar]
- Yufen, W.; Zhanqiang, F.; Liuchun, Z.; Pokeung, T.E. Biosynthesized iron nanoparticles in aqueous extracts of Eichhornia crassipes and its mechanism in the hexavalent chromium removal. Appl. Surf. Sci. 2017, 399, 322–329. [Google Scholar]
- Liu, T.; Zhao, L.; Sun, D.; Tan, X. Entrapment of nanoscale zerovalent iron in chitosan beads for hexavalent chromium removal from wastewater. J. Hazard. Mater. 2010, 184, 727–730. [Google Scholar] [CrossRef]
- Shu, J.; Liu, R.; Liu, Z.; Chen, H.; Tao, C. Leaching of manganese from electrolytic manganese residue by electro-reduction. Environ. Technol. 2017, 38, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Guo, J.; Liu, Z.; Tao, C. Direct advanced oxidation process for chromium(III) with sulfate free radicals. Sn Appl. Sci. 2019, 1, 14. [Google Scholar] [CrossRef]
- Okello, V.A.; Mwilu, S.; Noah, N.; Zhou, A.; Chong, J.; Knipfing, M.T.; Doetschman, D.; Sadik, O.A. Reduction of hexavalent chromium using naturally-derived flavonoids. Environ. Sci. Technol. 2014, 46, 10743–10751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, W.; Yin, Y.; Chen, Z.; Qiu, R.; Simonnot, M.-O.; Wang, X. Adsorption-reduction removal of Cr(VI) by tobacco petiole pyrolytic biochar: Batch experiment, kinetic and mechanism studies. Bioresour. Technol. 2018, 268, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Han, J.; Mu, Y.; Yu, H.; Qin, L. Two-stage chromium isotope fractionation during microbial Cr(VI) reduction. Water Res. 2019, 148, 10–18. [Google Scholar] [CrossRef]
- Garcia-Seguraa, S.; Eiband, M.M.S.G.; Melo, J.V.; AlbertoMartínez-Huitle, C. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef] [PubMed]
- Xiancai, F.; Wenxia, S.; Tianyang, Y.; Wenhua, H. Physical Chemistry; Higher Education Press: Beijing, China, 2005. [Google Scholar]
- Peng, H.; Liu, Z.; Tao, C. Adsorption Process of Vanadium (V) with Melamine. Water Air Soil Pollut. 2017, 228, 272. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J.; Zheng, X.; Liu, Z.; Tao, C. Leaching kinetics of vanadium from calcification roasting converter vanadium slag in acidic medium. J. Environ. Chem. Eng. 2018, 6, 5119–5124. [Google Scholar] [CrossRef]


| Kobs | R2 | |
|---|---|---|
| [H2SO4] | ||
| 100 g/L | 0.01276 | 0.9985 |
| 80 g/L | 0.01077 | 0.9993 |
| 60 g/L | 0.00712 | 0.9978 |
| 40 g/L | 0.00712 | 0.9849 |
| 20 g/L | 0.00608 | 0.9964 |
| Current density | ||
| 50 A/m2 | 0.01276 | 0.9985 |
| 40 A/m2 | 0.01163 | 0.9987 |
| 30 A/m2 | 0.01312 | 0.9975 |
| 20 A/m2 | 0.01249 | 0.9836 |
| 10 A/m2 | 0.01191 | 0.9946 |
| Reaction temperature | ||
| 70 °C | 0.01276 | 0.9985 |
| 60 °C | 0.01242 | 0.9980 |
| 50 °C | 0.01238 | 0.9983 |
| 40 °C | 0.01115 | 0.9967 |
| 30 °C | 0.01027 | 0.0017 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Leng, Y.; Guo, J. Electrochemical Removal of Chromium (VI) from Wastewater. Appl. Sci. 2019, 9, 1156. https://doi.org/10.3390/app9061156
Peng H, Leng Y, Guo J. Electrochemical Removal of Chromium (VI) from Wastewater. Applied Sciences. 2019; 9(6):1156. https://doi.org/10.3390/app9061156
Chicago/Turabian StylePeng, Hao, Yumeng Leng, and Jing Guo. 2019. "Electrochemical Removal of Chromium (VI) from Wastewater" Applied Sciences 9, no. 6: 1156. https://doi.org/10.3390/app9061156
APA StylePeng, H., Leng, Y., & Guo, J. (2019). Electrochemical Removal of Chromium (VI) from Wastewater. Applied Sciences, 9(6), 1156. https://doi.org/10.3390/app9061156






