Chemosensory Event-Related Potentials and Power Spectrum Could Be a Possible Biomarker in 3M Syndrome Infants?
Abstract
1. Introduction
1.1. CUL7 Mutation and Potential Olfactory Involvement
1.2. Olfactory Perception and Chemosensory Event-Related Potentials (CSERPs) in Infants
2. Materials and Methods
2.1. Subjects
2.2. OERP Assessment
2.3. EEG Recording
2.4. Data Processing
2.4.1. OERP Pre-Processing
2.4.2. EEG Signal Pre-Processing
3. Results
3.1. OERP Data Analysis
3.2. EEG Spectral Analysis
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marik, I.; Marikova, O.; Kuklik, M.; Zemkova, D.; Kozlowski, K. 3-M syndrome in two sisters. J. Paediatr. Child. Health 2002, 38, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Holder-Espinasse, M. 3-M Syndrome. In GeneReviews((R)); University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Hanson, D.; Stevens, A.; Murray, P.G.; Black, G.C.M.; Clayton, P.E. Identifying biological pathways that underlie primordial short stature using network analysis. J. Mol. Endocrinol. 2014, 52, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.G.; Hanson, D.; Coulson, T.; Stevens, A.; Whatmore, A.; Poole, R.L.; Mackay, D.J.; Black, G.C.M.; Clayton, P.E. 3-M syndrome: A growth disorder associated with IGF2 silencing. Endocr. Connect. 2013, 2, 225–235. [Google Scholar] [CrossRef]
- Clayton, P.E.; Hanson, D.; Magee, L.; Murray, P.G.; Saunders, E.; Abu-Amero, S.N.; Moore, G.E.; Black, G.C.M. Exploring the spectrum of 3-M syndrome, a primordial short stature disorder of disrupted ubiquitination. Clin. Endocrinol. (Oxf). 2012, 77, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Hanson, D.; Murray, P.G.; Coulson, T.; Sud, A.; Omokanye, A.; Stratta, E.; Sakhinia, F.; Bonshek, C.; Wilson, L.C.; Wakeling, E.; et al. Mutations in CUL7, OBSL1 and CCDC8 in 3-M syndrome lead to disordered growth factor signalling. J. Mol. Endocrinol. 2012, 49, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Sarikas, A.; Xu, X.; Field, L.J.; Pan, Z.Q. The cullin7 E3 ubiquitin ligase: A novel player in growth control. Cell Cycle 2008, 7, 3154–3161. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, F.; Toth, C. Insulin and the brain. Curr. Diabetes Rev. 2013, 9, 102–116. [Google Scholar]
- Lacroix, M.C.; Badonnel, K.; Meunier, N.; Tan, F.; Le Poupon, C.S.; Durieux, D.; Monnerie, R.; Baly, C.; Congar, P.; Salesse, R.; et al. Expression of insulin system in the olfactory epithelium: First approaches to its role and regulation. J. Neuroendocrinol. 2008, 20, 1176–1190. [Google Scholar] [CrossRef]
- Litterman, N.; Ikeuchi, Y.; Gallardo, G.; O’Connell, B.C.; Sowa, M.E.; Gygi, S.P.; Harper, J.W.; Bonni, A. An OBSL1-CUl7 Fbxw8 ubiquitin ligase signaling mechanism regulates golgi morphology and dendrite patterning. PLoS Biol. 2011, 9, 17–19. [Google Scholar] [CrossRef]
- Varendi, H.; Porter, R.H. Breast odour as the only maternal stimulus elicits crawling towards the odour source. Acta Paediatr. 2001, 90, 372–375. [Google Scholar] [CrossRef]
- Macfarlane, A. Olfaction in the Development of Social Preferences in the Human Neonate. Ciba Found. Symp. 1975, 33, 103–117. [Google Scholar] [CrossRef]
- Delaunay-El Allam, M.; Soussignan, R.; Patris, B.; Marlier, L.; Schaal, B. Long-lasting memory for an odor acquired at the mother’s breast. Dev. Sci. 2010, 13, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Astruc, D.; Messer, J.; Marlier, L. Exploring the olfactory environment of premature newborns: A French survey of health care and cleaning products used in neonatal units. Acta Paediatr. Int. J. Paediatr. 2011, 100, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Gauthaman, G.; Jayachandran, L.; Prabhakar, K. Olfactory reflexes in newborn infants. Indian J. Pediatr. 1984, 51, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Schriever, V.A.; Góis-Eanes, M.; Schuster, B.; Huart, C.; Hummel, T. Olfactory event-related potentials in infants. J. Pediatr. 2014, 165, 372–375.e2. [Google Scholar] [CrossRef]
- Kobal, G.; Hummel, T. Olfactory (chemosensory) event-related potentials. Tox. Ind. Heal. 1994, 10, 587–596. [Google Scholar]
- Invitto, S.; Grasso, A. Chemosensory Perception: A Review on Electrophysiological Methods in “Cognitive Neuro-Olfactometry”. Chemosensors 2019, 7, 45. [Google Scholar] [CrossRef]
- Pause, B.M.; Sojka, B.; Krauel, K.; Ferstl, R. The nature of the late positive complex within the olfactory event- related potential (OERP). Psychophysiology 1996, 33, 376–384. [Google Scholar] [CrossRef]
- Pause, B.M.; Krauel, K. Chemosensory event-related potentials (CSERP) as a key to the psychology of odors. Int. J. Psychophysiol. 2000, 36, 105–122. [Google Scholar] [CrossRef]
- Brodal, A. The hippocampus and the sense of smell: A review. Brain 1947, 70, 179–222. [Google Scholar] [CrossRef]
- Mormann, F. Independent delta/theta rhythms in the human hippocampus and entorhinal cortex. Front. Hum. Neurosci. 2008, 2, 3. [Google Scholar] [CrossRef][Green Version]
- Nunes, M.L.; Khan, R.L.; Gomes Filho, I.; Booij, L.; da Costa, J.C. Maturational changes of neonatal electroencephalogram: A comparison between intra uterine and extra uterine development. Clin. Neurophysiol. 2014, 125, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Invitto, S.; Capone, S.; Montagna, G.; Siciliano, P.A. MI2014A001344 Method and System for Measuring Physiological Parameters of a Subject Undergoing an Olfactory Stimulation. U.S. Patent No. 20170127971A1, 23 July 2014. [Google Scholar]
- Soussignan, R.; Schaal, B.; Marlier, L. Olfactory alliesthesia in human neonates: Prandial state and stimulus familiarity modulate facial and autonomic responses to milk odors. Dev. Psychobiol. 1999, 35, 3–14. [Google Scholar] [CrossRef]
- Soussignan, R.; Schaal, B.; Marlier, L.; Jiang, T. Facial and autonomic responses to biological and artificial olfactory stimuli in human neonates: Re-examining early hedonic discrimination of odors. Physiol. Behav. 1997, 62, 745–758. [Google Scholar] [CrossRef]
- Sirous, M.; Sinning, N.; Schneider, T.R.; Friese, U.; Lorenz, J.; Engel, A.K. Chemosensory Event-Related Potentials in Response to Nasal Propylene Glycol Stimulation. Front. Hum. Neurosci. 2019, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Stuck, B.A.; Moutsis, T.T.; Bingel, U.; Sommer, J.U. Chemosensory stimulation during sleep—Arousal responses to gustatory stimulation. Neuroscience 2016, 322, 326–332. [Google Scholar] [CrossRef]
- Heiser, C.; Baja, J.; Lenz, F.; Sommer, J.U.; Hörmann, K.; Herr, R.M.; Stuck, B.A. Effects of an artificial smoke on arousals during human sleep. Chemosens. Percept. 2012, 5, 274–279. [Google Scholar] [CrossRef]
- Badre, G.; Wloszczynski, M.; Croy, I. Neural activation of putative sleep-wake affecting and relaxing promoting odors. Sleep Med. 2019, 64, S19. [Google Scholar] [CrossRef]
- Luck, S.J. An Introduction to Event-Related Potentials and Their Neural Origins. An. Introd. to event-related potential Tech. 2005, 78, 388. [Google Scholar]
- Pause, B.M.; Sojka, B.; Ferstl, R. Central processing of odor concentration is a temporal phenomenon as revealed by chemosensory event-related potentials (CSERP). Chem. Senses 1997, 22, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Pause, B.M.; Sojka, B.; Krauel, K.; Fehm-Wolfsdorf, G.; Ferstl, R. Olfactory information processing during the course of the menstrual cycle. Biol. Psychol. 1996, 44, 31–54. [Google Scholar] [CrossRef]
- Invitto, S.; Piraino, G.; Mignozzi, A.; Capone, S.; Montagna, G.; Siciliano, P.A.; Mazzatenta, A.; Rocco, G.; De Feudis, I.; Trotta, G.F.; et al. Smell and meaning: An OERP study. Smart Innov. Syst. Technol. 2017, 69, 289–300. [Google Scholar]
- Goelman, G.; Dan, R. Multiple-region directed functional connectivity based on phase delays. Hum. Brain Mapp. 2017, 38, 1374–1386. [Google Scholar] [CrossRef]
- Mognon, A.; Jovicich, J.; Bruzzone, L.; Buiatti, M. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology 2011, 48, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Radüntz, T.; Scouten, J.; Hochmuth, O.; Meffert, B. EEG artifact elimination by extraction of ICA-component features using image processing algorithms. J. Neurosci. Methods 2015, 243, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Placidi, G.; Avola, D.; Petracca, A.; Sgallari, F.; Spezialetti, M. Basis for the implementation of an EEG-based single-trial binary brain computer interface through the disgust produced by remembering unpleasant odors. Neurocomputing 2015, 160, 308–318. [Google Scholar] [CrossRef]
- James, G.; Key, B.; Beverdam, A. The E3 ubiquitin ligase Mycbp2 genetically interacts with Robo2 to modulate axon guidance in the mouse olfactory system. Brain Struct. Funct. 2014, 219, 861–874. [Google Scholar] [CrossRef]
- Lötsch, J.; Hummel, T. The clinical significance of electrophysiological measures of olfactory function. Behav. Brain Res. 2006, 170, 78–83. [Google Scholar] [CrossRef]
- Koessler, L.; Maillard, L.; Benhadid, A.; Vignal, J.P.; Felblinger, J.; Vespignani, H.; Braun, M. Automated cortical projection of EEG sensors: Anatomical correlation via the international 10-10 system. Neuroimage 2009, 46, 64–72. [Google Scholar] [CrossRef]
- Dade, L.A.; Zatorre, R.J.; Evans, A.C.; Jones-Gotman, M. Working memory in another dimension: Functional imaging of human olfactory working memory. Neuroimage 2001, 14, 650–660. [Google Scholar] [CrossRef]
- Han, P.; Schriever, V.A.; Peters, P.; Olze, H.; Uecker, F.C.; Hummel, T. Influence of airflow rate and stimulus concentration on olfactory event-related potentials (OERP) in humans. Chem. Senses 2018, 43, 89–96. [Google Scholar] [CrossRef]
- Piarulli, A.; Zaccaro, A.; Laurino, M.; Menicucci, D.; De Vito, A.; Bruschini, L.; Berrettini, S.; Bergamasco, M.; Laureys, S.; Gemignani, A. Ultra-slow mechanical stimulation of olfactory epithelium modulates consciousness by slowing cerebral rhythms in humans. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Kim, S. Influence of fragrances on human psychophysiological activity: With special reference to human electroencephalographic response. Sci. Pharm. 2016, 84, 724. [Google Scholar] [CrossRef] [PubMed]
- Filiou, R.P.; Lepore, F.; Bryant, B.; Lundström, J.N.; Frasnelli, J. Perception of trigeminal mixtures. Chem. Senses 2015, 40, 61–69. [Google Scholar] [CrossRef] [PubMed]
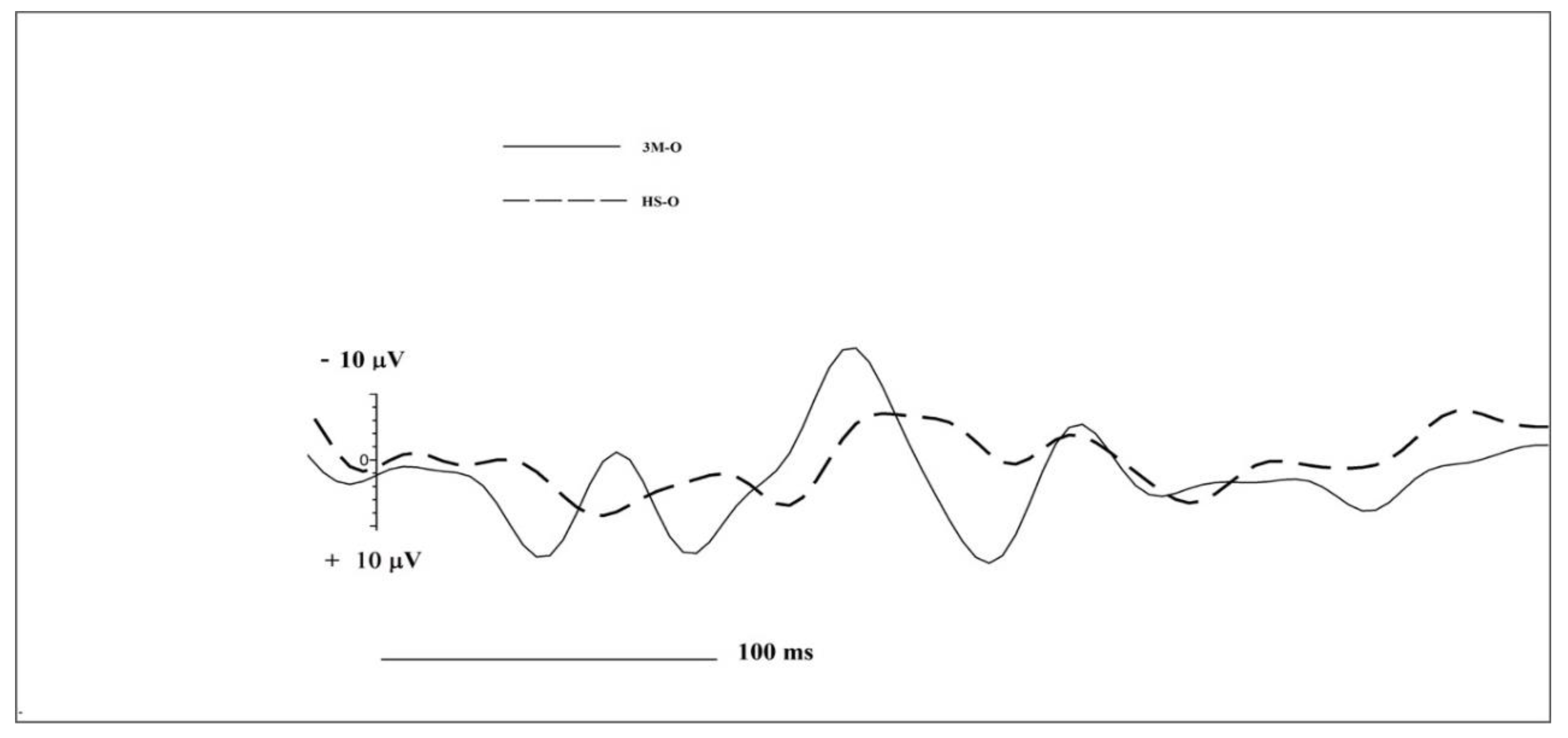
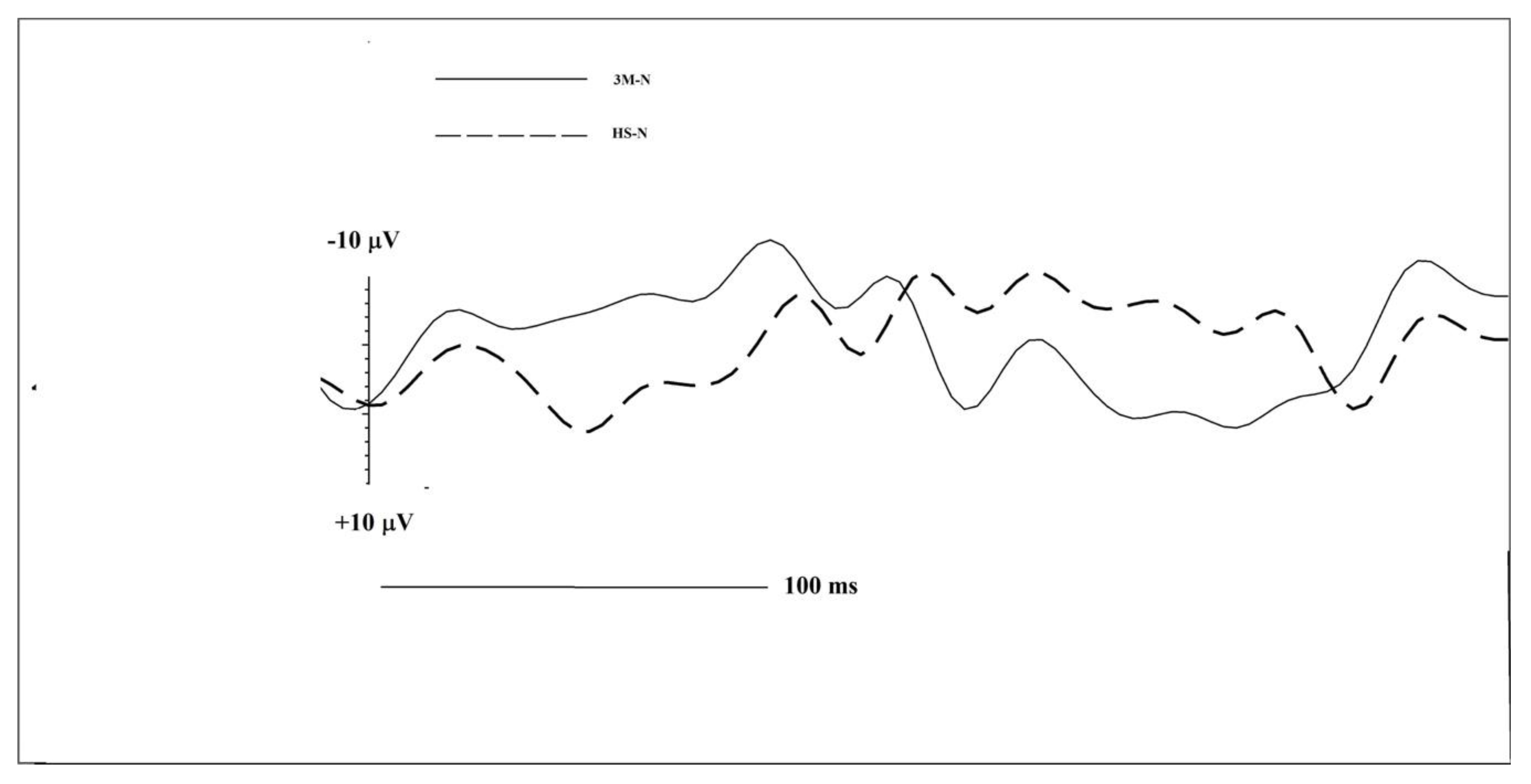
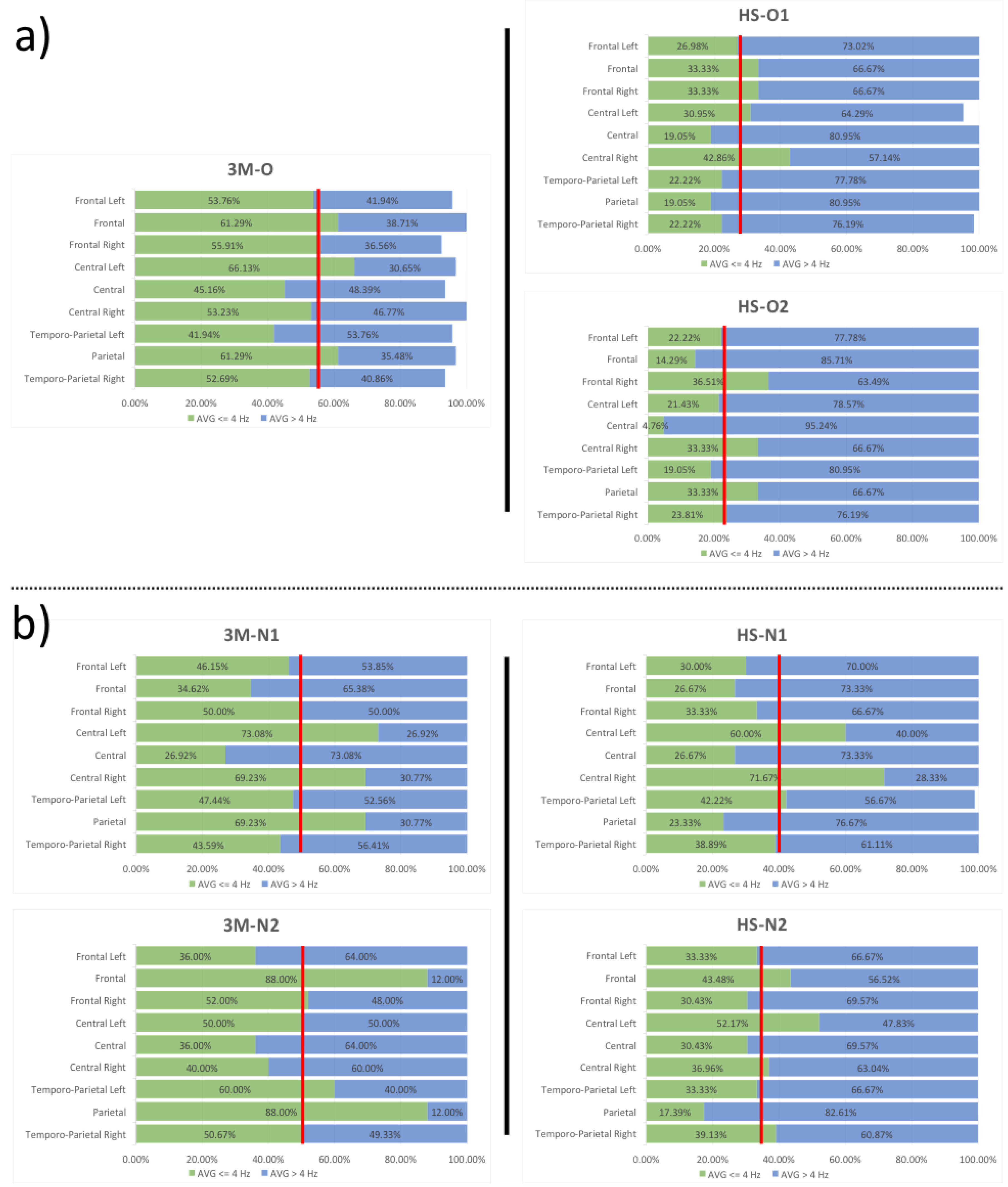

| Group | N1 Amplitude | N1 Latency | LPC Amplitude | LPC Latency | |
|---|---|---|---|---|---|
| Frontal Left | 3M-O | −17.34 | 66 | 33.32 | 191 |
| HS-O | −1.42 | 137 | 10.05 | 328 | |
| Frontal Right | 3M-O | −25.37 | 125 | 13.40 | 230 |
| HS-O | −12.55 | 234 | 3.85 | 188 | |
| Central Left | 3M-O | −11.17 | 141 | 24.02 | 180 |
| HS-O | −4.10 | 160 | 12.9 | 402 | |
| Central Right | 3M-O | −7.97 | 164 | 9.75 | 282 |
| HS-O | −2.70 | 102 | 12.23 | 203 | |
| Temporal Left | 3M-O | −6.80 | 140 | 24.4 | 188 |
| HS-O | −2.20 | 105 | 18.28 | 227 | |
| Temporal Right | 3M-O | −4.52 | 148 | 14.83 | 2,93 |
| HS-O | −14.5 | 113 | 8.83 | 2,54 | |
| Cz | 3M-O | −4.28 | 109 | 1.51 | 328 |
| HS-O | −11.80 | 129 | 1.20 | 262 | |
| Fz | 3M-O | −6.97 | 164 | 2.57 | 254 |
| HS-O | −28.24 | 133 | 9.38 | 191 | |
| Pz | 3M-O | −2.80 | 160 | 4.26 | 246 |
| HS-O | −1.34 | 113 | 22.06 | 156 |
| Group | N1 Amplitude | N1 Latency | LPC Amplitude | LPC Latency | |
|---|---|---|---|---|---|
| Frontal Left | 3M-N | −3.23 | 121 | 6.56 | 267 |
| HS-N | −8.94 | 203 | 4.89 | 297 | |
| Frontal Right | 3M-N | −9.32 | 188 | -- | -- |
| HS-N | −2.38 | 184 | -- | -- | |
| Central Left | 3M-N | −7.68 | 121 | 5.88 | 262 |
| HS-N | −5.76 | 168 | 4.19 | 297 | |
| Central Right | 3M-N | −1.47 | 195 | 1.30 | 215 |
| HS-N | −6.49 | 148 | -- | -- | |
| Temporal Left | 3M-N | −5.85 | 105 | 0.311 | 297 |
| HS-N | −9.26 | 172 | 5.08 | 316 | |
| Temporal Right | 3M-N | −0.988 | 137 | 6.07 | 207 |
| HS-N | −2.15 | 164 | 7.85 | 270 | |
| Cz | 3M-N | −16.02 | 180 | -- | -- |
| HS-N | −11.80 | 129 | 1.20 | 262 | |
| Fz | 3M-N | −8.95 | 109 | 5.78 | 160 |
| HS-N | −6.67 | 223 | 9.58 | 250 | |
| Pz | 3M-N | −6.12 | 0,27 | 14.27 | 164 |
| HS-N | −9.96 | 105 | 5.58 | 188 | |
| ROI | 3M-O/HS-O1 | 3M-O/HS-O2 | 3M-N1/HS-N1 | 3M-N1/HS-N2 | 3M-N2/HS-N1 | 3M-N2/HS-N2 |
|---|---|---|---|---|---|---|
| Temporo-Parietal Right | 57.82% | 54.81% | 10.78% | 10.23% | 23.25% | 22.77% |
| Parietal | 68.92% | 45.61% | 66.30% | 74.88% | 73.48% | 80.24% |
| Temporo-Parietal Left | 47.01% | 54.58% | 10.99% | 29.73% | 29.63% | 44.44% |
| Central Right | 19.48% | 37.37% | −3.52% | 46.62% | −79.17% | 7.61% |
| Central | 57.82% | 89.46% | 0.95% | −13.04% | 25.93% | 15.46% |
| Central Left | 53.19% | 67.60% | 17.89% | 28.60% | −20.00% | −4.35% |
| Frontal Right | 40.38% | 34.71% | 33.33% | 39.13% | 35.90% | 41.47% |
| Frontal | 45.61% | 76.69% | 22.96% | −25.60% | 69.70% | 50.59% |
| Frontal Left | 49.81% | 58.67% | 35.00% | 27.78% | 16.67% | 7.41% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Invitto, S.; Grasso, A.; Lofrumento, D.D.; Ciccarese, V.; Paladini, A.; Paladini, P.; Marulli, R.; De Pascalis, V.; Polsinelli, M.; Placidi, G. Chemosensory Event-Related Potentials and Power Spectrum Could Be a Possible Biomarker in 3M Syndrome Infants? Brain Sci. 2020, 10, 201. https://doi.org/10.3390/brainsci10040201
Invitto S, Grasso A, Lofrumento DD, Ciccarese V, Paladini A, Paladini P, Marulli R, De Pascalis V, Polsinelli M, Placidi G. Chemosensory Event-Related Potentials and Power Spectrum Could Be a Possible Biomarker in 3M Syndrome Infants? Brain Sciences. 2020; 10(4):201. https://doi.org/10.3390/brainsci10040201
Chicago/Turabian StyleInvitto, Sara, Alberto Grasso, Dario Domenico Lofrumento, Vincenzo Ciccarese, Angela Paladini, Pasquale Paladini, Raffaella Marulli, Vilfredo De Pascalis, Matteo Polsinelli, and Giuseppe Placidi. 2020. "Chemosensory Event-Related Potentials and Power Spectrum Could Be a Possible Biomarker in 3M Syndrome Infants?" Brain Sciences 10, no. 4: 201. https://doi.org/10.3390/brainsci10040201
APA StyleInvitto, S., Grasso, A., Lofrumento, D. D., Ciccarese, V., Paladini, A., Paladini, P., Marulli, R., De Pascalis, V., Polsinelli, M., & Placidi, G. (2020). Chemosensory Event-Related Potentials and Power Spectrum Could Be a Possible Biomarker in 3M Syndrome Infants? Brain Sciences, 10(4), 201. https://doi.org/10.3390/brainsci10040201








