Motor Cortical Activity during Observing a Video of Real Hand Movements versus Computer Graphic Hand Movements: An MEG Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
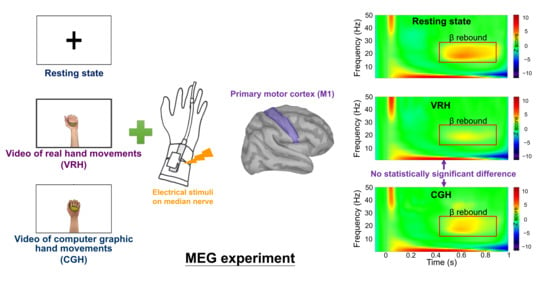
2.2. Experimental Design
2.3. MEG Recordings
2.4. MEG Data Analysis
2.5. Statistical Analysis
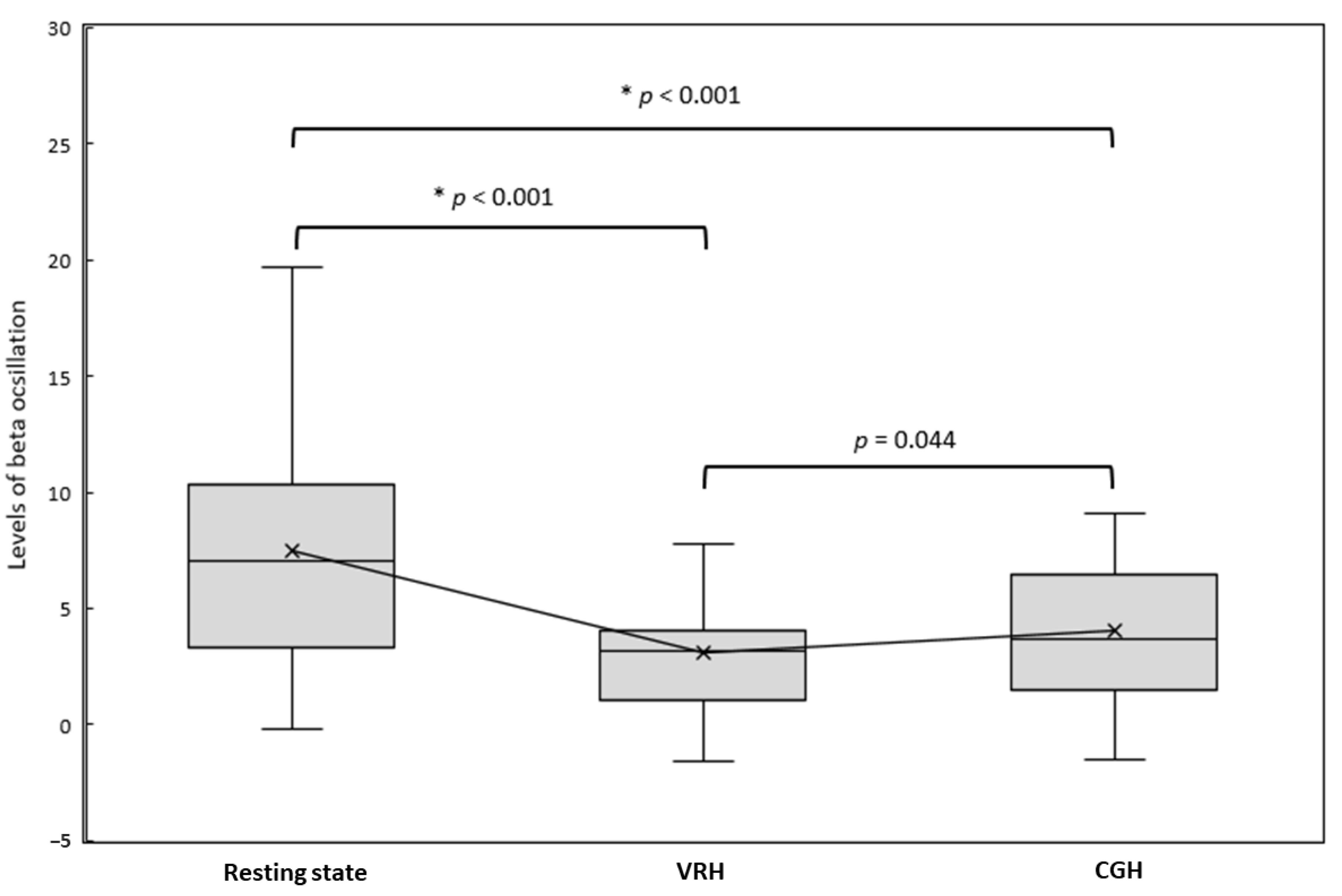
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keysers, C.; Gazzola, V. Social neuroscience: Mirror neurons recorded in humans. Curr. Biol. 2010, 20, R353–R354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sale, P.; Ceravolo, M.G.; Franceschini, M. Action observation therapy in the subacute phase promotes dexterity recovery in right-hemisphere stroke patients. BioMed Res. Int. 2014, 2014, 457538. [Google Scholar] [CrossRef] [PubMed]
- Buccino, G. Action observation treatment: A novel tool in neurorehabilitation. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130185. [Google Scholar] [CrossRef] [PubMed]
- Small, S.L.; Buccino, G.; Solodkin, A. The mirror neuron system and treatment of stroke. Dev. Psychobiol. 2012, 54, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Sale, P.; Franceschini, M. Action observation and mirror neuron network: A tool for motor stroke rehabilitation. Eur. J. Phys. Rehabil. Med. 2012, 48, 313–318. [Google Scholar]
- Liepert, J.; Greiner, J.; Dettmers, C. Motor excitability changes during action observation in stroke patients. J. Rehabil. Med. 2014, 46, 400–405. [Google Scholar] [CrossRef] [Green Version]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Stroke 2018, 49, E160–E161. [Google Scholar] [CrossRef]
- Bagce, H.F.; Saleh, S.; Adamovich, S.V.; Tunik, E. Visuomotor gain distortion alters online motor performance and enhances primary motor cortex excitability in patients with stroke. Neuromodulation 2012, 15, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Saleh, S.; Adamovich, S.V.; Tunik, E. Mirrored feedback in chronic stroke: Recruitment and effective connectivity of ipsilesional sensorimotor networks. Neurorehabil. Neural Repair 2014, 28, 344–354. [Google Scholar] [CrossRef] [Green Version]
- Tunik, E.; Saleh, S.; Adamovich, S.V. Visuomotor discordance during visually-guided hand movement in virtual reality modulates sensorimotor cortical activity in healthy and hemiparetic subjects. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Perani, D.; Fazio, F.; Borghese, N.A.; Tettamanti, M.; Ferrari, S.; Decety, J.; Gilardi, M.C. Different Brain Correlates for Watching Real and Virtual Hand Actions. NeuroImage 2001, 14, 749–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, P.J.; Meltzoff, A.N. Neural mirroring systems: Exploring the EEG μ rhythm in human infancy. Dev. Cogn. Neurosci. 2011, 1, 110–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meltzoff, A.N.; Marshall, P.J. Human infant imitation as a social survival circuit. Curr. Opin. Behav. Sci. 2018, 24, 130–136. [Google Scholar] [CrossRef]
- Meltzoff, A.N.; Ramírez, R.R.; Saby, J.N.; Larson, E.; Taulu, S.; Marshall, P.J. Infant brain responses to felt and observed touch of hands and feet: An MEG study. Dev. Sci. 2018, 21, e12651. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Sun, H.H.; Weng, J.Q.; Tseng, Y.J. Differential motor cortex excitability during observation of normal and abnormal goal-directed movement patterns. Neurosci. Res. 2017, 123, 36–42. [Google Scholar] [CrossRef]
- Zhu, J.D.; Cheng, C.H.; Tseng, Y.J.; Chou, C.C.; Chen, C.C.; Hsieh, Y.W.; Liao, Y.H. Modulation of Motor Cortical Activities by Action Observation and Execution in Patients with Stroke: An MEG Study. Neural Plast. 2019, 2019, 8481371. [Google Scholar] [CrossRef]
- Wilson, T.W.; Slason, E.; Asherin, R.; Kronberg, E.; Reite, M.L.; Teale, P.D.; Rojas, D.C. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 2010, 73, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Hari, R.; Salmelin, R. Human cortical oscillations: A neuromagnetic view through the skull. Trends Neurosci. 1997, 20, 44–49. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Hari, R.; Forss, N.; Avikainen, S.; Kirveskari, E.; Salenius, S.; Rizzolatti, G. Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc. Natl. Acad. Sci. USA 1998, 95, 15061–15065. [Google Scholar] [CrossRef] [Green Version]
- Taulu, S.; Simola, J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys. Med. Biol. 2006, 51, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.X.; Mosher, J.C.; Leahy, R.M. A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Phys. Med. Biol. 1999, 44, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Tseng, Y.J.; Chen, R.S.; Lin, Y.Y. Reduced functional connectivity of somatosensory network in writer’s cramp patients. Brain Behav. 2016, 6, e00433. [Google Scholar] [CrossRef] [PubMed]
- Kilteni, K.; Grau-Sanchez, J.; De Las Heras, M.V.; Rodriguez-Fornells, A.; Slater, M. Decreased Corticospinal Excitability after the Illusion of Missing Part of the Arm. Front. Hum. Neurosci. 2016, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Schutz-Bosbach, S.; Mancini, B.; Aglioti, S.M.; Haggard, P. Self and other in the human motor system. Curr. Biol. 2006, 16, 1830–1834. [Google Scholar] [CrossRef] [Green Version]
- Tsakiris, M.; Carpenter, L.; James, D.; Fotopoulou, A. Hands only illusion: Multisensory integration elicits sense of ownership for body parts but not for non-corporeal objects. Exp. Brain Res. 2010, 204, 343–352. [Google Scholar] [CrossRef]
- Diers, M.; Kamping, S.; Kirsch, P.; Rance, M.; Bekrater-Bodmann, R.; Foell, J.; Trojan, J.; Fuchs, X.; Bach, F.; Maaß, H.; et al. Illusion-related brain activations: A new virtual reality mirror box system for use during functional magnetic resonance imaging. Brain Res. 2015, 1594, 173–182. [Google Scholar] [CrossRef]
- Bekrater-Bodmann, R.; Foell, J.; Diers, M.; Flor, H. The perceptual and neuronal stability of the rubber hand illusion across contexts and over time. Brain Res. 2012, 1452, 130–139. [Google Scholar] [CrossRef]
- Brand, J.; Piccirelli, M.; Hepp-Reymond, M.-C.; Eng, K.; Michels, L. Brain Activation During Visually Guided Finger Movements. Front. Hum. Neurosci. 2020, 14, 309. [Google Scholar] [CrossRef]
- Brinkman, L.; Stolk, A.; Dijkerman, H.C.; de Lange, F.P.; Toni, I. Distinct roles for alpha- and beta-band oscillations during mental simulation of goal-directed actions. J. Neurosci. 2014, 34, 14783–14792. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, Y.-W.; Lee, M.-T.; Lin, Y.-H.; Chuang, L.-L.; Chen, C.-C.; Cheng, C.-H. Motor Cortical Activity during Observing a Video of Real Hand Movements versus Computer Graphic Hand Movements: An MEG Study. Brain Sci. 2021, 11, 6. https://doi.org/10.3390/brainsci11010006
Hsieh Y-W, Lee M-T, Lin Y-H, Chuang L-L, Chen C-C, Cheng C-H. Motor Cortical Activity during Observing a Video of Real Hand Movements versus Computer Graphic Hand Movements: An MEG Study. Brain Sciences. 2021; 11(1):6. https://doi.org/10.3390/brainsci11010006
Chicago/Turabian StyleHsieh, Yu-Wei, Meng-Ta Lee, Yu-Hsuan Lin, Li-Ling Chuang, Chih-Chi Chen, and Chia-Hsiung Cheng. 2021. "Motor Cortical Activity during Observing a Video of Real Hand Movements versus Computer Graphic Hand Movements: An MEG Study" Brain Sciences 11, no. 1: 6. https://doi.org/10.3390/brainsci11010006