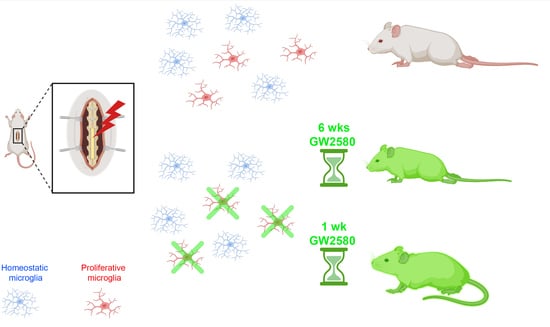
Unlike Brief Inhibition of Microglia Proliferation after Spinal Cord Injury, Long-Term Treatment Does Not Improve Motor Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Approval
2.2. Spinal Cord Injury
2.3. GW2580 Oral Treatment
2.4. Behavior
2.5. Ex Vivo Diffusion MRI (DW-MRI)
2.6. Histology
2.7. Imaging and Quantifications
2.8. Statistics
3. Results
3.1. Long-Term GW2580 Treatment after SCI Does Not Improve Motor Recovery
3.2. Long-Term GW2580 Treatment after SCI Has No Effect on Tissue Reorganization
3.3. IBA1-Microglial Expression Is Decreased 6 Weeks after SCI in the GW2580-Treated Mice
3.4. Astrocytic Reactivity Is Increased 6 Weeks after SCI with a Long-Term GW2580 Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Orr, M.B.; Gensel, J.C. Interactions of primary insult biomechanics and secondary cascades in spinal cord injury: Implications for therapy. Neural Regen. Res. 2017, 12, 1618. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of Secondary Spinal Cord Injury. Front. Cell. Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noristani, H.; Gerber, Y.N.; Sabourin, J.-C.; Le Corre, M.; Lonjon, N.; Mestre-Frances, N.; Hirbec, H.; Perrin, F.E. RNA-Seq Analysis of Microglia Reveals Time-Dependent Activation of Specific Genetic Programs following Spinal Cord Injury. Front. Mol. Neurosci. 2017, 10, 90. [Google Scholar] [CrossRef] [Green Version]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.-E.; Vernoux, N.; Tremblay, M.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ritzel, R.M.; Khan, N.; Cao, T.; He, J.; Lei, Z.; Matyas, J.J.; Sabirzhanov, B.; Liu, S.; Li, H.; et al. Delayed microglial depletion after spinal cord injury reduces chronic inflammation and neurodegeneration in the brain and improves neurological recovery in male mice. Theranostics 2020, 10, 11376–11403. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, Y.; Hu, D.; Wang, S.; Yu, T.; Zhang, L. Depletion of microglia exacerbates injury and impairs function recovery after spinal cord injury in mice. Cell Death Dis. 2020, 11, 528. [Google Scholar] [CrossRef]
- Conway, J.G.; McDonald, B.; Parham, J.; Keith, B.; Rusnak, D.W.; Shaw, E.; Jansen, M.; Lin, P.; Payne, A.; Crosby, R.M.; et al. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc. Natl. Acad. Sci. USA 2005, 102, 16078–16083. [Google Scholar] [CrossRef] [Green Version]
- Gerber, Y.N.; Saint-Martin, G.; Bringuier, C.M.; Bartolami, S.; Goze-Bac, C.; Noristani, H.; Perrin, F.E. CSF1R Inhibition Reduces Microglia Proliferation, Promotes Tissue Preservation and Improves Motor Recovery After Spinal Cord Injury. Front. Cell. Neurosci. 2018, 12, 368. [Google Scholar] [CrossRef] [Green Version]
- Poulen, G.; Aloy, E.; Bringuier, C.M.; Mestre-Francés, N.; Artus, E.V.; Cardoso, M.; Perez, J.-C.; Goze-Bac, C.; Boukhaddaoui, H.; Lonjon, N.; et al. Inhibiting microglia proliferation after spinal cord injury improves recovery in mice and nonhuman primates. Theranostics 2021, 11, 8640–8659. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Aliberti, J.; Graemmel, P.; Sunshine, M.J.; Kreutzberg, G.W.; Sher, A.; Littman, D.R. Analysis of Fractalkine Receptor CX 3 CR1 Function by Targeted Deletion and Green Fluorescent Protein Reporter Gene Insertion. Mol. Cell. Biol. 2000, 20, 4106–4114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noristani, H.N.; Sabourin, J.C.; Boukhaddaoui, H.; Chan-Seng, E.; Gerber, Y.N.; Perrin, F.E. Spinal cord injury induces astroglial conversion towards neuronal lineage. Mol. Neurodegener. 2016, 11, 68. [Google Scholar] [CrossRef] [Green Version]
- Noristani, H.N.; They, L.; Perrin, F.E. C57BL/6 and Swiss Webster Mice Display Differences in Mobility, Gliosis, Microcavity Formation and Lesion Volume After Severe Spinal Cord Injury. Front. Cell. Neurosci. 2018, 12. [Google Scholar] [CrossRef]
- Gerber, Y.N.; Privat, A.; Perrin, F.E. Gacyclidine improves the survival and reduces motor deficits in a mouse model of amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2013, 7, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, Y.N.; Sabourin, J.-C.; Rábano, M.; Vivanco, M.D.M.; Perrin, F.E. Early Functional Deficit and Microglial Disturbances in a Mouse Model of Amyotrophic Lateral Sclerosis. PLoS ONE 2012, 7, e36000. [Google Scholar] [CrossRef] [Green Version]
- Noristani, H.N.; Saint-Martin, G.P.; Cardoso, M.; Sidiboulenouar, R.; Catteau, M.; Coillot, C.; Goze-Bac, C.; Perrin, F.E. Longitudinal MRI analysis and histological characterization after spinal cord injury in two mouse strains with different functional recovery: Gliosis as a key factor. J. Neurotrauma 2018, 35, 2924–2940. [Google Scholar] [CrossRef] [PubMed]
- Coillot, C.; Sidiboulenouar, R.; Nativel, E.; Zanca, M.; Alibert, E.; Cardoso, M.; Saintmartin, G.; Noristani, H.; Lonjon, N.; Lecorre, M.; et al. Signal modeling of an MRI ribbon solenoid coil dedicated to spinal cord injury investigations. J. Sensors Sens. Syst. 2016, 5, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Le Corre, M.; Noristani, H.; Mestre-Frances, N.; Saint-Martin, G.P.; Coillot, C.; Goze-Bac, C.; Lonjon, N.; Perrin, F.E. A Novel Translational Model of Spinal Cord Injury in Nonhuman Primate. Neurotherapeutics 2018, 15, 751–769. [Google Scholar] [CrossRef] [Green Version]
- Mukaino, M.; Nakamura, M.; Yamada, O.; Okada, S.; Morikawa, S.; Renault-Mihara, F.; Iwanami, A.; Ikegami, T.; Ohsugi, Y.; Tsuji, O.; et al. Anti-IL-6-receptor antibody promotes repair of spinal cord injury by inducing microglia-dominant inflammation. Exp. Neurol. 2010, 224, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.L.; Fleming, S.M.; Budge, K.M.; Boyle, A.M.; Kim, C.; Alam, G.; Beier, E.E.; Wu, L.; Richardson, J.R. Pharmacological inhibition of CSF1R by GW2580 reduces microglial proliferation and is protective against neuroinflammation and dopaminergic neurodegeneration. FASEB J. 2020, 34, 1679–1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Muriana, A.; Mancuso, R.; Quijorna, I.F.; Olmos-Alonso, A.; Osta, R.; Perry, V.H.; Navarro, X.; Gomez-Nicola, D.; López-Vales, R. CSF1R blockade slows the progression of amyotrophic lateral sclerosis by reducing microgliosis and invasion of macrophages into peripheral nerves. Sci. Rep. 2016, 6, 25663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olmos-Alonso, A.; Schetters, S.T.T.; Sri, S.; Askew, K.; Mancuso, R.; Vargas-Caballero, M.; Holscher, C.; Perry, V.H.; Gomez-Nicola, D. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain J. Neurol. 2016, 139, 891–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Nicola, D.; Fransen, N.L.; Suzzi, S.; Perry, V.H. Regulation of Microglial Proliferation during Chronic Neurodegeneration. J. Neurosci. 2013, 33, 2481–2493. [Google Scholar] [CrossRef]
- Catale, C.; Bisicchia, E.; Carola, V.; Viscomi, M.T. Early life stress exposure worsens adult remote microglia activation, neuronal death, and functional recovery after focal brain injury. Brain Behav. Immun. 2021, 94, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Sasaki, T.; Shimizu, A.; Yoshida, K.; Iwai, H.; Koya, I.; Kobayashi, Y.; Itakura, G.; Shibata, S.; Ebise, H.; et al. Global gene expression analysis following spinal cord injury in non-human primates. Exp. Neurol. 2014, 261, 171–179. [Google Scholar] [CrossRef]
- Perez, J.C.; Perrin, F.E.; Gerber, Y.N. Dynamic diversity of glial response among species in spinal cord injury. Front. Aging Neurosci 2021, 13, 769548. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Kawaguchi, R.; Zhang, Y.; Wang, Q.; Monavarfeshani, A.; Yang, Z.; Chen, B.; Shi, Z.; Meng, H.; et al. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature 2020, 587, 613–618. [Google Scholar] [CrossRef]
- Henry, R.J.; Ritzel, R.; Barrett, J.P.; Doran, S.J.; Jiao, Y.; Leach, J.B.; Szeto, G.L.; Wu, J.; Stoica, B.A.; Faden, A.I.; et al. Microglial Depletion with CSF1R Inhibitor During Chronic Phase of Experimental Traumatic Brain Injury Reduces Neurodegeneration and Neurological Deficits. J. Neurosci. 2020, 40, 2960–2974. [Google Scholar] [CrossRef]
- Wyatt-Johnson, S.K.; Sommer, A.L.; Shim, K.Y.; Brewster, A.L. Suppression of Microgliosis With the Colony-Stimulating Factor 1 Receptor Inhibitor PLX3397 Does Not Attenuate Memory Defects During Epileptogenesis in the Rat. Front. Neurol. 2021, 12, 651096. [Google Scholar] [CrossRef]
- Sosna, J.; Philipp, S.; Albay, R.; Reyes-Ruiz, J.M.; Baglietto-Vargas, D.; LaFerla, F.M.; Glabe, C.G. Early long-term administration of the CSF1R inhibitor PLX3397 ablates microglia and reduces accumulation of intraneuronal amyloid, neuritic plaque deposition and pre-fibrillar oligomers in 5XFAD mouse model of Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhu, K.; Zhang, X.-M.; Harris, R.A. Enforced microglial depletion and repopulation as a promising strategy for the treatment of neurological disorders. Glia 2019, 67, 217–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Wang, Y.-Y.; Wang, H.-F.; Liu, X.-K.; Li, R.; Zhang, P.; Chu, Z.; Wang, C.-L.; Liu, H.-R.; Qi, J.; et al. Effect of glial cells on remyelination after spinal cord injury. Neural Regen. Res. 2017, 12, 1724–1732. [Google Scholar] [CrossRef]
- Ohtake, Y.; Li, S. Molecular mechanisms of scar-sourced axon growth inhibitors. Brain Res. 2015, 1619, 22–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, S.; Hara, M.; Kobayakawa, K.; Matsumoto, Y.; Nakashima, Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 2018, 126, 39–43. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K. Glial Cells Shape Pathology and Repair After Spinal Cord Injury. Neurotherapeutics 2018, 15, 554–577. [Google Scholar] [CrossRef] [Green Version]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulen, G.; Bartolami, S.; Noristani, H.N.; Perrin, F.E.; Gerber, Y.N. Unlike Brief Inhibition of Microglia Proliferation after Spinal Cord Injury, Long-Term Treatment Does Not Improve Motor Recovery. Brain Sci. 2021, 11, 1643. https://doi.org/10.3390/brainsci11121643
Poulen G, Bartolami S, Noristani HN, Perrin FE, Gerber YN. Unlike Brief Inhibition of Microglia Proliferation after Spinal Cord Injury, Long-Term Treatment Does Not Improve Motor Recovery. Brain Sciences. 2021; 11(12):1643. https://doi.org/10.3390/brainsci11121643
Chicago/Turabian StylePoulen, Gaëtan, Sylvain Bartolami, Harun N. Noristani, Florence E. Perrin, and Yannick N. Gerber. 2021. "Unlike Brief Inhibition of Microglia Proliferation after Spinal Cord Injury, Long-Term Treatment Does Not Improve Motor Recovery" Brain Sciences 11, no. 12: 1643. https://doi.org/10.3390/brainsci11121643