Microsurgical Anatomy of the Anterior Circulation of the Brain Adjusted to the Neurosurgeon’s Daily Practice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Specimens
2.2. Procurement and Preservation of Cadaveric Head
2.3. Irrigation
2.4. Injection
3. Results
3.1. Cervical
3.2. Petrous
3.3. Lacerum
3.4. Cavernous
3.5. Clinoid
3.6. Ophthalmic
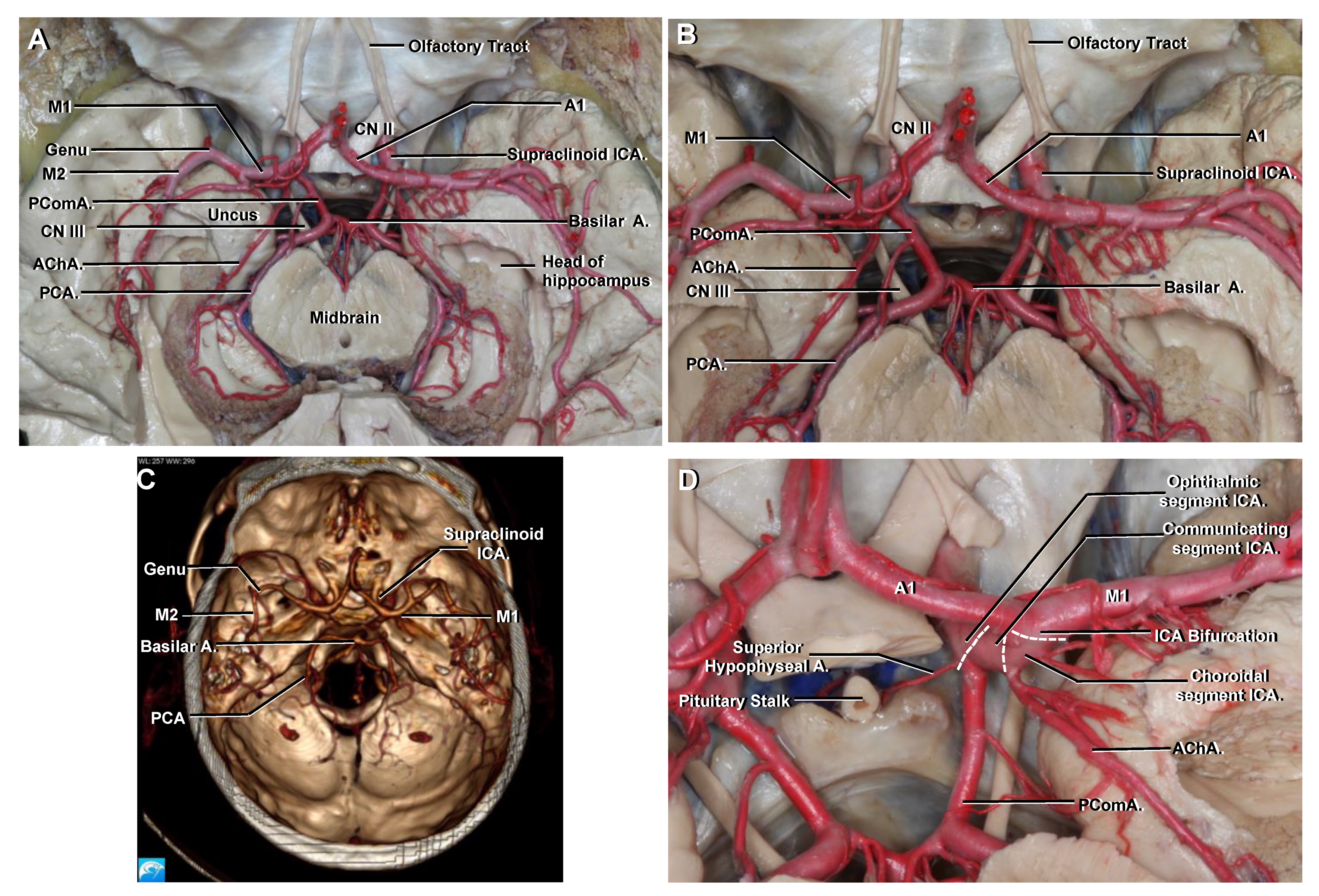
3.7. Communicating
3.8. Choroidal
3.9. Terminal Branches of the ICA
3.9.1. Middle Cerebral Artery (MCA)
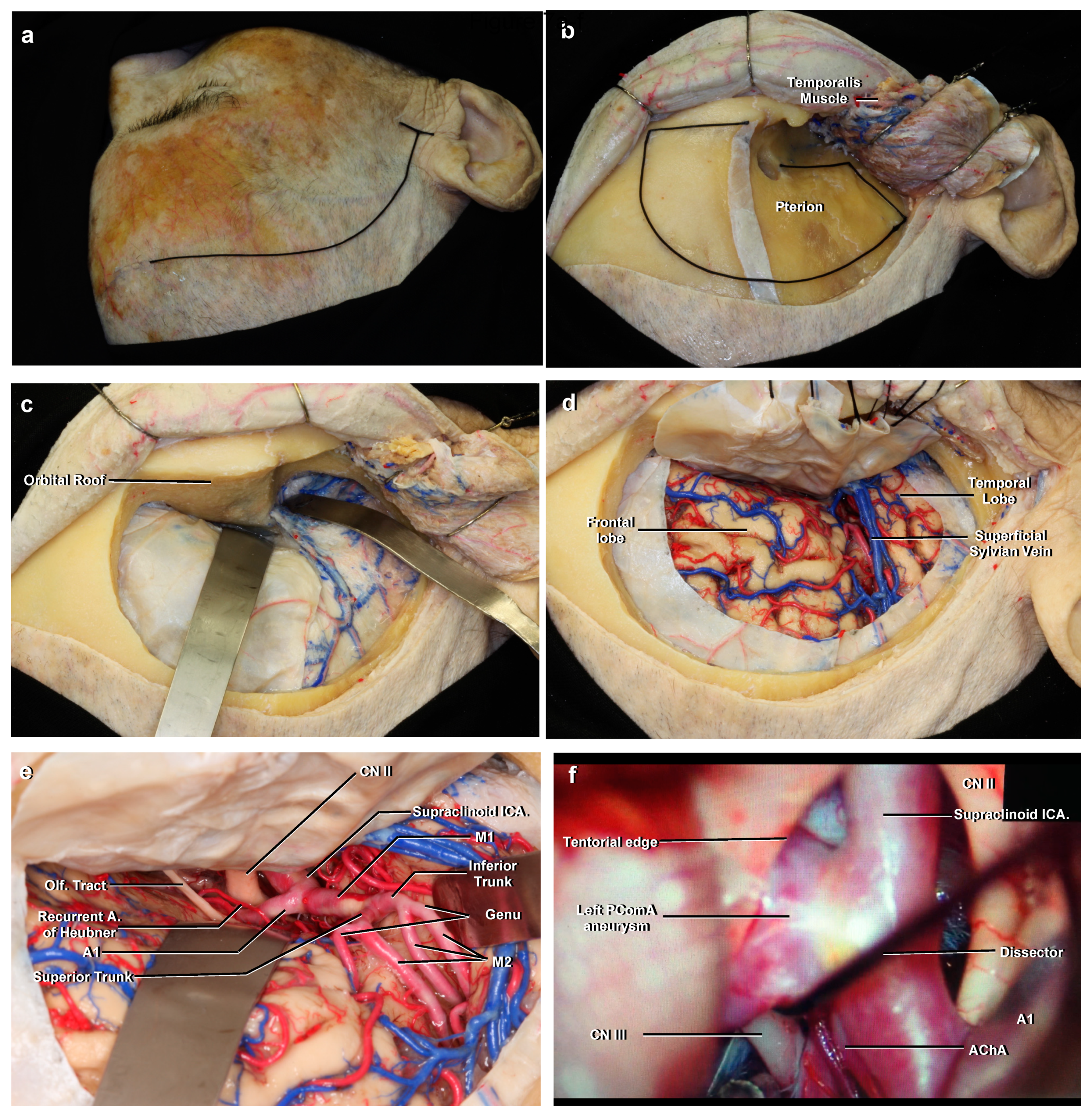
3.9.2. M1
3.9.3. M2
3.9.4. M3
3.9.5. M4
3.10. Anterior Cerebral Artery (ACA)
3.10.1. A1
3.10.2. A2
3.10.3. A3
3.10.4. A4 and A5
3.11. Circle of Willis
4. Discussion
4.1. In Relation to Different ICA Classifications
4.2. Anatomic Information as Applied to Angiography
Anteroposterior Projection (AP)
4.3. Anatomic Information as Applied to Computed Tomographic Angiography (CTA)
4.4. Anatomic Information as Applied to Surgery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rhoton, A.L., Jr. The supratentorial arteries. Neurosurgery 2002, 51, S53–S120. [Google Scholar] [CrossRef] [PubMed]
- Yasargil, M.G. Microsurgical anatomy of the basal cisterns and vessels of the brain, diagnostic studies, general operative tech-niques. In Microneurosurgery Vol 1; Thieme Medical Publishers: New York, NY, USA, 1984; pp. 54–168. [Google Scholar]
- Winn, H. Surgical anatomy of the brain. In Youmans and Winn Neurological Surgery, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 38–62. [Google Scholar]
- Standring, S. Vascular supply and drainage of the brain. In Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 41st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 280–290. [Google Scholar]
- Jacquesson, T.; Simon, E.; Dauleac, C.; Margueron, L.; Robinson, P.; Mertens, P. Stereoscopic three-dimensional visualization: Interest for neuroanatomy teaching in medical school. Surg. Radiol. Anat. 2020, 42, 719–727. [Google Scholar] [CrossRef]
- Sanan, A.; Abdel, A.K.M.; Janjua, R.M.; Van Loveren, H.R.; Keller, J.T. Colored silicone injection for use in neurosurgical dissec-tions: Anatomical technical note. Neurosurgery 1999, 45, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Poblete, T.; Soto, M.; Casanova, D.; Rojas, X. Descripción de la técnica de Rhoton modificada para la preparación de encéfalos en cadáveres y su práctica en el adiestramiento neuroquirúrgico en Chile. Rev. Chil. Neurocir. 2020, 46, 8–14. (In Spanish) [Google Scholar]
- Bouthillier, A.; van Loveren, H.R.; Keller, J.T. Segments of the internal carotid artery: A new Classification. Neurosurgery 1998, 38, 425–433. [Google Scholar]
- Gibo, H.; Lenkey, C.; Rhoton, A.L., Jr. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J. Neu-rosurg 1981, 55, 560–574. [Google Scholar] [CrossRef] [PubMed]
- Osborn, A.G. The internal carotid artery. In Diagnostic Cerebral Angiography, 2nd ed.; Lipincott Williams & Wilkins: Philadelphia, PA, USA, 1998; pp. 57–104. [Google Scholar]
- Charalambous, S.; Hatzidakis, A.; Peteinarakis, I.; Megremis, S.; Karantanas, A. Common left carotid bifurcation at C7-Th1 level: A rare anatomical variant. Surg. Radiol. Anat. 2019, 41, 227–229. [Google Scholar] [CrossRef]
- Singh, M.; Vashistha, A.; Chaudhary, M.; Kaur, G. Forgotten triangles of neck. Ann. Maxillofac Surg. 2016, 6, 91–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuta, S.; Iwanaga, J.; Kusukawa, J.; Tubbs, R.S. Triangles of the neck: A review with clinical/surgical applications. Anat. Cell Biol. 2019, 52, 120–127. [Google Scholar] [CrossRef]
- Osawa, S.; Rhoton, A.L., Jr.; Tanriover, N.; Shimizu, S.; Fujii, K. Microsurgical anatomy and surgical exposure of the petrous segment of the internal carotid artery. Neurosurgery 2008, 63, 210–239. [Google Scholar] [CrossRef]
- Yasuda, A.; Campero, A.; Martins, C.; Rhoton, A.L., Jr.; de Oliveira, E.; Ribas, G.C. Microsurgical anatomy and approaches to the cavernous sinus. Neurosurgery 2005, 56, 4–27. [Google Scholar] [PubMed]
- Seoane, E.; Rhoton, A.L., Jr.; de Oliveira, E. Microsurgical anatomy of the dural collar (carotid collar) and rings around the clinoid segment of the internal carotid artery. Neurosurgery 1998, 42, 869–886. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.T. Ophthalmic artery aneurysms. In Seven Aneurysm; Thieme Medical Publishers: New York, NY, USA, 2010; pp. 121–146. [Google Scholar]
- Samson, D.; Batjer, H.; White, J.; Trammell, J.; Eddleman, C. Aneurysms of the Internal Carotid Artery at the Origins of the Posterior Communicating and Anterior Choroidal Arteries. In Intracranial Aneurysm Surgery: Basic Principles and Techniques; Thieme Medical Publishers: New York, NY, USA, 2011; pp. 15–32. [Google Scholar]
- Tanriover, N.; Kucukyuruk, B.; Ulu, M.O.; Isler, C.; Sam, B.; Abuzayed, B.; Uzan, M.; Ak, H.; Tuzgen, S. Microsurgical anatomy of the cisternal anterior choroidal artery with special emphasis on the preoptic and postoptic subdivisions. J. Neurosurg. 2014, 120, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.T.; Rhoton, A.L., Jr.; de Oliveira, E.; Cardoso, A.C.; Tedeschi, H.; Baccanelli, M.; Marino, R., Jr. Microsurgical anatomy of the temporal lobe: Part 1: Mesial temporal lobe anatomy and its vascular relationships as applied to amygdalohippocampectomy. Neurosurgery 1999, 45, 549–592. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S. Intracranial arterial system: Basal perforating arteries. In Neurovascular Imaging; Springer: Berlin/Heidelberg, Germany, 2011; pp. 55–130. [Google Scholar]
- Osborn, A.G. The middle cerebral artery. In Diagnostic Cerebral Angiography, 2nd ed.; Lipincott Williams & Wilkins: Philadelphia, PA, USA, 1998; pp. 135–151. [Google Scholar]
- Gibo, H.; Carver, C.C.; Rhoton, A.L., Jr.; Lenkey, C.; Mitchell, R.J. Microsurgical anatomy of the middle cerebral artery. J. Neuro-surg. 1981, 54, 151–169. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, S. Perforating branches of the anterior communicating artery: Anatomy and infarction. In Neurovascular Imaging; Springer: Berlin/Heidelberg, Germany, 2011; pp. 189–1196. [Google Scholar]
- Fischer, E. Die Lageabweichungen der vorderen hirnarterie im gefässbild. Zentralbl. Neurochir. 1938, 3, 300–313. [Google Scholar]
- Abdulrauf, S.I.; Ashour, A.M.; Marvin, E.; Coppens, J.; Kang, B.; Hsieh, T.Y.; Nery, B.; Penanes, J.R.; Alsahlawi, A.K.; Moore, S.; et al. Proposed clinical internal carotid artery classification system. J. Craniovertebr. Junction Spine 2016, 7, 161–170. [Google Scholar] [CrossRef]
- Gonzalez, L.F.; Walker, M.T.; Zabramski, J.M.; Partovi, S.; Wallace, R.C.; Spetzler, R.F. Distinction between paraclinoid and cav-ernous sinus aneurysms with computed tomographic angiography. Neurosurgery 2003, 52, 1131–1139. [Google Scholar]
- Winn, H. Neurovascular imaging. In Youmans and Winn Neurological Surgery, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3603–3614. [Google Scholar]
- Wen, H.T.; Rhoton, A.L., Jr.; de Oliveira, E.; Castro, L.H.; Figueiredo, E.G.; Teixeira, M.J. Microsurgical anatomy of the temporal lobe: Part 2—sylvian fissure region and its clinical application. Neurosurgery 2009, 65, 1–36. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, L.J.; Poon, C.S.; Wu, S.Y.; Zhou, C.S.; Luo, S.; Wang, M.; Lu, G.M. Digital subtraction CT angiography for detection of intracranial aneurysms: Comparison with three-dimensional digital subtraction angiography. Radiology 2012, 262, 605–612. [Google Scholar] [CrossRef]
- Kamide, T.; Burkhardt, J.K.; Tabani, H.; Safaee, M.M.; Lawton, M.T. Preoperative prediction of the necessity for anterior clinoidec-tomy during microsurgical clipping of ruptured posterior communicating artery aneurysms. World Neurosurg. 2018, 109, e493–e501. [Google Scholar] [CrossRef]
- Yasargil, M.G. Microsurgical anatomy of the basal cisterns and vessels of the brain, diagnostic studies, general operative tech-niques. In Microneurosurgery Vol 1; Thieme Medical Publishers: New York, NY, USA, 1984; pp. 215–233. [Google Scholar]
- Yasargil, M.G.; Reichman, M.V.; Kubik, S. Preservation of the frontotemporal branch of the facial nerve using the interfascial temporalis flap for pterional craniotomy. Technical. Artic. J. Neurosurg. 1987, 67, 463–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poblete, T.; Jiang, X.; Komune, N.; Matsushima, K.; Rhoton, A.L., Jr. Preservation of the nerves to the frontalis muscle during pterional craniotomy. J. Neurosurg. 2015, 122, 1274–1282. [Google Scholar] [CrossRef]
- Javalkar, V.; Banerjee, A.D.; Nanda, A. Paraclinoid carotid aneurysms. J. Clin. Neurosci. 2011, 18, 13–22. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poblete, T.; Casanova, D.; Soto, M.; Campero, A.; Mura, J. Microsurgical Anatomy of the Anterior Circulation of the Brain Adjusted to the Neurosurgeon’s Daily Practice. Brain Sci. 2021, 11, 519. https://doi.org/10.3390/brainsci11040519
Poblete T, Casanova D, Soto M, Campero A, Mura J. Microsurgical Anatomy of the Anterior Circulation of the Brain Adjusted to the Neurosurgeon’s Daily Practice. Brain Sciences. 2021; 11(4):519. https://doi.org/10.3390/brainsci11040519
Chicago/Turabian StylePoblete, Tomas, Daniel Casanova, Miguel Soto, Alvaro Campero, and Jorge Mura. 2021. "Microsurgical Anatomy of the Anterior Circulation of the Brain Adjusted to the Neurosurgeon’s Daily Practice" Brain Sciences 11, no. 4: 519. https://doi.org/10.3390/brainsci11040519





