Exploring Inflammatory Status in Febrile Seizures Associated with Urinary Tract Infections: A Two-Step Cluster Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Paraclinical Investigations in the Evaluation of the UTIs
2.3. Data Analysis
3. Results
3.1. General Description
3.2. Clinical Data Comparison: UTI versus Non-UTI Groups and U-UTI versus L-UTI Subgroups
3.3. Laboratory Parameters Comparison in UTI versus Non-UTI Groups and U-UTI versus L-UTI Subgroups
3.4. Two Steps Cluster Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
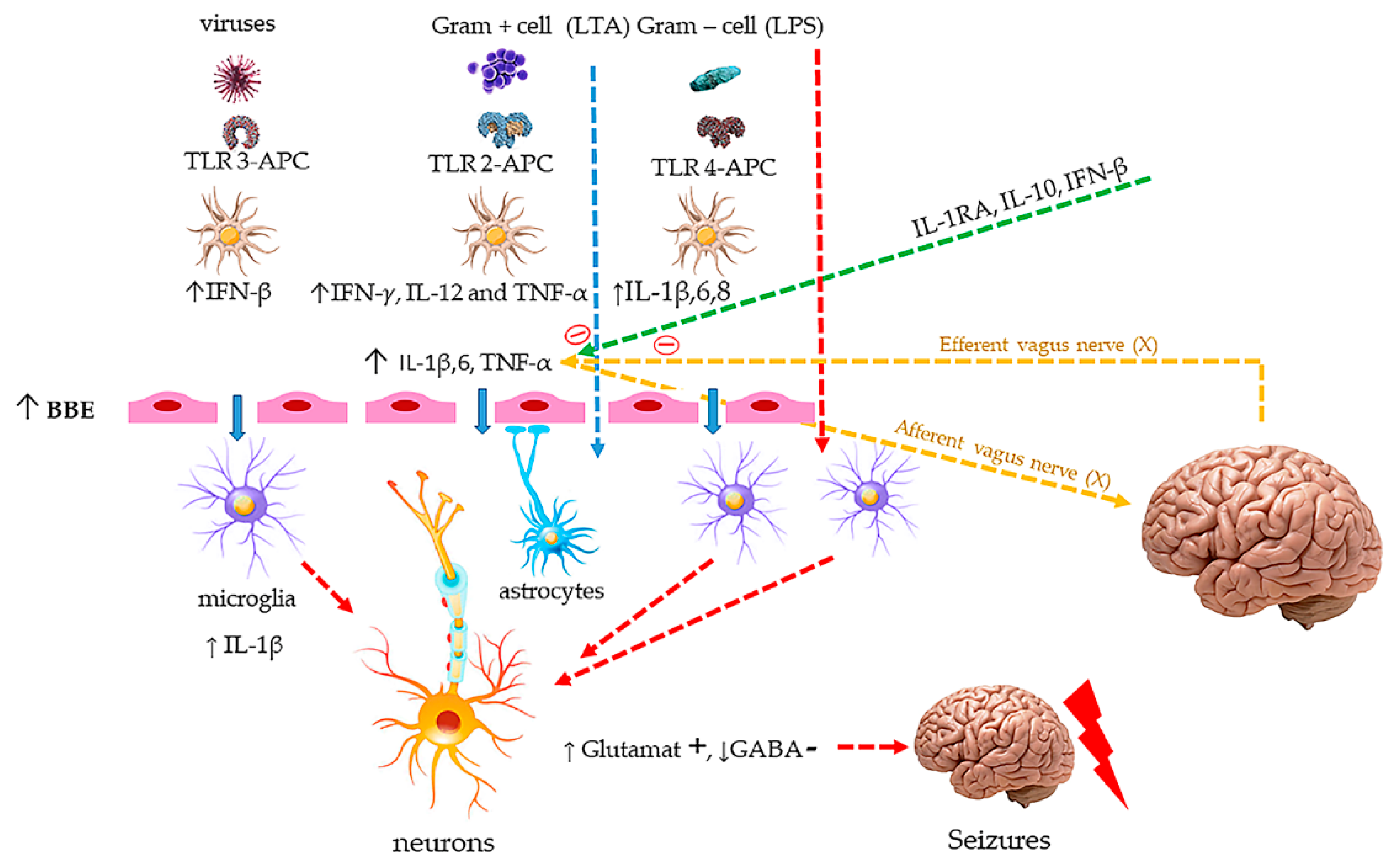
Appendix B
| Non-UTI 177 (89.8%) | UTI 20 (10.2%) | p | U-UTI 13 (65%) | L-UTI 7 (35%) | p | ||
|---|---|---|---|---|---|---|---|
| Gender | M | 88(49.72) | 12(60.00) | 0.383 | 6(46.15) | 6(85.71) | 0.085 |
| F | 89(50.28) | 8(40.00) | 7(53.85) | 1(14.29) | |||
| Age | <6 | 5(2.82) | 0(0.00) | 0.294 | 0(0.00) | 0(0.00) | 0.061 |
| 6–12 | 24(13.56) | 5(25.00) | 2(15.38) | 3(42.86) | |||
| 13–24 | 81(45.76) | 8(40.00) | 7(53.85) | 1(14.29) | |||
| 25–36 | 41(23.16) | 2(10.00) | 0(0.00) | 2(28.57) | |||
| >36 | 26(14.69) | 5(25.00) | 4(30.77) | 1(14.29) | |||
| M ± SD | 23.14 ± 12.01 | 24 ± 16.10 | 25.77 ± 17.89 | 20.71 ± 12.70 | |||
| Temperature | <38 | 20(11.30) | 6(30.00) | 0.106 | 4(30.77) | 2(28.57) | 0.737 |
| 38–39 | 49(27.68) | 7(35.00) | 5(38.46) | 2(28.57) | |||
| 39–40 | 78(44.07) | 6(30.00) | 3(23.08) | 3(42.86) | |||
| 40–41 | 25(14.12) | 1(5.00) | 1(7.69) | 0(0.00) | |||
| >41 | 5(2.82) | 0(0.00) | 0(0.00) | 0(0.00) | |||
| FS episode Type | S | 143(80.79) | 13(65.00) | 0.099 | 9(69.23) | 4(57.14) | 0.589 |
| C | 34(19.21) | 7(35.00) | 4(30.77) | 3(42.86) | |||
| Seizure duration | <1 | 25(14.12) | 4(20.00) | 0.843 | 2(15.38) | 2(28.57) | 0.731 |
| 1–4.9 | 84(47.46) | 9(45.00) | 7(53.85) | 2(28.57) | |||
| 5–14.9 | 55(31.07) | 5(25.00) | 3(23.08) | 2(28.57) | |||
| ≥15 | 13(7.34) | 2(10.00) | 1(7.69) | 1(14.29) | |||
| Recurrence/24 h | Yes | 15(8.47) | 3(15.00) | 0.337 | 2(15.38) | 1(14.29) | 0.948 |
| Non-UTI | UTI | p | U-UTI | L-UTI | p | |
|---|---|---|---|---|---|---|
| CRP | 27.21 ± 35.85 | 38.05 ± 42.30 | 0.054 | 45.92 ± 48.26 (4–171) 30 (13–46) | 21.00 ± 18.48 | 0.292 |
| (2–209) | (4–171) | (5–52) | ||||
| 15 | 30 | 14.50 | ||||
| (5–35) | (10–46) | (6–34) | ||||
| PDW | 9.95 ± 1.54 | 9.78 ± 1.00 | 0.941 | 9.85 ± 1.04 (8.20–12.20) 9.70 (9.20–10.20) | 9.65 ± 1.01 | 0.960 |
| (7–16.30) | (7.80–12.20) | (7.80–10.70) | ||||
| 9.80 | 9.70 | 9.85 | ||||
| (8.80–10.60) | (9.40–10.20) | (9.50–10.20) | ||||
| P-LCR | 15.82 ± 6.43 | 16.02 ± 5.25 | 0.415 | 16.47 ± 5.55 | 15.13 ± 4.97 | 0.963 |
| (6.20–44.10) | (6.60–31.90) | (10.20–31.90) | (6.60–21.50) | |||
| 14.20 | 15.65 | 15.65 | 15.45 | |||
| (11.90–19.00) | (14.10–17.70) | (13.50–17.65) | (14.10–17.70) | |||
| MPV | 8.63 ± 0.95 | 8.73 ± 0.82 | 0.442 | 8.78 ± 0.82 | 8.63 ± 0.88 | 0.880 |
| (6.70–12.40) | (7.10–10.80) | (7.90–10.80) | (7.10–9.70) | |||
| 8.50 | 8.60 | 8.60 | 8.70 | |||
| (8.10–9.10) | (8.40–8.90) | (8.40–8.90) | (8.40–9.20) | |||
| PLT | 327.41 ± 118.03 | 355.75 ± 163.13 | 0.711 | 374.62 ± 188.19 | 320.71 ± 105.99 | 0.606 |
| (98–665) | (120–716) | (120–716) | (188–461) | |||
| 303 | 315.50 | 326 | 305 | |||
| (247–391) | (228.50–478) | (237–518) | (210–450) | |||
| Neutrophils | 8.88 ± 5.87 | 9.57 ± 4.92 | 0.341 | 10.87 ± 5.25 | 6.75 ± 2.62 | 0.054 |
| (0.88–38.16) | (0.23–21.17) | (0.23–21.17) | (3.44–10.31) | |||
| 7.83 | 8.59 | 10.72 | 6.98 | |||
| (4.73–11.38) | (6.61–13.86) | (7.54–14.31) | (4.22–8.57) | |||
| Lymphocytes | 2.98 ± 1.94 | 2.59 ± 1.67 | 0.328 | 3.02 ± 1.83 | 1.67 ± 0.75 | 0.136 |
| (0.54–13.74) | (0.62–6.43) | (0.62–6.43) | (0.80–2.73) | |||
| 2.52 | 2.27 | 2.77 | 1.58 | |||
| (1.75–3.64) | (1.27–4.11) | (1.55–4.31) | (1.07–2.27) | |||
| NLR | 3.93 ± 3.33 | 5.36 ± 5.10 | 0.171 | 5.49 ± 5.81 | 5.07 ± 3.56 | 0.930 |
| (0.21–22.75) | (0.14–22.35) | (0.14–22.35) | (1.55–10.71) | |||
| 3.04 | 4.06 | 4.26 | 3.64 | |||
| (1.59–5.08) | (2.38–5.89) | (2.38–5.37) | (2.77–8.12) | |||
| RBC | 4.46 ± 0.40 | 4.50 ± 0.36 | 0.898 | 4.55 ± 0.34 | 4.38 ± 0.40 | 0.254 |
| (3.27–6.22) | (4.03–5.26) | (4.07–5.26) | (4.03–5.05) | |||
| 4.46 | 4.42 | 4.45 | 4.26 | |||
| (4.23–4.70) | (4.25–4.69) | (4.33–4.69) | (4.05–4.63) | |||
| Hb | 11.32 ± 1.31 | 11.08 ± 1.38 | 0.487 | 10.97 ± 1.55 | 11.32 ± 0.99 | 0.930 |
| (5.10–13.90) | (7.80–13.10) | (7.80–13.10) | (9.80–12.90) | |||
| 11.45 | 11.40 | 11.40 | 11.20 | |||
| (10.70–12.10) | (10.40–11.80) | (10.40–11.80) | (11.20–11.60) | |||
| Ht | 32.93 ± 2.98 | 32.12 ± 3.02 | 0.253 | 32.16 ± 3.45 | 32.03 ± 2.07 | 0.861 |
| (19.70–40.00) | (26.10–37.90) | (26.10–37.90) | (29.70–35.30) | |||
| 32.95 | 31.70 | 32.40 | 31.70 | |||
| (31.10–34.90) | (30.30–34.60) | (30.60–34.60) | (30.30–33.50) | |||
| MCV | 73.52 ± 6.31 | 71.58 ± 6.37 | 0.305 | 70.68 ± 7.15 | 73.74 ± 3.65 | 0.598 |
| (51.90–89.00) | (59.00–78.20) | (59.00–77.20) | (69.10–78.20) | |||
| 73.90 | 74.10 | 74.65 | 72.40 | |||
| (70.90–77.50) | (69.10–76.10) | (63.40–75.80) | (72.40–76.60) | |||
| MCH | 25.51 ± 2.94 | 24.69 ± 2.93 | 0.244 | 24.15 ± 3.34 | 25.88 ± 1.33 | 0.510 |
| (13.70–32.40) | (17.60–27.80) | (17.60–27.70) | (24.20–27.80) | |||
| 25.80 | 25.60 | 26.10 | 25.55 | |||
| (24.40–27.25) | (24.20–26.50) | (21.90–26.40) | (25.10–27.10) | |||
| MCHC | 34.21 ± 2.17 | 34.39 ± 1.79 | 0.891 | 33.98 ± 1.83 | 35.28 ± 1.42 | 0.095 |
| (23.10–37.30) | (29.90–37.00) | (29.90–36.40) | (33.00–37.00) | |||
| 34.60 | 34.60 | 34.30 | 35.30 | |||
| (33.55–35.40) | (33.00–35.30) | (33.00–34.90) | (34.60–36.50) | |||
| RDW-CV | 14.63 ± 2.38 | 14.72 ± 1.92(12.50–19.60) 14.60(13.20–16.40) | 0.716 | 15.11 ± 1.99 | 13.88 ± 1.59 | 0.160 |
| (8.00–26.90) | (13.00–19.60) | (12.50–16.60) | ||||
| 14.10 | 14.70 | 13.35 | ||||
| (13.20–15.20) | (13.60–16.40) | (12.60–14.90) | ||||
| RDW-SD | 38.14 ± 4.18 | 37.18 ± 3.10 | 0.316 | 37.61 ± 2.86 | 36.25 ± 3.66 | 0.219 |
| (32.50–73.40) | (33.00–43.10) | (33.20–43.10) | (33.00–42.70) | |||
| 37.60 | 36.70 | 37.50 | 34.95 | |||
| (35.80–39.40) | (34.60–39.70) | (35.00–39.70) | (33.70–38.20) | |||
| Na+ | 130.05 ± 2.82 | 129.89 ± 3.02 | 0.744 | 130.08 ± 3.58 | 129.57 ± 1.90 | 0.966 |
| (122–140) | (126–135) | (126–135) | (126–132) | |||
| 130 | 129 | 129 | 130 | |||
| (128–132) | (128–132) | (127–133.50) | (129–131) | |||
| Cl− | 107.54 ± 3.40 | 106.58 ± 4.21 | 0.19 | 106.54 ± 3.86 | 106.67 ± 5.24 | 0.887 |
| (98.60–116) | (100–115) | (100–115) | (100–115) | |||
| 107 | 105.70 | 105.70 | 106.50 | |||
| (105.05–110) | (105–109) | (105–109) | (103–109) | |||
| K+ | 4.01 ± 0.63 | 4.04 ± 0.49 | 0.788 | 3.88 ± 0.43 | 4.30 ± 0.50 | 0.175 |
| (0.92–5.80) | (3.10–5.10) | (3.10–4.50) | (3.80–5.10) | |||
| 4 | 4 | 4 | 4.30 | |||
| (3.60–4.40) | (3.80–4.40) | (3.55–4.15) | (3.80–4.70) | |||
| pH− | 7.47 ± 0.08 | 7.47 ± 0.06 | 0.888 | 7.48 ± 0.07 | 7.46 ± 0.05 | 0.341 |
| (7.214–7.670) | (7.350–7.605) | (7.350–7.605) | (7.401–7.553) | |||
| 7.47 | 7.47 | 7.49 | 7.45 | |||
| (7.42–7.52) | (7.43–7.51) | (7.43–7.53) | (7.43–7.48) | |||
| pCO2 | 25.79 ± 5.92 | 24.58 ± 5.04 | 0.535 | 23.14 ± 4.70 | 26.84 ± 5.03 | 0.063 |
| (13.90–43.70) | (15.80–33.10) | (15.80–32.70) | (17.80–33.10) | |||
| 25 | 24.25 | 23.60 | 26.60 | |||
| (22–29) | (22.20–27.90) | (19.10–24.80) | (24.40–30.50) | |||
| HCO3− | 20.97 ± 2.29 | 20.67 ± 1.80 | 0.431 | 20.32 ± 1.79 | 21.23 ± 1.81 | 0.297 |
| (11.70–27.40) | (17.70–24.00) | (17.70–24.00) | (18.50–23.90) | |||
| 21.20 | 20.70 | 20.40 | 21.30 | |||
| (19.50–22.50) | (19.40–21.40) | (18.60–21.40) | (19.70–22.40) | |||
| lactate | 25.14 ± 13.45 | 22.78 ± 9.37 | 0.813 | 23.40 ± 9.57 | 21.40 ± 9.84 | 1.000 |
| (9–95) | (8–49) | (15–49) | (8–32) | |||
| 21 | 19.70 | 19.40 | 20 | |||
| (16–29) | (17.50–28.50) | (18–28) | (17–30) |
References
- International league against epilepsy, guidelines for epidemiologic studies on epilepsy, commission on epidemiology and prognosis. Epilepsia 1993, 34, 592–596. [CrossRef]
- Carman, K.B.; Calik, M.; Karal, Y.; Isikay, S.; Kocak, O.; Ozcelik, A.; Yazar, A.S.; Nuhoglu, C.; Sag, C.; Kilic, O.; et al. Viral etiological causes of febrile seizures for respiratory pathogens. Hum. Vaccines Immunother. 2019, 15, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, X.; Zhang, M.; Huang, X.; Bai, J.; Pan, Z.; Lin, X.; Yu, D.; Zeng, H.; Wan, R.; et al. The role of mean platelet volume/platelet count ratio and neutrophil to lymphocyte ratio on the risk of febrile seizure. Sci. Rep. 2018, 8, 15123. [Google Scholar] [CrossRef] [PubMed]
- Mosili, P.; Maikoo, S.; Mabandla, M.V.; Qulu, L. The pathogenesis of fever-induced febrile seizures and its current state. Neurosci. Insights 2020, 15, 2633105520956973. [Google Scholar] [CrossRef]
- Skovbjerg, S.; Martner, A.; Hynsjö, L.; Hessle, C.; Olsen, I.; Dewhirst, F.E.; Tham, W.; Wold, A.E. Gram-positive and gram-negative bacteria induce different patterns of cytokine production in human mononuclear cells irrespective of taxonomic relatedness. J. Interferon Cytokine Res. 2010, 30, 23–32. [Google Scholar] [CrossRef]
- Coulthard, M.G. Using urine nitrite sticks to test for urinary tract infection in children aged <2 years: A meta-analysis. Pediatric Nephrol. 2019, 34, 1283–1288. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, J.; Temple-Smith, M.; Sanci, L. Urinary tract infections in children: An overview of diagnosis and management. BMJ Paediatr. Open 2019, 3, e000487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costea, R.; Maniu, I.; Dragomir, A.; Banciu, D.D.; Neamtu, B.M. Cluster Analysis a Profiling Tool in Children With Febrile Seizures. In Proceedings of the 7th IEEE International Conference on E-Health and Bioengineering (EHB), Iasi, Romania, 21 November 2019; pp. 1–4. [Google Scholar] [CrossRef]
- Costea, R.M.; Maniu, I.; Dobrota, L.; Neamtu, B. Stress hyperglycemia as predictive factor of recurrence in children with febrile seizures. Brain Sci. 2020, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Sachdev, R.; Tiwari, A.K.; Goel, S.; Raina, V.; Sethi, M. Establishing biological reference intervals for novel platelet parameters (immature platelet fraction, high immature platelet fraction, platelet distribution width, platelet large cell ratio, platelet-X, plateletcrit, and platelet distribution width) and their correlations among each other. Indian J. Pathol. Microbiol. 2014, 57, 231–235. [Google Scholar]
- Barbacariu, L. Infectiatractuluiurinar la copil. Supliment Urologie 2016. Available online: https://www.medichub.ro/reviste/medic-ro/infectia-tractului-urinar-la-copilid-275-cmsid-51 (accessed on 21 May 2020).
- Popescu, V. Urinary tract infections in children. Rom. J. Pediatrics 2007, 3, 267–281. [Google Scholar]
- Abraham, H.M.; Stoller, M.L. Infection and urinary stones. Curr. Opin. Urol. 2003, 13, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Bauchner, H.; Dashefsky, B.; Philipp, B.; Klein, J.O. Prevalence of bacteriuria in febrile children. Pediatric Infect. Dis. J. 1987, 6, 239–242. [Google Scholar] [CrossRef]
- American Academy of Pediatrics; Committee on Quality Improvement; Subcommittee on Urinary Tract Infection. Practice parameter: The diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics 1999, 103, 843–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitsori, M.; Galanakis, E. Pediatric Urinary Tract Infections, Diagnosis and Treatment. Expert Rev. Anti-Infect. Ther. 2012, 10, 1153–1164. [Google Scholar] [CrossRef]
- Neamțu, B.M.; Visa, G.; Maniu, I.; Ognean, M.L.; Pérez-Elvira, R.; Dragomir, A.; Agudo, M.; Șofariu, C.R.; Gheonea, M.; Pitic, A.; et al. A decision-tree approach to assist in forecasting the outcomes of the neonatal brain injury. Int. J. Environ. Res. Public Health 2021, 18, 4807. [Google Scholar] [CrossRef]
- Abedi, A.; Ashrafi, M.; Moghtaderi, M. Prevalence of urinary tract infection among children with febrile convulsion. Int. J. Nephrol. Kidney Fail. 2017. [Google Scholar] [CrossRef]
- Kazeminezhad, B.; Borji, M.; Seymohammadi, R.; Taghinejad, H. Evaluation of the prevalence of urinary tract infection in children with febrile seizure. J. Compr. Pediatrics 2018, 9, e62557. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, N.; Bost, J.E.; Farrell, M.H. Prevalence of urinary tract infection in childhood: A meta-analysis. Pediatric Infect. Dis. J. 2008, 27, 302–308. [Google Scholar] [CrossRef]
- Karmazyn, B.K.; Alazraki, A.L.; Anupindi, S.A.; Dempsey, M.E.; Dillman, J.R.; Dorfman, S.R.; Garber, M.D.; Moore, S.G.; Peters, C.A.; Rice, H.E.; et al. ACR appropriateness criteria. Urinary tract infection child. J. Am. Coll. Radiol. 2017, 14, S362–S371. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.J.; Kennedy, W.A.; Shortliffe, L.D. Urinary Tract Infection in Children: When to Worry. Urol. Clin. 2010, 37, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Korbel, L.; Howell, M.; Spencer, J.D. The clinical diagnosis and management of urinary tract infections in children and adolescents. Paediatr. Int. Child Health 2017, 37, 273–279. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Hon, K.L.; Leung, A.A.M. Urinary tract infection in children. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Wiswell, T.E.; Roscelli, J.D. Corroborative evidence for the decreased incidence of urinary tract infections in circumcised male infants. Pediatrics 1986, 78, 96–99. [Google Scholar] [CrossRef]
- Mahyar, A.; Ayazi, P.; Azimi, E.; Dalirani, R.; Barikani, A.; Esmaeily, S. The Relation between Urinary Tract Infection and Febrile Seizure. Iran. J. Child Neurol. 2018, 12, 120–126. [Google Scholar] [PubMed]
- Chon, C.; Lar, F.; Shortliffe, L.M. Pediatric urinary tract infections. Pediatric Clin. N. Am. 2001, 48, 1443. [Google Scholar] [CrossRef]
- Grigore, N.; Pîrvuț, V.; Totan, M.; Bratu, D.; Mitariu, S.I.; Mițariu, M.C.; Chicea, R.; Sava, M.; Hasegan, A. The evaluation of biochemical and microbiological parameters in the diagnosis of emphysematous pyelonephritis. Rev. Chim. 2017, 68, 1285–1288. [Google Scholar] [CrossRef]
- Hasegan, A.; Totan, M.; Antonescu, E.; Bumbu, A.G.; Pantis, C.; Furau, C.; Urducea, C.B.; Grigore, N. Prevalence of urinary tract infections in children and changes in sensitivity to antibiotics of E. coli Strains. Rev. Chim. 2019, 70, 3788–3792. [Google Scholar] [CrossRef]
- Srinivas, P.; Gopu, S.; Krishna, V.M.; Kumar, M.A. A study of prevalence of urinary tract infection among children with febrile seizures in a tertiary care hospital. IOSR J. Dent. Med. Sci. 2017, 16, 46–48. [Google Scholar] [CrossRef]
- Bryan, C.S.; Reynolds, K.L. Community acquired bacteremic urinary tract infection: Epidemiology and Outcome. J. Urol. 1984, 132, 934. [Google Scholar] [CrossRef]
- Bagga, A.; Sharma, J. Urinary tract infections clinical features, evaluation and treatment. Pediatric Today 2000, 3, 395–401. [Google Scholar]
- Silva, A.C.; Oliveira, E.A. Update on the approach of urinary tract infection in childhood. J. Pediatric 2015, 91 (Suppl. 1), S2–S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okarska-Napierala, M.; Wasilewska, A.; Kuchar, E. Urinary tract infection in children: Diagnosis, treatment, imaging-comparison of current guidelines. J. Pediatric Urol. 2017, 13, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Simões, E.; Silva, A.C.; Oliveira, E.A.; Mak, R.H. Urinary tract infection in pediatrics: An overview. J. Pediatr. 2020, 96 (Suppl. 1), 65–79. [Google Scholar] [CrossRef]
- Larcombe, J. Urinary tract infection in children. Am. Fam. Physician 2010, 82, 1252. [Google Scholar] [PubMed]
- Larcombe, J. Urinary tract infection in children: Recurrent infections. BMJ Clin. Evid. 2015, 2015, 306. [Google Scholar]
- Schaffer, J.N.; Pearson, M.M. Proteus mirabilis and Urinary Tract Infections. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Schlager, T.A. Urinary tract infections in infants and children. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Hudson, A.; Romao, R.L.P.; MacLellan, D. Urinary tract infection in children. CMAJ 2017, 189, E608. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.E.; Mattoo, T.K. Update on childhood urinary tract infection and vesicoureteral reflux. Semin. Nephrol. 2009, 29, 349–359. [Google Scholar] [CrossRef]
- Jung, N.; Byun, H.J.; Park, J.H.; Kim, J.S.; Kim, H.W.; Ha, J.Y. Diagnostic accuracy of urinary biomarkers in infants younger than 3 months with urinary tract infection. Korean J. Pediatr. 2018, 61, 24–29. [Google Scholar] [CrossRef]
- Balighian, E.; Burke, M. Urinary Tract Infections in Children. Pediatr. Rev. 2018, 39, 3–12. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Xu, L.; Zhao, J. Prediction of acute pyelonephritis from urinary tract infection in children with fever using detection of CRP level: A diagnostic meta-analysis. Int. J. Clin. Exp. Med. 2018, 11, 2988–2999. [Google Scholar]
- Fretzayas, A.; Moustaki, M.; Gourgiotis, D.; Bossios, A.; Koukoutsakis, P.; Stavrinadis, C. Polymorphonuclear elastase as a diagnostic marker of acute pyelonephritis in children. Pediatrics 2000, 105, E28. [Google Scholar] [CrossRef] [Green Version]
- Leroy, S.; Fernandez-Lopez, A.; Nikfar, R.; Romanello, C.; Bouissou, F.; Gervaix, A.; Gurgoze, M.K.; Bressan, S.; Smolkin, V.; Tuerlinckx, D.; et al. Association of procalcitonin with acute pyelonephritis and renal scars in pediatric UTI. Pediatrics 2013, 131, 870–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecile, P.; Miorin, E.; Romanello, C.; Falleti, E.; Valent, F.; Giacomuzzi, F.; Tenore, A. Procalcitonin: A marker of severity of acute pyelonephritis among children. Pediatrics 2004, 114, e249–e254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari Gilani, K.; ModaresiEsfeh, J.; Gholamrezanezhad, A.; Gholami, A.; Mamishi, S.; Eftekhari, M.; Beiki, D.; Fard-Esfahani, A.; Fallahi, B.; Anvari, A. Predictors of abnormal renal cortical scintigraphy in children with first urinary tract infection: The importance of time factor. Int. Urol. Nephrol. 2010, 42, 1041–1047. [Google Scholar] [CrossRef]
- Kuil, S.D.; Hidad, S.; Fischer, J.C.; Harting, J.; Hertogh, C.M.; Prins, J.M.; van Leth, F.; de Jong, M.D.; Schneeberger, C. Sensitivity of point-of-care testing C reactive protein and procalcitonin to diagnose urinary tract infections in Dutch nursing homes: Progress study protocol. BMJ Open 2019, 9, e031269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaikh, N.; Borrell, J.L.; Evron, J.; Leeflang, M.M.G. Procalcitonin, C-reactive protein, and erythrocyte sedimentation rate for the diagnosis of acute pyelonephritis in children. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.H.; Park, S.J.; Lee, K.H.; Park, J.H.; Kronbichler, A.; Eisenhut, M.; Kim, J.H.; Lee, J.W.; Shin, J.I. The value of delta neutrophil index in young infants with febrile urinary tract infection. Sci. Rep. 2017, 7, 41265. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Choi, S.A.; Kim, S.Y.; Kim, H.; Lim, B.C.; Hwang, H.; Chae, J.H.; Kim, K.J.; Oh, S.; Kim, E.Y.; et al. Association analysis of interleukin-1β, interleukin-6, and HMGB1 variants with postictal serum cytokine levels in children with febrile seizure and generalized epilepsy with febrile seizure plus. J. Clin. Neurol. 2019, 15, 555–563. [Google Scholar] [CrossRef]
- Kwon, A.; Kwak, B.O.; Kim, K.; Ha, J.; Kim, S.J.; Bae, S.H.; Son, J.S.; Kim, S.N.; Lee, R. Cytokine levels in febrile seizure patients: A systematic review and meta-analysis. Seizure 2018, 59, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Maniu, I.; Costea, R.; Maniu, G.; Neamtu, B.M. Inflammatory Biomarkers in Febrile Seizure: A Comprehensive Bibliometric, Review and Visualization Analysis. Brain Sci. 2021, 11, 1077. [Google Scholar] [CrossRef]
- Surbatovic, M.; Popovic, N.; Vojvodic, D.; Milosevic, I.; Acimovic, G.; Stojicic, M.; Veljovic, M.; Jevdjic, J.; Djordjevic, D.; Radakovic, S. Cytokine profile in severe Gram-positive and Gram-negative abdominal sepsis. Sci. Rep. 2015, 5, 11355. [Google Scholar] [CrossRef] [PubMed]
- Rini, T.Y.; Abadi, S.; Katu, S.; Bakri, S.; Rasyid, H.; Kasim, H.; Fachruddin, A.; Halim, R.; Seweng, A. Association of bacterial/viral infections with neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio, and platelet-lymphocyte ratio in patients presenting with fever. Eur. J. Mol. Clin. Med. 2020, 7, 1500–1509. [Google Scholar]
- Heffernan, A.J.; Denny, K.J. Host diagnostic biomarkers of infection in the ICU: Where are we and where are we going? Curr. Infect. Dis. Rep. 2021, 23, 4. [Google Scholar] [CrossRef]
- Ji, T.; Yang, Z.; Liu, Q.; Liao, J.; Yin, F.; Chen, Y.; Zou, L.; Li, B.; Gao, Y.; Shu, X.; et al. Vagus nerve stimulation for pediatric patients with intractable epilepsy between 3 and 6 years of age: Study protocol for a double-blind, randomized control trial. Trials 2019, 20, 44. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Carbonell, L.; Faulkner, H.; Higgins, S.; Koutroumanidis, M.; Leschziner, G. Vagus nerve stimulation for drug-resistant epilepsy. Pract. Neurol. 2020, 20, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Elvira, R.; Oltra-Cucarella, J.; Carrobles, J.A.; Teodoru, M.; Bacila, C.; Neamtu, B. Individual alpha peak frequency, an important biomarker for live z-score training neurofeedback in adolescents with learning disabilities. Brain Sci. 2021, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Elvira, R.; Oltra-Cucarella, J.; Carrobles, J.A.; Moltó, J.; Flórez, M.; Parra, S.; Agudo, M.; Saez, C.; Guarino, S.; Costea, R.M.; et al. Enhancing the effects of neurofeedback training: The motivational value of the reinforcers. Brain Sci. 2021, 11, 457. [Google Scholar] [CrossRef]
- Walker, J.E.; Kozlowski, G.P. Neurofeedback treatment of epilepsy. Child Adolesc. Psychiatr. Clin. 2005, 14, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.; Imran, I.; Pervaiz, H.; Ashraf, W.; Perveen, N.; Rasool, M.F.; Alasmari, A.F.; Alharbi, M.; Samad, N.; Alqarni, S.A.; et al. Non-pharmacological interventions for intractable epilepsy. Saudi Pharm. J. 2020, 28, 951–962. [Google Scholar] [CrossRef] [PubMed]
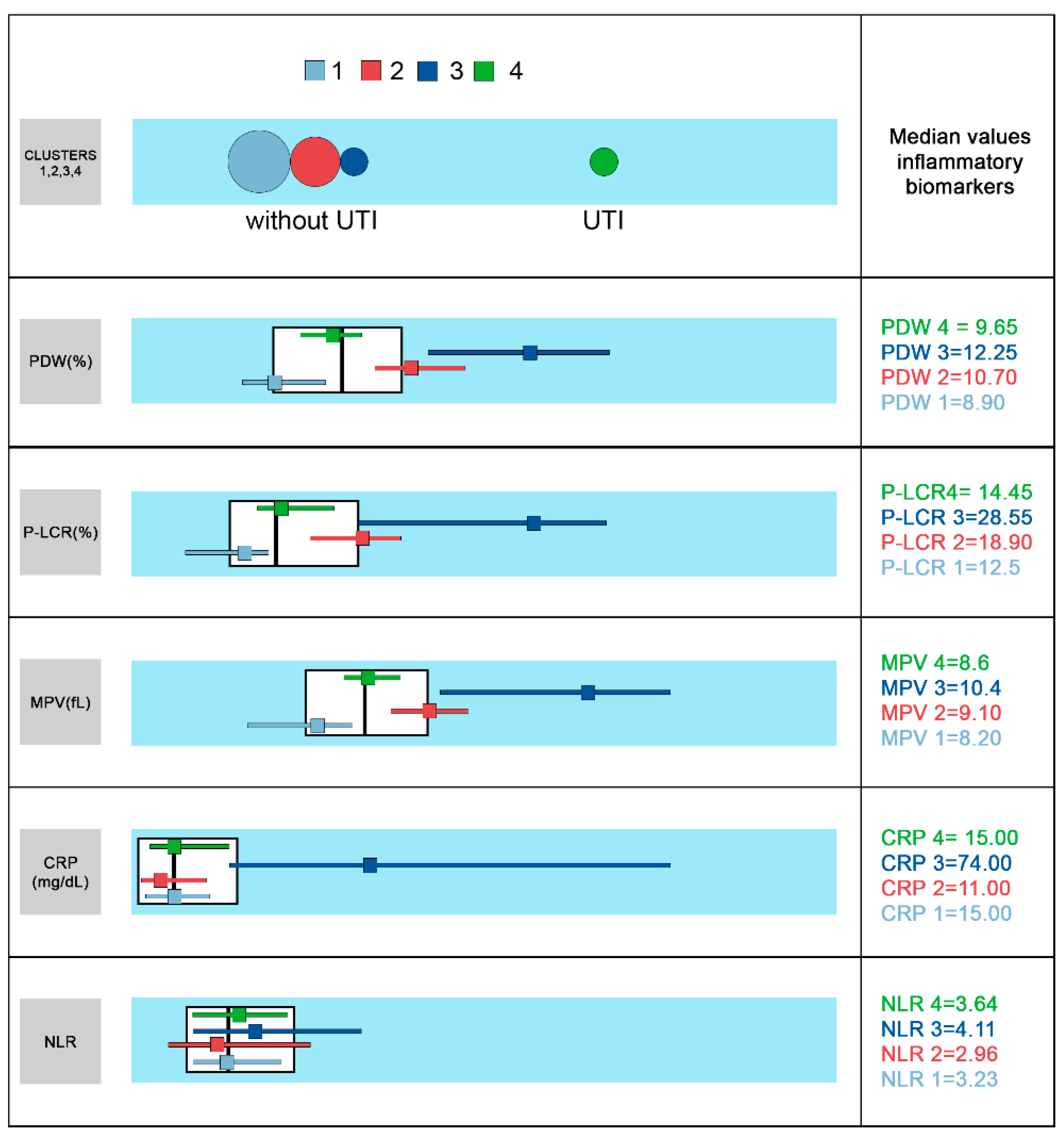
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | p | |
|---|---|---|---|---|---|
| CRP | 22.87 ± 26.24 | 18.13 ± 0.02 | 92.36 ± 0.35 | 21.29 ± 0.90 | 0.000 |
| (2.00–114.00) | (4.00–75.00) | (4.00–209.00) | (4.00–52.00) | ||
| 15.00 | 11.00 | 74.00 | 15.00 | ||
| (5.00–26.00) | (5.00–26.00) | (26.00–171.00) | (7.00–32.00) | ||
| PDW | 8.97 ± 0.70 | 10.91 ± 2.97 | 12.31 ± 1.01 | 9.51 ± 8.79 | 0.000 |
| (7.00–10.20) | (9.20–13.20) | (9.10–16.30) | (7.80–10.70) | ||
| 8.90 | 10.70 | 12.25 | 9.65 | ||
| (8.50–9.60) | (10.20–11.40) | (10.70–13.40) | (9.20–10.10) | ||
| P-LCR | 11.85 ± 2.91 | 19.13 ± 8.70 | 27.21 ± 9.26 | 14.79 ± 6.85 | 0.000 |
| (6.20–20.50) | (11.80–27.20) | (13.90–44.10) | (6.60–21.50) | ||
| 12.50 | 18.90 | 28.55 | 14.45 | ||
| (9.70–13.80) | (15.90–20.80) | (16.30–32.80) | (12.80–17.70) | ||
| MPV | 8.06 ± 0.54 | 9.14 ± 0.52 | 10.24 ± 1.30 | 8.59 ± 0.68 | 0.000 |
| (6.70–9.20) | (8.20–10.40) | (8.10–12.40) | (7.10–9.70) | ||
| 8.20 | 9.10 | 10.40 | 8.60 | ||
| (7.60–8.50) | (8.80–9.40) | (8.90–11.20) | (8.40–8.90) | ||
| PLT | 345.06 ± 0.75 | 326.95 ± 0.78 | 271.36 ± 0.00 | 395.29 ± 0.77 | 0.042 |
| (111.00–650.00) | (106.00–665.00) | (120.00–655.00) | (210.00–716.00) | ||
| 322.00 | 284.00 | 218.50 | 331.00 | ||
| (277.00–411.00) | (247.00–393.00) | (170.00–304.00) | (261.00–495.00) | ||
| NLR | 4.18 ± 5.69 | 4.03 ± 2.33 | 5.77 ± 6.75 | 4.07 ± 1.80 | 0.462 |
| (0.56–22.75) | (0.27–13.64) | (0.60–22.35) | (0.14–10.71) | ||
| 3.23 | 2.96 | 4.11 | 3.64 | ||
| (1.79–5.03) | (1.44–6.47) | (2.11–7.58) | (2.03–5.37) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costea, R.M.; Maniu, I.; Dobrota, L.; Pérez-Elvira, R.; Agudo, M.; Oltra-Cucarella, J.; Dragomir, A.; Bacilă, C.; Banciu, A.; Banciu, D.D.; et al. Exploring Inflammatory Status in Febrile Seizures Associated with Urinary Tract Infections: A Two-Step Cluster Approach. Brain Sci. 2021, 11, 1168. https://doi.org/10.3390/brainsci11091168
Costea RM, Maniu I, Dobrota L, Pérez-Elvira R, Agudo M, Oltra-Cucarella J, Dragomir A, Bacilă C, Banciu A, Banciu DD, et al. Exploring Inflammatory Status in Febrile Seizures Associated with Urinary Tract Infections: A Two-Step Cluster Approach. Brain Sciences. 2021; 11(9):1168. https://doi.org/10.3390/brainsci11091168
Chicago/Turabian StyleCostea, Raluca Maria, Ionela Maniu, Luminita Dobrota, Rubén Pérez-Elvira, Maria Agudo, Javier Oltra-Cucarella, Andrei Dragomir, Ciprian Bacilă, Adela Banciu, Daniel Dumitru Banciu, and et al. 2021. "Exploring Inflammatory Status in Febrile Seizures Associated with Urinary Tract Infections: A Two-Step Cluster Approach" Brain Sciences 11, no. 9: 1168. https://doi.org/10.3390/brainsci11091168






