Intranasal Oxytocin and Pain Reduction: Testing a Social Cognitive Mediation Model
Abstract
:1. Introduction
1.1. Oxytocin and Pain
1.2. Mechanisms
1.3. Life History Considerations
1.4. Biological and Physiological Considerations
1.5. A Conceptual Framework
2. Material and Methods
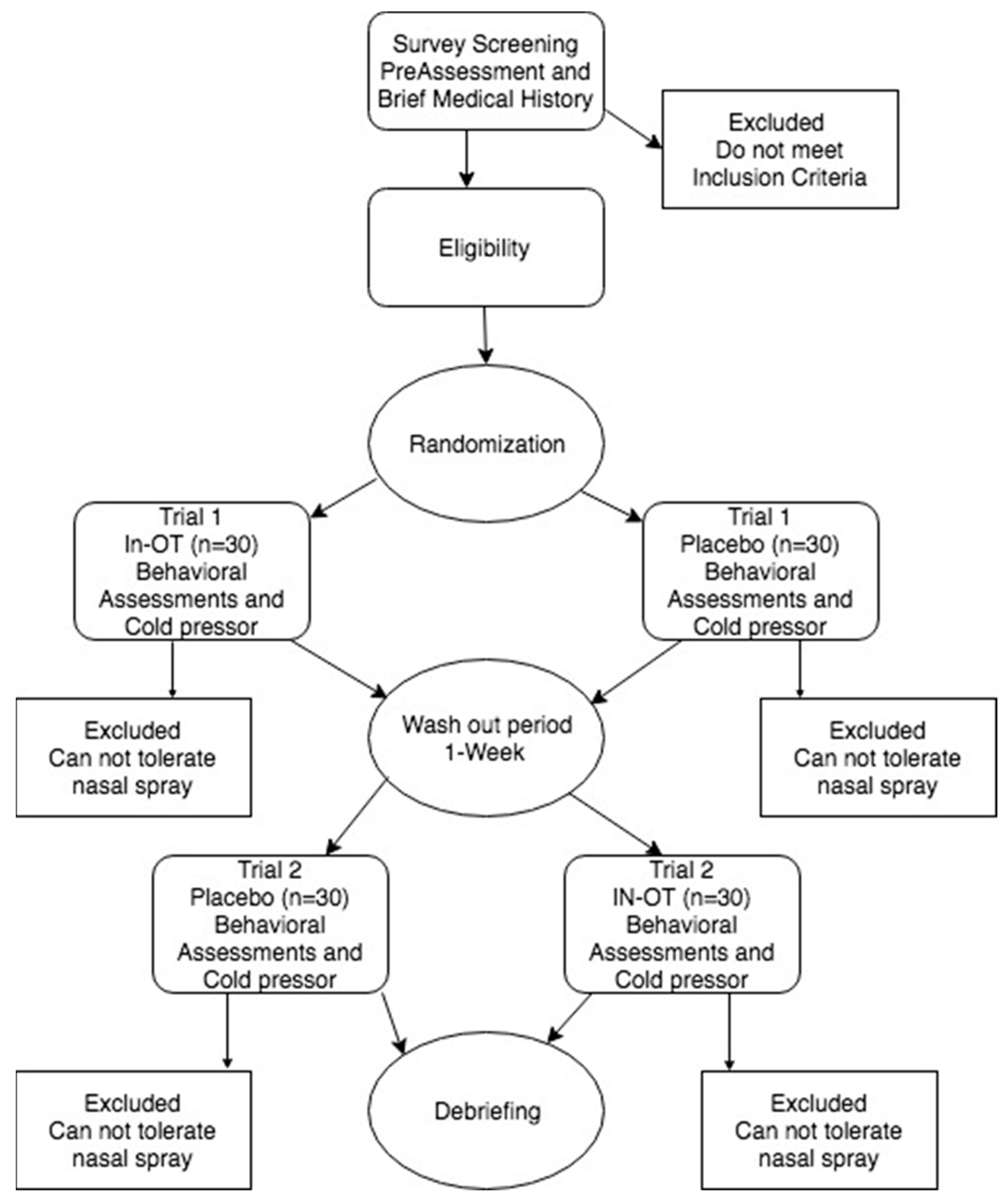
2.1. Subject Sample and Procedure
2.2. Laboratory Testing
2.3. Intranasal Oxytocin Administration
2.4. Measurements
2.5. Barratt Impulsiveness Scale-11 (BIS-11)
2.6. Psychological Safety Scale
2.7. The Interpersonal Trust Scale (ITS)
2.8. Trust Game Dilemmas
2.9. Parent Love-Withdrawal Scale
2.10. Expectancy Bias Scale
2.11. The Visual Analog Scale
2.12. Beck Anxiety Inventory (BAI)
2.13. Delayed Discounting Measure (DDM)
2.14. Loss Aversion Scale (LAS)
2.15. Chronic Pain Grade Scale (CPGS)
2.16. Cold Pressor Task
2.17. Urinary Oxytocin Extraction
2.18. Urinary Oxytocin and Creatinine Analysis
2.19. DNA Extraction
2.20. SNP Genotyping
2.21. Study Design
3. Results
3.1. Data Exploration
3.2. Main Effects
3.3. OT and Social Cognitions (H1−3)
3.4. Analysis of Covariance Mean Difference Test for Cooperation by Condition with Controls (n = 43)
3.5. Frequency of Trust Decisions Made by Gender and Condition, n = 43
3.6. Mediations (H4)
3.7. Decision Making and OT (H5)
3.8. Post Hoc Analysis
3.9. Exploratory Genetics
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, A. Oxytocin and Human Social Behavior. Personal. Soc. Psychol. Rev. 2010, 14, 281–295. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.; MacDonald, T.M. The Peptide That Binds. Harv. Rev. Psychiatry 2010, 18, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Uvnäs-Moberg, K.; Ekström-Bergström, A.; Berg, M.; Buckley, S.; Pajalic, Z.; Hadjigeorgiou, E.; Kotłowska, A.; Lengler, L.; Kielbratowska, B.; Leon-Larios, F.; et al. Maternal plasma levels of oxytocin during physiological childbirth—A systematic review with implications for uterine contractions and central actions of oxytocin. BMC Pregnancy Childbirth 2019, 19, 285. [Google Scholar] [CrossRef] [PubMed]
- Roels, R.; Rehman, U.S.; Carter, C.S.; Nazarloo, H.P.; Janssen, E. The link between oxytocin plasma levels and observed communication behaviors during sexual and nonsexual couple discussions: An exploratory study. Psychoneuroendocrinology 2021, 129, 105265. [Google Scholar] [CrossRef]
- Walter, M.H.; Abele, H.; Plappert, C.F. The Role of Oxytocin and the Effect of Stress During Childbirth: Neurobiological Basics and Implications for Mother and Child. Front. Endocrinol. 2021, 12, 742236. [Google Scholar] [CrossRef]
- Carter, C.S. Sex, love and oxytocin: Two metaphors and a molecule. Neurosci. Biobehav. Rev. 2022, 143, 104948. [Google Scholar] [CrossRef] [PubMed]
- Nersesyan, Y.; Demirkhanyan, L.; Cabezas-Bratesco, D.; Oakes, V.; Kusuda, R.; Dawson, T.; Sun, X.; Cao, C.; Cohen, A.M.; Chelluboina, B.; et al. Oxytocin Modulates Nociception as an Agonist of Pain-Sensing TRPV1. Cell Rep. 2017, 21, 1681–1691. [Google Scholar] [CrossRef]
- Kingsbury, M.A.; Bilbo, S.D. The inflammatory event of birth: How oxytocin signaling may guide the development of the brain and gastrointestinal system. Front. Neuroendocrinol. 2019, 55, 100794. [Google Scholar] [CrossRef]
- Hilfiger, L.; Zhao, Q.; Kerspern, D.; Inquimbert, P.; Andry, V.; Goumon, Y.; Darbon, P.; Hibert, M.; Charlet, A. A Nonpeptide Oxytocin Receptor Agonist for a Durable Relief of Inflammatory Pain. Sci. Rep. 2020, 10, 3017. [Google Scholar] [CrossRef]
- Nishimura, H.; Yoshimura, M.; Shimizu, M.; Sanada, K.; Sonoda, S.; Nishimura, K.; Baba, K.; Ikeda, N.; Motojima, Y.; Maruyama, T.; et al. Endogenous oxytocin exerts anti-nociceptive and anti-inflammatory effects in rats. Commun. Biol. 2022, 5, 907. [Google Scholar] [CrossRef]
- Boll, S.; Almeida de Minas, A.C.; Raftogianni, A.; Herpertz, S.C.; Grinevich, V. Oxytocin and Pain Perception: From Animal Models to Human Research. Neuroscience 2018, 387, 149–161. [Google Scholar] [CrossRef]
- Losin, E.A.R.; Anderson, S.R.; Wager, T.D. Feelings of Clinician-Patient Similarity and Trust Influence Pain: Evidence from Simulated Clinical Interactions. J. Pain 2017, 18, 787–799. [Google Scholar] [CrossRef]
- Ito, E.; Shima, R.; Yoshioka, T. A novel role of oxytocin: Oxytocin-induced well-being in humans. Biophys. Physicobiol. 2019, 16, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Osório, F.d.L. Effects of intranasal oxytocin on pain perception among human subjects: A systematic literature review and meta-analysis. Horm. Behav. 2023, 147, 105282. [Google Scholar] [CrossRef] [PubMed]
- You, D.S.; Haney, R.; Albu, S.; Meagher, M.W. Generalized Pain Sensitization and Endogenous Oxytocin in Individuals with Symptoms of Migraine: A Cross-Sectional Study. Headache J. Head Face Pain 2018, 58, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Bartz, J.A.; Zaki, J.; Bolger, N.; Ochsner, K.N. Social effects of oxytocin in humans: Context and person matter. Trends Cogn. Sci. 2011, 15, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.H.; Guastella, A.J. The Role of Oxytocin in Human Affect. Curr. Dir. Psychol. Sci. 2011, 20, 222–231. [Google Scholar] [CrossRef]
- Harari-Dahan, O.; Bernstein, A. A general approach-avoidance hypothesis of Oxytocin: Accounting for social and non-social effects of oxytocin. Neurosci. Biobehav. Rev. 2014, 47, 506–519. [Google Scholar] [CrossRef]
- Rash, J.A.; Aguirre-Camacho, A.; Campbell, T.S. Oxytocin and pain: A systematic review and synthesis of findings. Clin. J. Pain 2014, 30, 453–462. [Google Scholar] [CrossRef]
- Moberg, K.U.; Handlin, L.; Petersson, M. Neuroendocrine mechanisms involved in the physiological effects caused by skin-to-skin contact—With a particular focus on the oxytocinergic system. Infant Behav. Dev. 2020, 61, 101482. [Google Scholar] [CrossRef]
- Freeman, H.; Scholl, J.L.; AnisAbdellatif, M.; Gnimpieba, E.; Forster, G.L.; Jacob, S. I only have eyes for you: Oxytocin administration supports romantic attachment formation through diminished interest in close others and strangers. Psychoneuroendocrinology 2021, 134, 105415. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Herrera-Melendez, A.L.; Pestke, K.; Feeser, M.; Aust, S.; Otte, C.; Pruessner, J.C.; Böker, H.; Bajbouj, M.; Grimm, S. Early life stress modulates amygdala-prefrontal functional connectivity: Implications for oxytocin effects. Hum. Brain Mapp. 2014, 35, 5328–5339. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Kim, Y.-K. Possible oxytocin-related biomarkers in anxiety and mood disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 116, 110531. [Google Scholar] [CrossRef] [PubMed]
- Kreuder, A.-K.; Wassermann, L.; Wollseifer, M.; Ditzen, B.; Eckstein, M.; Stoffel-Wagner, B.; Hennig, J.; Hurlemann, R.; Scheele, D. Oxytocin enhances the pain-relieving effects of social support in romantic couples. Hum. Brain Mapp. 2019, 40, 242–251. [Google Scholar] [CrossRef]
- van Ijzendoorn, M.H.; Huffmeijer, R.; Alink, L.R.A.; Bakermans-Kranenburg, M.J.; Tops, M. The Impact of Oxytocin Administration on Charitable Donating Is Moderated by Experiences of Parental Love-Withdrawal. Front. Psychol. 2011, 2, 258. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, R.J.; McInnis, O.A.; Stead, J.D.; Matheson, K.; Anisman, H. A paradoxical association of an oxytocin receptor gene polymorphism: Early-life adversity and vulnerability to depression. Front. Neurosci. 2013, 7, 128. [Google Scholar] [CrossRef]
- Feng, C.; Lori, A.; Waldman, I.D.; Binder, E.B.; Haroon, E.; Rilling, J.K. A common oxytocin receptor gene (OXTR) polymorphism modulates intranasal oxytocin effects on the neural response to social cooperation in humans. Genes Brain Behav. 2015, 14, 516–525. [Google Scholar] [CrossRef]
- Kosaka, H.; Okamoto, Y.; Munesue, T.; Yamasue, H.; Inohara, K.; Fujioka, T.; Anme, T.; Orisaka, M.; Ishitobi, M.; Jung, M. Oxytocin efficacy is modulated by dosage and oxytocin receptor genotype in young adults with high-functioning autism: A 24-week randomized clinical trial. Transl. Psychiatry 2016, 6, e872. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Su, Y. The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. J. Affect. Disord. 2012, 138, 468–472. [Google Scholar] [CrossRef]
- Feldman, R.; Zagoory-Sharon, O.; Weisman, O.; Schneiderman, I.; Gordon, I.; Maoz, R.; Shalev, I.; Ebstein, R.P. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol. Psychiatry 2012, 72, 175–181. [Google Scholar] [CrossRef]
- Wang, C.; Gao, J.; Ma, Y.; Zhu, C.; Dong, X.W. Physical pain increases interpersonal trust in females. Eur. J. Pain 2018, 22, 150–160. [Google Scholar] [CrossRef]
- Hamel, A.; Bastien, C.; Jacques, C.; Moreau, A.; Giroux, I. Sleep or Play Online Poker?: Gambling Behaviors and Tilt Symptoms While Sleep Deprived. Front. Psychiatry 2021, 11, 600092. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, V.A.; Sosa, Y.; Krauss, B.R.; Thomas, S.P.; Fredrickson, B.E.; Levy, R.E.; Harden, N.R.; Chialvo, D.R. Chronic pain patients are impaired on an emotional decision-making task. Pain 2004, 108, 129–136. [Google Scholar] [CrossRef]
- Timm, A.; Schmidt-Wilcke, T.; Blenk, S.; Studer, B. Altered social decision making in patients with chronic pain. Psychol. Med. 2023, 53, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Ma, Q. Nociceptors—Noxious Stimulus Detectors. Neuron 2007, 55, 353–364. [Google Scholar] [CrossRef]
- Pontén, M.; Fust, J.; D’Onofrio, P.; Dorp, R.V.; Sunnergård, L.; Ingre, M.; Axelsson, J.; Jensen, K. The pain alarm response—An example of how conscious awareness shapes pain perception. Sci. Rep. 2019, 9, 12478. [Google Scholar] [CrossRef]
- Carson, D.S.; Guastella, A.J.; Taylor, E.R.; McGregor, I.S. A brief history of oxytocin and its role in modulating psychostimulant effects. J. Psychopharmacol. 2013, 27, 231–247. [Google Scholar] [CrossRef]
- Wilson, A.D.; Golonka, S. Embodied Cognition Is Not What You Think It Is. Front. Psychol. 2013, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Frith, C.D.; Singer, T. The role of social cognition in decision making. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 3875–3886. [Google Scholar] [CrossRef]
- Downey, H.; Haynes, J.M.; Johnson, H.M.; Odum, A.L. Deprivation Has Inconsistent Effects on Delay Discounting: A Review. Front. Behav. Neurosci. 2022, 16, 787322. [Google Scholar] [CrossRef]
- Wallace, L.S.; Wexler, R.K.; McDougle, L.; Miser, W.F.; Haddox, J.D. Voices that may not otherwise be heard: A qualitative exploration into the perspectives of primary care patients living with chronic pain. J. Pain Res. 2014, 7, 291–299. [Google Scholar] [CrossRef]
- Walteros, C.; Sánchez-Navarro, J.P.; Muñoz, M.A.; Martínez-Selva, J.M.; Chialvo, D.; Montoya, P. Altered associative learning and emotional decision making in fibromyalgia. J. Psychosom. Res. 2011, 70, 294–301. [Google Scholar] [CrossRef]
- Berger, S.E.; Baria, A.T.; Baliki, M.N.; Mansour, A.; Herrmann, K.M.; Torbey, S.; Huang, L.; Parks, E.L.; Schnizter, T.J.; Apkarian, A.V. Risky monetary behavior in chronic back pain is associated with altered modular connectivity of the nucleus accumbens. BMC Res. Notes 2014, 7, 739. [Google Scholar] [CrossRef]
- Hess, L.E.; Haimovici, A.; Muñoz, M.A.; Montoya, P. Beyond pain: Modeling decision-making deficits in chronic pain. Front. Behav. Neurosci. 2014, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, R. Effective Choice in the Prisoner’s Dilemma. J. Confl. Resolut. 1980, 24, 3–25. [Google Scholar] [CrossRef]
- Berg, J.; Dickhaut, J.; McCabe, K. Trust, Reciprocity, and Social History. Games Econ. Behav. 1995, 10, 122–142. [Google Scholar] [CrossRef]
- Gächter, S.; Herrmann, B.; Thöni, C. Trust, voluntary cooperation, and socio-economic background: Survey and experimental evidence. J. Econ. Behav. Organ. 2004, 55, 505–531. [Google Scholar] [CrossRef]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676. [Google Scholar] [CrossRef]
- Gereke, J.; Schaub, M.; Baldassarri, D. Ethnic diversity, poverty and social trust in Germany: Evidence from a behavioral measure of trust. PLoS ONE 2018, 13, e0199834. [Google Scholar] [CrossRef]
- Kleinert, T.; Schiller, B.; Fischbacher, U.; Grigutsch, L.-A.; Koranyi, N.; Rothermund, K.; Heinrichs, M. The Trust Game for Couples (TGC): A new standardized paradigm to assess trust in romantic relationships. PLoS ONE 2020, 15, e0230776. [Google Scholar] [CrossRef] [PubMed]
- Eckel, C.C.; Wilson, R.K. Is trust a risky decision? J. Econ. Behav. Organ. 2004, 55, 447–465. [Google Scholar] [CrossRef]
- Mischkowski, D.; Crocker, J.; Way, B.M. From painkiller to empathy killer: Acetaminophen (paracetamol) reduces empathy for pain. Soc. Cogn. Affect. Neurosci. 2016, 11, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Zunhammer, M.; Geis, S.; Busch, V.; Greenlee, M.W.; Eichhammer, P. Effects of intranasal oxytocin on thermal pain in healthy men: A randomized functional magnetic resonance imaging study. Psychosom. Med. 2015, 77, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the barratt impulsiveness scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef]
- Edmondson, A. Psychological Safety and Learning Behavior in Work Teams. Adm. Sci. Q. 1999, 44, 350–383. [Google Scholar] [CrossRef]
- Rotter, J.B. A new scale for the measurement of interpersonal trust1. J. Personal. 1967, 35, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D.; Tversky, A. Prospect Theory: An Analysis of Decision under Risk. Econometrica 1979, 47, 263. [Google Scholar] [CrossRef]
- Kirby, K.N.; Maraković, N.N. Modeling Myopic Decisions: Evidence for Hyperbolic Delay-Discounting within Subjects and Amounts. Organ. Behav. Hum. Decis. Process. 1995, 64, 22–30. [Google Scholar] [CrossRef]
- Stanford, M.S.; Mathias, C.W.; Dougherty, D.M.; Lake, S.L.; Anderson, N.E.; Patton, J.H. Fifty years of the Barratt Impulsiveness Scale: An update and review. Personal. Individ. Differ. 2009, 47, 385–395. [Google Scholar] [CrossRef]
- Schlenker, B.R.; Helm, B.; Tedeschi, J.T. The effects of personality and situational variables on behavioral trust. J. Personal. Soc. Psychol. 1973, 25, 419–427. [Google Scholar] [CrossRef]
- Tucker, A.W. The Mathematics of Tucker: A Sampler. Two-Year Coll. Math. J. 1983, 14, 228. [Google Scholar] [CrossRef]
- Galbally, M.; Lewis, A.J.; van Ijzendoorn, M.; Permezel, M. The Role of Oxytocin in Mother-Infant Relations: A Systematic Review of Human Studies. Harv. Rev. Psychiatry 2011, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.D.; Duncan, B.; Brown, J.; Sparks, J.; Claud, D. The outcome rating scale: A preliminary study of the reliability, validity, and feasibility of a brief visual analog measure. J. Brief Ther. 2003, 2, 91–100. [Google Scholar]
- Fydrich, T.; Dowdall, D.; Chambless, D.L. Reliability and validity of the beck anxiety inventory. J. Anxiety Disord. 1992, 6, 55–61. [Google Scholar] [CrossRef]
- Von Korff, M.; Ormel, J.; Keefe, F.J.; Dworkin, S.F. Grading the severity of chronic pain. Pain 1992, 50, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.; Pollard, B.; Johnston, M. What does the chronic pain grade questionnaire measure? Pain 2007, 130, 249–253. [Google Scholar] [CrossRef]
- Wolf, S.; Hardy, J.D. Studies on pain. Observations on pain due to local cooling and on factors involved in the “cold pressor” effect. J. Clin. Investig. 1941, 20, 521–533. [Google Scholar] [CrossRef]
- Crockford, C.; Wittig, R.M.; Langergraber, K.; Ziegler, T.E.; Zuberbühler, K.; Deschner, T. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc. Biol. Sci. 2013, 280, 20122765. [Google Scholar] [CrossRef] [PubMed]
- Finkenwirth, C.; van Schaik, C.; Ziegler, T.E.; Burkart, J.M. Strongly bonded family members in common marmosets show synchronized fluctuations in oxytocin. Physiol. Behav. 2015, 151, 246–251. [Google Scholar] [CrossRef]
- Reyes, T.L.; Galinsky, A.M.; Hoffmann, J.N.; You, H.M.; Ziegler, T.E.; McClintock, M.K. Social peptides: Measuring urinary oxytocin and vasopressin in a home field study of older adults at risk for dehydration. J. Gerontol. B Psychol. Sci. Soc. Sci. 2014, 69 (Suppl. S2), S229–S237. [Google Scholar] [CrossRef]
- Seltzer, L.J.; Ziegler, T.E.; Pollak, S.D. Social vocalizations can release oxytocin in humans. Proc. Biol. Sci. 2010, 277, 2661–2666. [Google Scholar] [CrossRef]
- Seltzer, L.J.; Ziegler, T.; Connolly, M.J.; Prososki, A.R.; Pollak, S.D. Stress-induced elevation of oxytocin in maltreated children: Evolution, neurodevelopment, and social behavior. Child Dev. 2014, 85, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.E. Measuring peripheral oxytocin and vasopressin in nonhuman primates. Am. J. Primatol. 2018, 80, e22871. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.E.; Scheffler, G.; Snowdon, C.T. The Relationship of Cortisol Levels to Social Environment and Reproductive Functioning in Female Cotton-Top Tamarins, Saguinus oedipus. Horm. Behav. 1995, 29, 407–424. [Google Scholar] [CrossRef]
- De Dreu, C.K.W.; Greer, L.L.; Van Kleef, G.A.; Shalvi, S.; Handgraaf, M.J.J. Oxytocin promotes human ethnocentrism. Proc. Natl. Acad. Sci. USA 2011, 108, 1262–1266. [Google Scholar] [CrossRef] [PubMed]
- DeWall, C.N.; Gillath, O.; Pressman, S.D.; Black, L.L.; Bartz, J.A.; Moskovitz, J.; Stetler, D.A. When the Love Hormone Leads to Violence. Soc. Psychol. Personal. Sci. 2014, 5, 691–697. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Domes, G.; Kirsch, P.; Heinrichs, M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011, 12, 524–538. [Google Scholar] [CrossRef]
- Yong, E. Dark side of the love hormone. New Sci. 2012, 213, 39–41. [Google Scholar] [CrossRef]
- Zhang, H.; Gross, J.; De Dreu, C.; Ma, Y. Oxytocin promotes coordinated out-group attack during intergroup conflict in humans. eLife 2019, 8, e40698. [Google Scholar] [CrossRef]
- Scheele, D.; Striepens, N.; Güntürkün, O.; Deutschländer, S.; Maier, W.; Kendrick, K.M.; Hurlemann, R. Oxytocin modulates social distance between males and females. J. Neurosci. 2012, 32, 16074–16079. [Google Scholar] [CrossRef]
- Eckstein, M.; Markett, S.; Kendrick, K.M.; Ditzen, B.; Liu, F.; Hurlemann, R.; Becker, B. Oxytocin differentially alters resting state functional connectivity between amygdala subregions and emotional control networks: Inverse correlation with depressive traits. NeuroImage 2017, 149, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Steinman, M.Q.; Duque-Wilckens, N.; Trainor, B.C. Complementary Neural Circuits for Divergent Effects of Oxytocin: Social Approach Versus Social Anxiety. Biol. Psychiatry 2019, 85, 792–801. [Google Scholar] [CrossRef] [PubMed]


| Min | Max | Mean | SD | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Descriptives | ||||||||||||||||
| 1. Impulsivity | 1.54 | 3.02 | 2.17 | 0.37 | – | |||||||||||
| 2. Chronic Pain | 1.00 | 5.29 | 2.45 | 1.09 | 0.06 | – | ||||||||||
| 3. Anxiety | 0.00 | 44.00 | 15.07 | 11.99 | 0.48 ** | 0.15 | – | |||||||||
| 4. Expectations | 1.50 | 5.00 | 3.64 | 0.62 | −0.28 ** | 0.22 | −0.31 * | – | ||||||||
| 5. Parent History | 1.14 | 4.00 | 2.03 | 0.68 | 0.30 ** | −0.10 | 0.48 ** | −0.35 * | – | |||||||
| Baseline Descriptives | ||||||||||||||||
| 6. Cooperation | 44.62 | 90.11 | 61.71 | 10.87 | −0.16 | 0.13 | −0.13 | 0.16 | −0.19 * | – | ||||||
| 7. Safety | 15.00 | 29.00 | 23.04 | 3.67 | −0.32 ** | 0.18 * | −0.27 ** | 0.17 * | −0.17 | −0.01 | – | |||||
| 8. Trust | 1.92 | 3.28 | 2.54 | 0.35 | −0.19 * | −0.03 | −0.06 | −0.003 | −0.08 | 0.01 | 0.14 | – | ||||
| 9. Delayed Discounting | 0.00 | 0.03 | 0.02 | 0.01 | 0.08 | 0.10 | 0.09 | 0.08 | 0.05 | 0.08 | 0.02 | −0.08 | – | |||
| 10. Loss Aversion | 0.00 | 2.20 | 0.77 | 0.84 | 0.07 | −0.08 | 0.15 | −0.09 | 0.09 | −0.03 | −0.11 | 0.03 | 0.11 | – | ||
| Treatment | ||||||||||||||||
| 11. Oxytocin | 1.60 | 9.60 | 5.23 | 1.67 | 0.22 | 0.08 | −0.11 | 0.38 * | −0.24 | −0.12 | 0.12 | −0.21 | 0.10 | 0.15 | – | |
| 12. Placebo | 1.00 | 9.80 | 5.24 | 1.91 | 0.358 | 0.06 | −0.01 | 0.21 | −0.21 | 0.03 | 0.23 | 0.12 | 0.03 | −0.06 | 0.62 ** | – |
| Within Subjects Effects | |||||
|---|---|---|---|---|---|
| SS | df | MS | F | p | |
| Cooperation | 286.282 | 1 | 286.282 | 6.124 | 0.018 |
| Cooperation * Baseline | 180.278 | 1 | 180.278 | 3.856 | 0.053 |
| Cooperation * Parent-Love History | 161.369 | 1 | 161.369 | 3.452 | 0.071 |
| Cooperation * Procedural Order | 18.525 | 1 | 18.525 | 0.390 | 0.535 |
| Residual | 1869.896 | 40 | 46.747 | ||
| Count | Condition | ||
|---|---|---|---|
| OT | PB | ||
| Males | Trust | 9 | 13 |
| Do not Trust | 6 | 2 | |
| Females | Trust | 24 | 23 |
| Do not Trust | 4 | 5 | |
| Pearson Correlations | |||||
|---|---|---|---|---|---|
| GMPain | GMImpuls | GMExFunc | Cooperation | ||
| GMPain | Pearson’s r | — | |||
| p-value | — | ||||
| GMImpuls | Pearson’s r | −0.143 | — | ||
| p-value | 0.362 | — | |||
| GMExFunc | Pearson’s r | 0.681 *** | −0.064 | — | |
| p-value | <0.001 | 0.681 | — | ||
| Cooperation | Pearson’s r | −0.316 * | 0.314 * | −0.330 * | — |
| p-value | 0.039 | 0.040 | 0.031 | — | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, P.; Scholl, J.L.; Wang, X.; Kallsen, N.A.; Ehli, E.A.; Freeman, H. Intranasal Oxytocin and Pain Reduction: Testing a Social Cognitive Mediation Model. Brain Sci. 2023, 13, 1689. https://doi.org/10.3390/brainsci13121689
Long P, Scholl JL, Wang X, Kallsen NA, Ehli EA, Freeman H. Intranasal Oxytocin and Pain Reduction: Testing a Social Cognitive Mediation Model. Brain Sciences. 2023; 13(12):1689. https://doi.org/10.3390/brainsci13121689
Chicago/Turabian StyleLong, Preston, Jamie L. Scholl, Xiaotian Wang, Noah A. Kallsen, Erik A. Ehli, and Harry Freeman. 2023. "Intranasal Oxytocin and Pain Reduction: Testing a Social Cognitive Mediation Model" Brain Sciences 13, no. 12: 1689. https://doi.org/10.3390/brainsci13121689





