Analysis of Functional Neuroplastic Changes in the Cortical Language System in Relation to Different Growth Patterns of Glioblastoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Image Acquitision
2.3. Data Analysis
3. Results
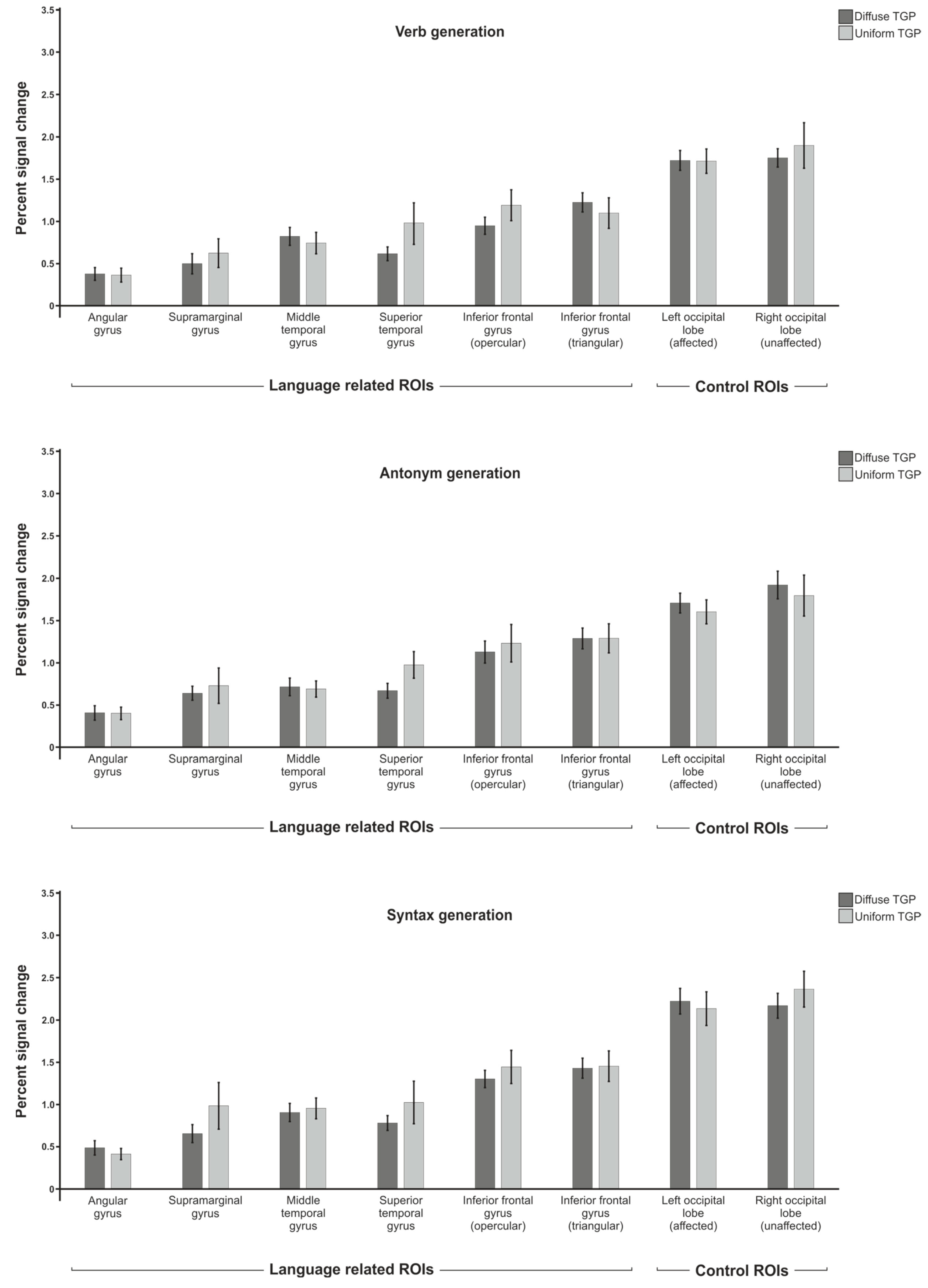
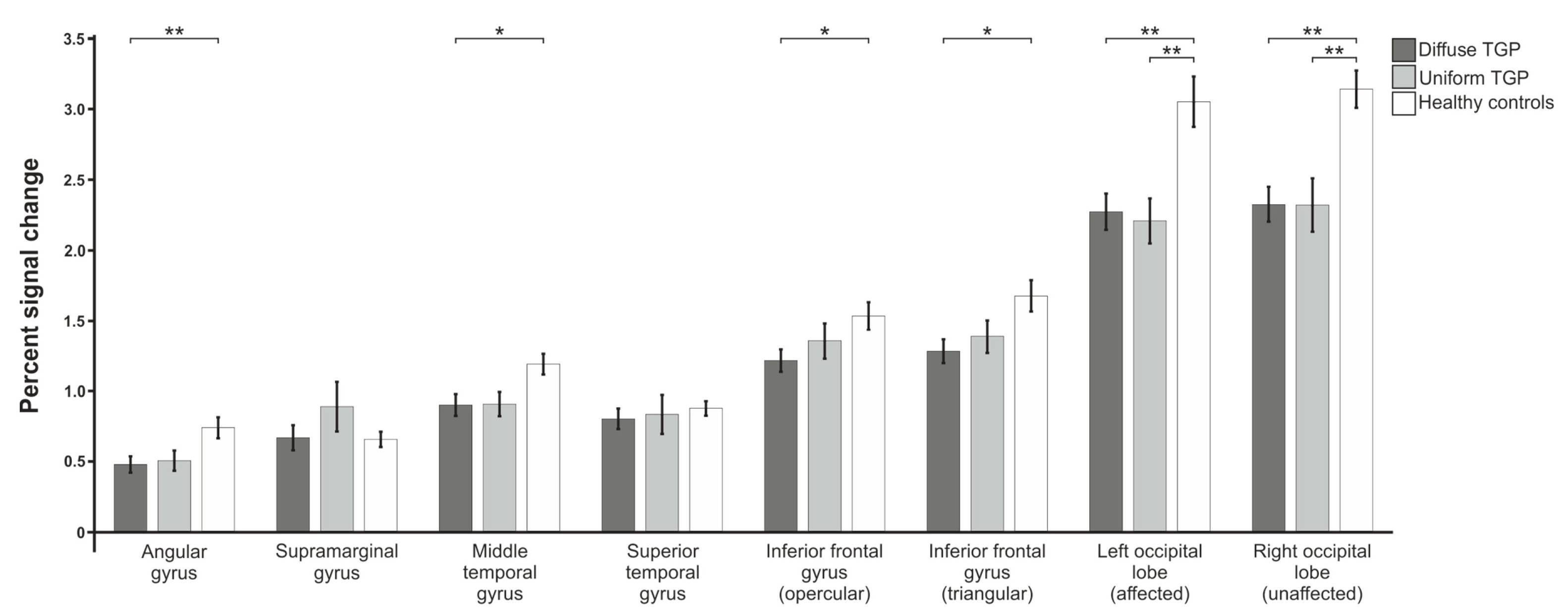
3.1. Percent Signal Change
3.1.1. Verb Generation
3.1.2. Antonym Generation
3.1.3. Syntax Generation
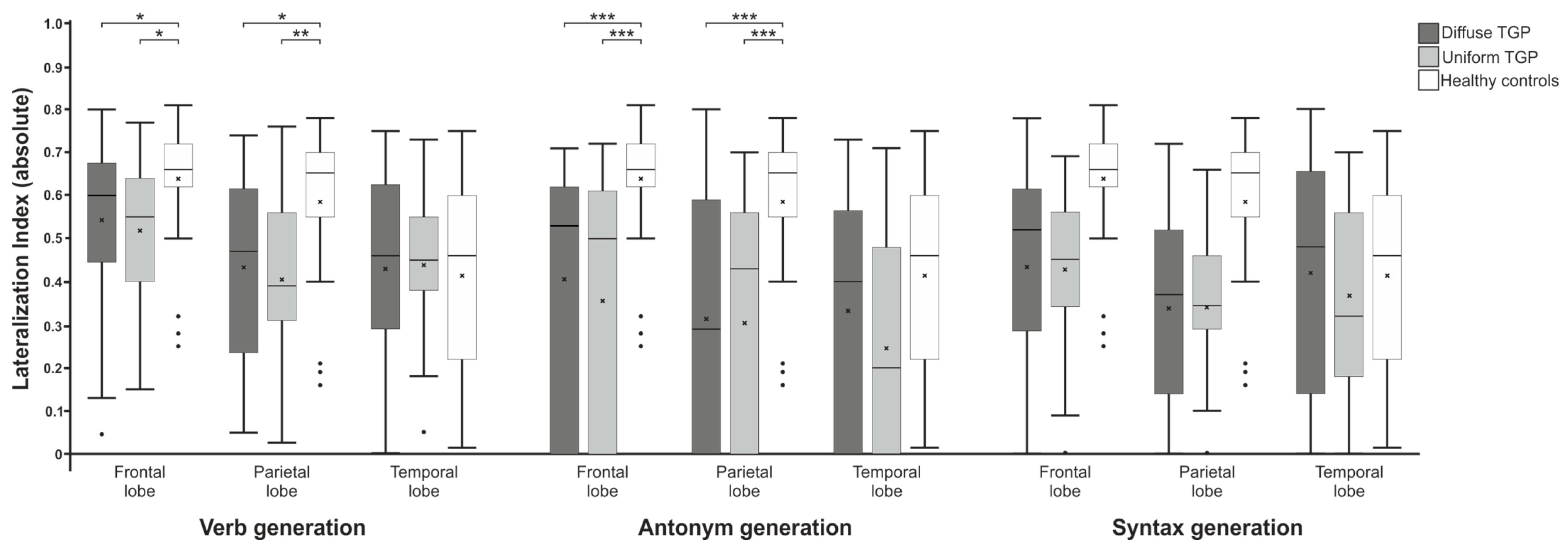
3.2. Laterality Index
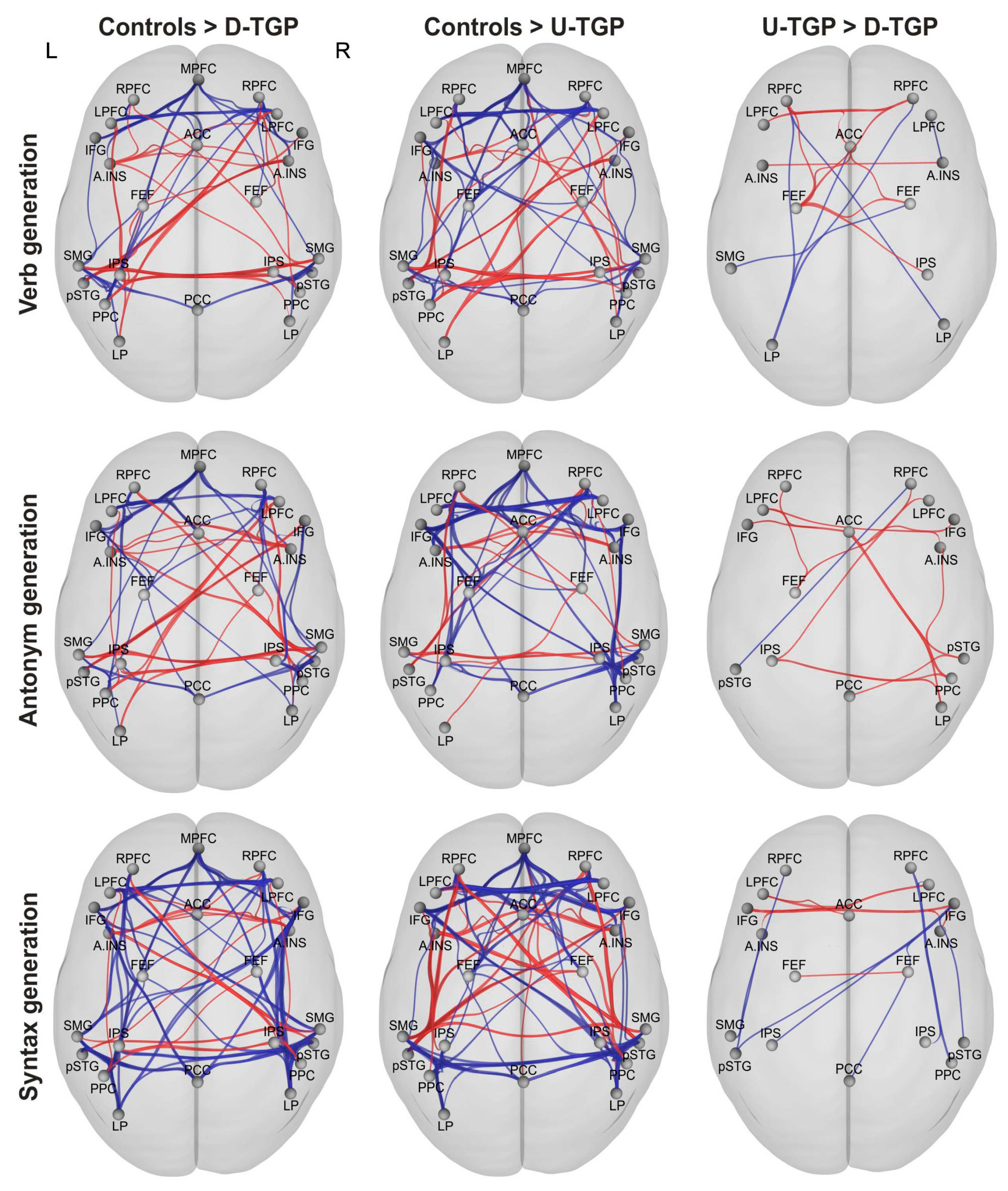
3.3. Functional Connectivity
3.3.1. Verb Generation
3.3.2. Antonym Generation
3.3.3. Syntax Generation
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
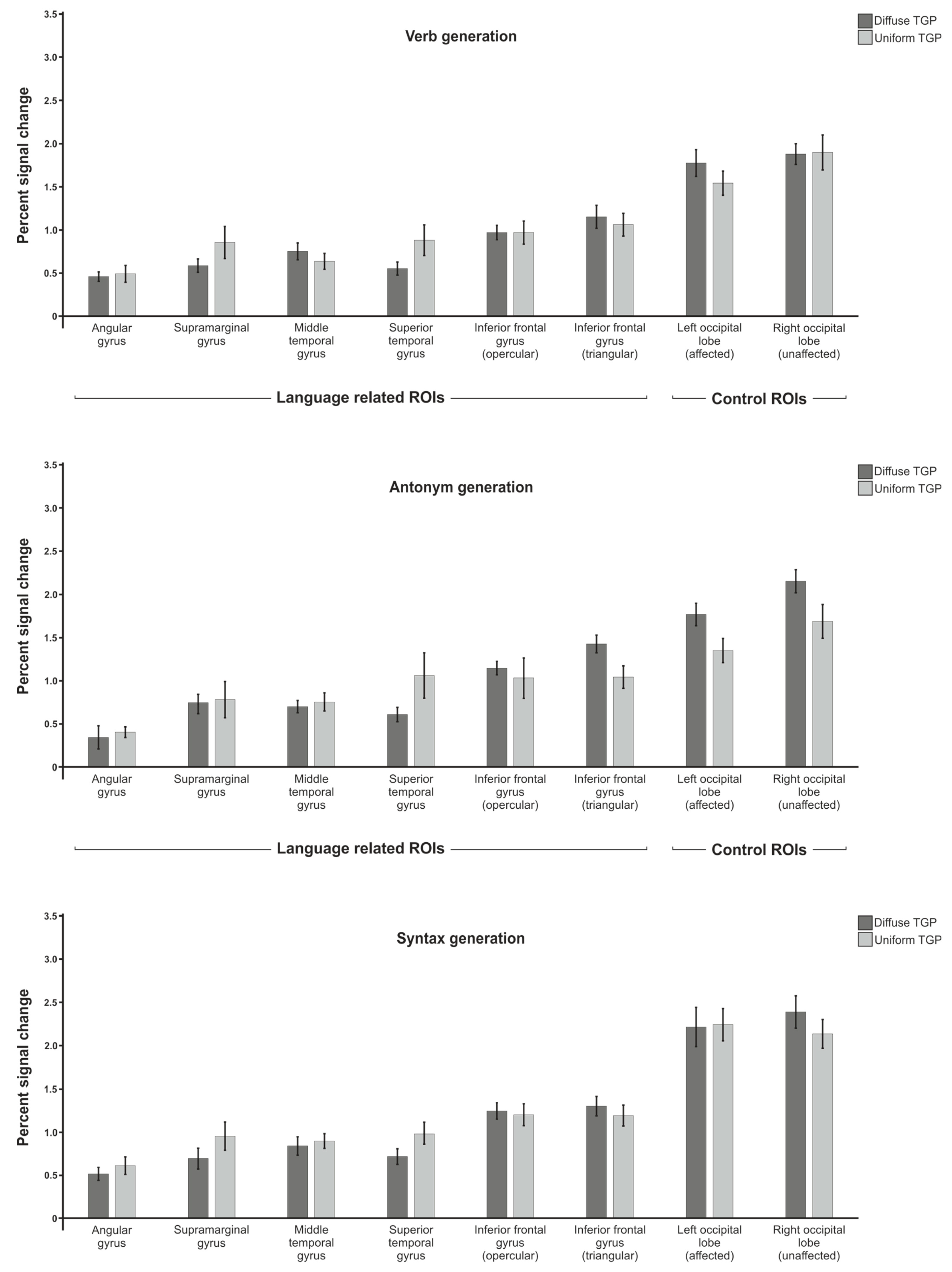
Appendix A

Appendix B


References
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef] [PubMed]
- Vysotski, S.; Madura, C.; Swan, B.; Holdsworth, R.; Lin, Y.; Munoz Del Rio, A.; Wood, J.; Kundu, B.; Penwarden, A.; Voss, J.; et al. Preoperative FMRI associated with decreased mortality and morbidity in brain tumor patients. Interdiscip. Neurosurg. 2018, 13, 40–45. [Google Scholar] [CrossRef]
- Wang, L.; Chen, D.; Olson, J.; Ali, S.; Fan, T.; Mao, H. Re-examine tumor-induced alterations in hemodynamic responses of BOLD fMRI: Implications in presurgical brain mapping. Acta Radiol. 2012, 53, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Krainik, A.; David, O.; Salon, C.; Troprès, I.; Hoffmann, D.; Pannetier, N.; Barbier, E.L.; Bombìn, E.R.; Warnking, J.; et al. Impaired fMRI activation in patients with primary brain tumors. NeuroImage 2010, 52, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Holodny, A.I.; Schulder, M.; Liu, W.C.; Maldjian, J.A.; Kalnin, A.J. Decreased BOLD functional MR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: Implications for image-guided neurosurgery. Am. J. Neuroradiol. 1999, 20, 609–612. [Google Scholar]
- Bogomolny, D.L.; Petrovich, N.M.; Hou, B.L.; Peck, K.K.; Kim, M.J.J.; Holodny, A.I. Functional MRI in the Brain Tumor Patient. Top. Magn. Reson. Imaging 2004, 15, 325–335. [Google Scholar] [CrossRef]
- Chen, C.M.; Hou, B.L.; Holodny, A.I. Effect of age and tumor grade on BOLD functional MR imaging in preoperative assessment of patients with glioma. Radiology 2008, 248, 971–978. [Google Scholar] [CrossRef]
- Fraga de Abreu, V.H.; Peck, K.K.; Petrovich-Brennan, N.M.; Woo, K.M.; Holodny, A.I. Brain Tumors: The Influence of Tumor Type and Routine MR Imaging Characteristics at BOLD Functional MR Imaging in the Primary Motor Gyrus. Radiology 2016, 281, 876–883. [Google Scholar] [CrossRef]
- Hou, B.L.; Bradbury, M.; Peck, K.K.; Petrovich, N.M.; Gutin, P.H.; Holodny, A.I. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. NeuroImage 2006, 32, 489–497. [Google Scholar] [CrossRef]
- Holodny, A.I.; Schulder, M.; Liu, W.C.; Wolko, J.; Maldjian, J.A.; Kalnin, A.J. The Effect of Brain Tumors on BOLD Functional MR Imaging Activation in the Adjacent Motor Cortex: Implications for Image-guided Neurosurgery. Am. J. Neuroradiol. 2000, 21, 1415–1422. [Google Scholar]
- Schreiber, A.; Hubbe, U.; Ziyeh, S.; Hennig, J. The influence of gliomas and nonglial space-occupying lesions on blood-oxygen-level-dependent contrast enhancement. Am. J. Neuroradiol. 2000, 21, 1055–1063. [Google Scholar]
- Harris, R.J.; Bookheimer, S.Y.; Cloughesy, T.F.; Kim, H.J.; Pope, W.B.; Lai, A.; Nghiemphu, P.L.; Liau, L.M.; Ellingson, B.M. Altered functional connectivity of the default mode network in diffuse gliomas measured with pseudo-resting state fMRI. J. Neurooncol 2014, 116, 373–379. [Google Scholar] [CrossRef]
- Liu, W.-C.; Feldman, S.C.; Schulder, M.; Kalnin, A.J.; Holodny, A.I.; Zimmerman, A.; Sinensky, R.; Rao, S. The effect of tumour type and distance on activation in the motor cortex. Neuroradiology 2005, 47, 813–819. [Google Scholar] [CrossRef]
- Chang, C.Y.; Peck, K.K.; Brennan, N.M.; Hou, B.L.; Gutin, P.H.; Holodny, A.I. Functional MRI in the presurgical evaluation of patients with brain tumors: Characterization of the statistical threshold. Stereotact. Funct. Neurosurg. 2010, 88, 35–41. [Google Scholar] [CrossRef]
- Lindauer, U.; Dirnagl, U.; Füchtemeier, M.; Böttiger, C.; Offenhauser, N.; Leithner, C.; Royl, G. Pathophysiological interference with neurovascular coupling—When imaging based on hemoglobin might go blind. Front. Neuroenergetics 2010, 2, 25. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, F.; Alberghina, C.; D’Aprile, S.; Pavone, A.M.; Longhitano, L.; Giallongo, S.; Tibullo, D.; Di Rosa, M.; Zappalà, A.; Cammarata, F.P.; et al. The Hallmarks of Glioblastoma: Heterogeneity, Intercellular Crosstalk and Molecular Signature of Invasiveness and Progression. Biomedicines 2022, 10, 806. [Google Scholar] [CrossRef]
- Xie, Y.; Bergström, T.; Jiang, Y.; Johansson, P.; Marinescu, V.D.; Lindberg, N.; Segerman, A.; Wicher, G.; Niklasson, M.; Baskaran, S.; et al. The Human Glioblastoma Cell Culture Resource: Validated Cell Models Representing All Molecular Subtypes. EBioMedicine 2015, 2, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Wangaryattawanich, P.; Hatami, M.; Wang, J.; Thomas, G.; Flanders, A.; Kirby, J.; Wintermark, M.; Huang, E.S.; Bakhtiari, A.S.; Luedi, M.M.; et al. Multicenter imaging outcomes study of The Cancer Genome Atlas glioblastoma patient cohort: Imaging predictors of overall and progression-free survival. Neuro Oncol. 2015, 17, 1525–1537. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Qin, L.; Bay, C.; Chen, X.; Yu, K.-H.; Miskin, N.; Li, A.; Xu, X.; Young, G. Deep Transfer Learning and Radiomics Feature Prediction of Survival of Patients with High-Grade Gliomas. Am. J. Neuroradiol. 2020, 41, 40–48. [Google Scholar] [CrossRef]
- Itakura, H.; Achrol, A.S.; Mitchell, L.A.; Loya, J.J.; Liu, T.; Westbroek, E.M.; Feroze, A.H.; Rodriguez, S.; Echegaray, S.; Azad, T.D.; et al. Magnetic resonance image features identify glioblastoma phenotypic subtypes with distinct molecular pathway activities. Sci. Transl. Med. 2015, 7, 303ra138. [Google Scholar] [CrossRef] [PubMed]
- Czarnek, N.; Clark, K.; Peters, K.B.; Mazurowski, M.A. Algorithmic three-dimensional analysis of tumor shape in MRI improves prognosis of survival in glioblastoma: A multi-institutional study. J. Neuro-Oncol. 2017, 132, 55–62. [Google Scholar] [CrossRef]
- Vigneau, M.; Beaucousin, V.; Hervé, P.Y.; Duffau, H.; Crivello, F.; Houdé, O.; Mazoyer, B.; Tzourio-Mazoyer, N. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage 2006, 30, 1414–1432. [Google Scholar] [CrossRef]
- Maldjian, J.A.; Laurienti, P.J.; Burdette, J.H. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage 2004, 21, 450–455. [Google Scholar] [CrossRef]
- Maldjian, J.A.; Laurienti, P.J.; Kraft, R.A.; Burdette, J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 2003, 19, 1233–1239. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Brett, M.; Anton, J.L.; Valabregue, R.; Poline, J.B. Region of interest analysis using an SPM toolbox. NeuroImage 2002, 16, 497. [Google Scholar]
- Wilke, M.; Lidzba, K. LI-tool: A new toolbox to assess lateralization in functional MR-data. J. Neurosci Methods 2007, 163, 128–136. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Weissenbacher, A.; Kasess, C.; Gerstl, F.; Lanzenberger, R.; Moser, E.; Windischberger, C. Correlations and anticorrelations in resting-state functional connectivity MRI: A quantitative comparison of preprocessing strategies. NeuroImage 2009, 47, 1408–1416. [Google Scholar] [CrossRef]
- Rathore, S.; Akbari, H.; Rozycki, M.; Abdullah, K.G.; Nasrallah, M.P.; Binder, Z.A.; Davuluri, R.V.; Lustig, R.A.; Dahmane, N.; Bilello, M.; et al. Radiomic MRI signature reveals three distinct subtypes of glioblastoma with different clinical and molecular characteristics, offering prognostic value beyond IDH1. Sci. Rep. 2018, 8, 5087. [Google Scholar] [CrossRef]
- Lüdemann, L.; Förschler, A.; Grieger, W.; Zimmer, C. BOLD signal in the motor cortex shows a correlation with the blood volume of brain tumors. J. Magn. Reson. Imaging 2006, 23, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Maesawa, S.; Bagarinao, E.; Fujii, M.; Futamura, M.; Motomura, K.; Watanabe, H.; Mori, D.; Sobue, G.; Wakabayashi, T. Evaluation of resting state networks in patients with gliomas: Connectivity changes in the unaffected side and its relation to cognitive function. PLoS ONE 2015, 10, e0118072. [Google Scholar] [CrossRef] [PubMed]
- Briganti, C.; Sestieri, C.; Mattei, P.A.; Esposito, R.; Galzio, R.J.; Tartaro, A.; Romani, G.L.; Caulo, M. Reorganization of functional connectivity of the language network in patients with brain gliomas. Am. J. Neuroradiol. 2012, 33, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Capper, D.; Jeibmann, A.; Habel, A.; Paulus, W.; Troost, D.; Deimling, A. von. Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch. Neurol. 2012, 69, 523–526. [Google Scholar] [CrossRef]
- Bartolomei, F.; Bosma, I.; Klein, M.; Baayen, J.C.; Reijneveld, J.C.; Postma, T.J.; Heimans, J.J.; van Dijk, B.W.; de Munck, J.C.; de Jongh, A.; et al. Disturbed functional connectivity in brain tumour patients: Evaluation by graph analysis of synchronization matrices. Clin. Neurophysiol. 2006, 117, 2039–2049. [Google Scholar] [CrossRef]
- Bartolomei, F.; Bosma, I.; Klein, M.; Baayen, J.C.; Reijneveld, J.C.; Postma, T.J.; Heimans, J.J.; Van Dijk, B.W.; De Munck, J.C.; De Jongh, A.; et al. How do brain tumors alter functional connectivity? A magnetoencephalography study. Ann. Neurol. 2006, 59, 128–138. [Google Scholar] [CrossRef]
- Daniel, A.G.S.; Park, K.Y.; Roland, J.L.; Dierker, D.; Gross, J.; Humphries, J.B.; Hacker, C.D.; Snyder, A.Z.; Shimony, J.S.; Leuthardt, E.C. Functional connectivity within glioblastoma impacts overall survival. Neuro Oncol. 2021, 23, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Esposito, R.; Mattei, P.A.; Briganti, C.; Romani, G.L.; Tartaro, A.; Caulo, M. Modifications of default-mode network connectivity in patients with cerebral glioma. PLoS ONE 2012, 7, e40231. [Google Scholar] [CrossRef] [PubMed]



| Total | Uniform Tumor Growth Pattern | Diffuse Tumor Growth Pattern | Sig. | ||
|---|---|---|---|---|---|
| Verb generation | n | 64 | 23 | 41 | |
| Age | 59.84 | 62.87 | 58.15 | 0.114 | |
| Sex (m/f) | 37/27 | 13/10 | 24/17 | ||
| Tumor location (frontal/parietal/temporal) | 5/4/14 | 13/9/19 | |||
| Tumor size in mm3 | 22,707.65 | 35,876.75 | 0.031 * | ||
| Edema size in mm3 | 52,534.35 | 56,229.12 | 0.707 | ||
| Antonym generation | n | 47 | 16 | 31 | |
| Age | 59.66 | 62.31 | 58.29 | 0.274 | |
| Sex (m/f) | 26/21 | 9/7 | 17/14 | ||
| Tumor location (frontal/parietal/temporal) | 2/4/10 | 9/8/14 | |||
| Tumor size in mm3 | 24,437.44 | 36,302.55 | 0.098 | ||
| Edema size in mm3 | 58,471.71 | 57,401.19 | 0.926 | ||
| Syntax generation | n | 57 | 22 | 35 | |
| Age | 59.86 | 63.90 | 57.83 | 0.095 | |
| Sex (m/f) | 33/24 | 13/9 | 20/15 | ||
| Tumor location (frontal/parietal/temporal) | 5/4/13 | 8/9/18 | |||
| Tumor size in mm3 | 22,536.14 | 34,112.34 | 0.076 | ||
| Edema size in mm3 | 52,936.82 | 54,296.62 | 0.894 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hense, K.; Deuter, D.; Greenlee, M.W.; Wendl, C.; Schmidt, N.O.; Stroszczynski, C.; Doenitz, C.; Ott, C.; Rosengarth, K. Analysis of Functional Neuroplastic Changes in the Cortical Language System in Relation to Different Growth Patterns of Glioblastoma. Brain Sci. 2023, 13, 867. https://doi.org/10.3390/brainsci13060867
Hense K, Deuter D, Greenlee MW, Wendl C, Schmidt NO, Stroszczynski C, Doenitz C, Ott C, Rosengarth K. Analysis of Functional Neuroplastic Changes in the Cortical Language System in Relation to Different Growth Patterns of Glioblastoma. Brain Sciences. 2023; 13(6):867. https://doi.org/10.3390/brainsci13060867
Chicago/Turabian StyleHense, Katharina, Daniel Deuter, Mark W. Greenlee, Christina Wendl, Nils Ole Schmidt, Christian Stroszczynski, Christian Doenitz, Christian Ott, and Katharina Rosengarth. 2023. "Analysis of Functional Neuroplastic Changes in the Cortical Language System in Relation to Different Growth Patterns of Glioblastoma" Brain Sciences 13, no. 6: 867. https://doi.org/10.3390/brainsci13060867






