Detecting Subtle Cognitive Impairment in Patients with Parkinson’s Disease and Normal Cognition: A Novel Cognitive Control Challenge Task (C3T)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Cognitive Assessment
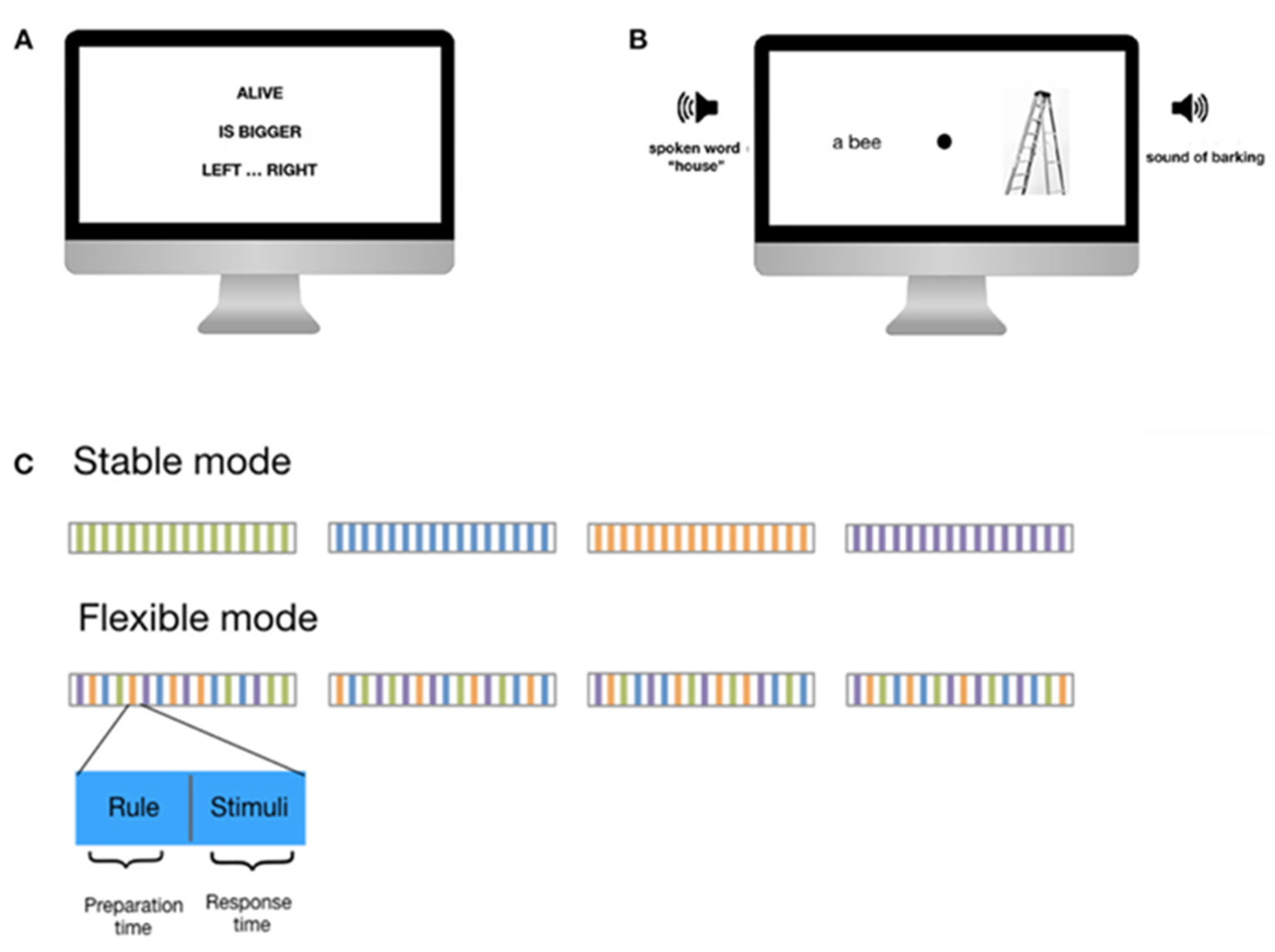
2.4. Cognitive Control Challenge Task
2.5. Statistical Analyses
3. Results
3.1. Standardized Neuropsychological Assessment Performance
3.2. Comparison of Performance on Standard Cognitive Tests and the C3T Task
3.3. C3T Performance Accuracy
3.4. C3T Response Times
4. Discussion
4.1. Performance Accuracy Declines with Age for Healthy Participants but Remains Impaired Regardless of Age in PD Patients
4.2. Overall Performance on the Cognitive Control Task Is Significantly Lower in Patients with PD Compared with That in Healthy Controls, Even When They Are within the Normal Range on Standardized Neuropsychological Assessments
4.3. Patients with PD Respond Significantly Faster Than Healthy Controls during the Flexible Cognitive Control Task
4.4. The C3T Task Engages Multiple Cognitive Systems in Healthy Subjects, Including Working Memory, Inhibition, and Task-Switching, and Provides a Valid Measure of Complex Cognitive Control
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fengler, S.; Liepelt-Scarfone, I.; Brockmann, K.; Schäffer, E.; Berg, D.; Kalbe, E. Cognitive Changes in Prodromal Parkinson’s Disease: A Review. Mov. Disord. 2017, 32, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive Decline in Parkinson Disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solla, P.; Masala, C.; Ercoli, T.; Frau, C.; Bagella, C.; Pinna, I.; Loy, F.; Defazio, G. Olfactory Impairment Correlates with Executive Functions Disorders and Other Specific Cognitive Dysfunctions in Parkinson’s Disease. Biology 2023, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Barker, R.A.; Sahakian, B.J.; Robbins, T.W. Enhanced or Impaired Cognitive Function in Parkinson’s Disease as a Function of Dopaminergic Medication and Task Demands. Cereb. Cortex 2001, 11, 1136–1143. [Google Scholar] [CrossRef] [Green Version]
- Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Breen, D.P.; Khoo, T.K.; Williams-Gray, C.H.; Barker, R.A.; Collerton, D.; Taylor, J.-P.; Burn, D.J. Cognitive Decline and Quality of Life in Incident Parkinson’s Disease: The Role of Attention. Park. Relat. Disord. 2016, 27, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-Motor Features of Parkinson Disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- McKeith, I.G.; Dickson, D.W.; Lowe, J.; Emre, M.; O’Brien, J.T.; Feldman, H.; Cummings, J.; Duda, J.E.; Lippa, C.; Perry, E.K.; et al. Diagnosis and Management of Dementia with Lewy Bodies: Third Report of the DLB Consortium. Neurology 2005, 65, 1863–1872. [Google Scholar] [CrossRef] [Green Version]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of Revised Diagnostic Criteria for the Behavioural Variant of Frontotemporal Dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Besser, L.M.; Litvan, I.; Monsell, S.E.; Mock, C.; Weintraub, S.; Zhou, X.-H.; Kukull, W. Mild Cognitive Impairment in Parkinson’s Disease versus Alzheimer’s Disease. Park. Relat. Disord. 2016, 27, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Caviness, J.N.; Driver-Dunckley, E.; Connor, D.J.; Sabbagh, M.N.; Hentz, J.G.; Noble, B.; Evidente, V.G.H.; Shill, H.A.; Adler, C.H. Defining Mild Cognitive Impairment in Parkinson’s Disease. Mov. Disord. 2007, 22, 1272–1277. [Google Scholar] [CrossRef]
- Zhang, Q.; Aldridge, G.M.; Narayanan, N.S.; Anderson, S.W.; Uc, E.Y. Approach to Cognitive Impairment in Parkinson’s Disease. Neurotherapeutics 2020, 17, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, G. Should I Stay or Should I Go? Conceptual Underpinnings of Goal-Directed Actions. Front. Syst. Neurosci. 2014, 8, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampshire, A.; Sharp, D.J. Contrasting Network and Modular Perspectives on Inhibitory Control. Trends Cogn. Sci. 2015, 19, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [Green Version]
- Diamond, A. Executive Functions. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 173, pp. 225–240. ISBN 978-0-444-64150-2. [Google Scholar]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The Unity and Diversity of Executive Functions and Their Contributions to Complex “Frontal Lobe” Tasks: A Latent Variable Analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavanagh, J.F.; Ryman, S.; Richardson, S.P. Cognitive Control in Parkinson’s Disease. Prog. Brain Res. 2022, 269, 137–152. [Google Scholar] [CrossRef]
- Leh, S.E.; Petrides, M.; Strafella, A.P. The Neural Circuitry of Executive Functions in Healthy Subjects and Parkinson’s Disease. Neuropsychopharmacology 2010, 35, 70–85. [Google Scholar] [CrossRef]
- Botvinick, M.M.; Braver, T.S.; Barch, D.M.; Carter, C.S.; Cohen, J.D. Conflict Monitoring and Cognitive Control. Psychol. Rev. 2001, 108, 624–652. [Google Scholar] [CrossRef]
- Braver, T.S. The Variable Nature of Cognitive Control: A Dual Mechanisms Framework. Trends Cogn. Sci. 2012, 16, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Lustig, C.; Eichenbaum, H. Editorial Overview: Cognitive Control: Diversity of Domains, Parallels in Mechanism. Curr. Opin. Behav. Sci. 2015, 1, iv–vii. [Google Scholar] [CrossRef]
- Cole, M.W.; Reynolds, J.R.; Power, J.D.; Repovs, G.; Anticevic, A.; Braver, T.S. Multi-Task Connectivity Reveals Flexible Hubs for Adaptive Task Control. Nat. Neurosci. 2013, 16, 1348–1355. [Google Scholar] [CrossRef] [Green Version]
- Dosenbach, N.U.F.; Fair, D.A.; Cohen, A.L.; Schlaggar, B.L.; Petersen, S.E. A Dual-Networks Architecture of Top-down Control. Trends Cogn. Sci. 2008, 12, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [Green Version]
- Politakis, V.A.; Slana Ozimič, A.; Repovš, G. Cognitive Control Challenge Task Across the Lifespan. Front. Psychol. 2022, 12, 789816. [Google Scholar] [CrossRef]
- Durstewitz, D.; Seamans, J.K. The Dual-State Theory of Prefrontal Cortex Dopamine Function with Relevance to Catechol-O-Methyltransferase Genotypes and Schizophrenia. Biol. Psychiatry 2008, 64, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Hikosaka, O. Dopamine-Mediated Learning and Switching in Cortico-Striatal Circuit Explain Behavioral Changes in Reinforcement Learning. Front. Behav. Neurosci. 2011, 5, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cools, R. Chemistry of the Adaptive Mind: Lessons from Dopamine. Neuron 2019, 104, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Cools, R. Dopaminergic Modulation of Cognitive Function-Implications for l-DOPA Treatment in Parkinson’s Disease. Neurosci. Biobehav. Rev. 2006, 30, 1–23. [Google Scholar] [CrossRef]
- Gruszka, A.; Hampshire, A.; Barker, R.A.; Owen, A.M. Normal Aging and Parkinson’s Disease Are Associated with the Functional Decline of Distinct Frontal-Striatal Circuits. Cortex 2017, 93, 178–192. [Google Scholar] [CrossRef]
- Kudlicka, A.; Clare, L.; Hindle, J.V. Executive Functions in Parkinson’s Disease: Systematic Review and Meta-Analysis. Mov. Disord. 2011, 26, 2305–2315. [Google Scholar] [CrossRef]
- Lange, F.; Seer, C.; Loens, S.; Wegner, F.; Schrader, C.; Dressler, D.; Dengler, R.; Kopp, B. Neural Mechanisms Underlying Cognitive Inflexibility in Parkinson’s Disease. Neuropsychologia 2016, 93, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.G.; Slabosz, A.; Robbins, T.W.; Barker, R.A.; Owen, A.M. Dopaminergic Basis for Deficits in Working Memory but Not Attentional Set-Shifting in Parkinson’s Disease. Neuropsychologia 2005, 43, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Monchi, O.; Petrides, M.; Doyon, J.; Postuma, R.B.; Worsley, K.; Dagher, A. Neural Bases of Set-Shifting Deficits in Parkinson’s Disease. J. Neurosci. 2004, 24, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Cools, R.; Rogers, R.; Barker, R.A.; Robbins, T.W. Top-down Attentional Control in Parkinson’s Disease: Salient Considerations. J. Cogn. Neurosci. 2010, 22, 848–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallon, S.J.; Hampshire, A.; Barker, R.A.; Owen, A.M. Learning to Be Inflexible: Enhanced Attentional Biases in Parkinson’s Disease. Cortex 2016, 82, 24–34. [Google Scholar] [CrossRef]
- Cools, R. The Costs and Benefits of Brain Dopamine for Cognitive Control: The Costs and Benefits of Brain Dopamine for Cognitive Control. WIREs Cogn. Sci. 2016, 7, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Fallon, S.J.; Williams-Gray, C.H.; Barker, R.A.; Owen, A.M.; Hampshire, A. Prefrontal Dopamine Levels Determine the Balance between Cognitive Stability and Flexibility. Cereb. Cortex 2013, 23, 361–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallon, S.J.; Smulders, K.; Esselink, R.A.; van de Warrenburg, B.P.; Bloem, B.R.; Cools, R. Differential Optimal Dopamine Levels for Set-Shifting and Working Memory in Parkinson’s Disease. Neuropsychologia 2015, 77, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ray, N.J.; Strafella, A.P. The Neurobiology and Neural Circuitry of Cognitive Changes in Parkinson’s Disease Revealed by Functional Neuroimaging. Mov. Disord. 2012, 27, 1484–1492. [Google Scholar] [CrossRef] [Green Version]
- van Schouwenburg, M.R.; O’Shea, J.; Mars, R.B.; Rushworth, M.F.S.; Cools, R. Controlling Human Striatal Cognitive Function via the Frontal Cortex. J. Neurosci. 2012, 32, 5631–5637. [Google Scholar] [CrossRef] [Green Version]
- Gauggel, S.; Rieger, M.; Feghoff, T.-A. Inhibition of Ongoing Responses in Patients with Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2004, 75, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, G.; Fragola, M.; Giannini, G.; Modugno, N.; Lakens, D. Inhibitory Control Is Not Lateralized in Parkinson’s Patients. Neuropsychologia 2017, 102, 177–189. [Google Scholar] [CrossRef]
- Di Caprio, V.; Modugno, N.; Mancini, C.; Olivola, E.; Mirabella, G. Early-Stage Parkinson’s Patients Show Selective Impairment in Reactive but Not Proactive Inhibition. Mov. Disord. 2020, 35, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Burgess, P.W.; Stuss, D.T. Fifty Years of Prefrontal Cortex Research: Impact on Assessment. J. Int. Neuropsychol. Soc. 2017, 23, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Marras, C.; Tröster, A.I.; Kulisevsky, J.; Stebbins, G.T. The Tools of the Trade: A State of the Art “How to Assess Cognition” in the Patient with Parkinson’s Disease: Assessing Cognition in Parkinson’s Disease. Mov. Disord. 2014, 29, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Hlavatá, P.; Linhartová, P.; Šumec, R.; Filip, P.; Světlák, M.; Baláž, M.; Kašpárek, T.; Bareš, M. Behavioral and Neuroanatomical Account of Impulsivity in Parkinson’s Disease. Front. Neurol. 2019, 10, 1338. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.; Braver, T.S. Learned Predictions of Error Likelihood in the Anterior Cingulate Cortex. Science 2005, 307, 1118–1121. [Google Scholar] [CrossRef]
- Schmitter-Edgecombe, M.; McAlister, C.; Weakley, A. Naturalistic Assessment of Everyday Functioning in Individuals with Mild Cognitive Impairment: The Day-out Task. Neuropsychology 2012, 26, 631–641. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.F.; Segal, M.; Veramonti, T.; Ferraro, M.; Buxbaum, L.J. The Naturalistic Action Test: A Standardised Assessment for Everyday Action Impairment. Neuropsychol. Rehabil. 2002, 12, 311–339. [Google Scholar] [CrossRef]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic Criteria for Mild Cognitive Impairment in Parkinson’s Disease: Movement Disorder Society Task Force Guidelines: PD-MCI Diagnostic Criteria. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Reitan, R.M.; Wolfson, D. The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical Interpretation; Reitan Neuropsychology: Mesa, AZ, USA, 1985. [Google Scholar]
- Shallice, T. Specific Impairments of Planning. Phil. Trans. R. Soc. Lond. B 1982, 298, 199–209. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of Interference in Serial Verbal Reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Rey, A. L’examen Psychologique Dans Les Cas d’ecephalopathie Traumatique [Psychological Examination of Traumatic Encephalopathy]. Arch. De Psychol. 1941, 28, 286–340. [Google Scholar]
- Unsworth, N.; Heitz, R.P.; Schrock, J.C.; Engle, R.W. An Automated Version of the Operation Span Task. Behav. Res. Methods 2005, 37, 498–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogačnik, V. Test Verbalne Sposobnosti “Tujke”—TVS, 2. Izdaja [Verbal Ability Test—“Foreign Words”]. 2005. Available online: https://hrcak.srce.hr/clanak/133672 (accessed on 7 June 2023).
- Raven, J.C. Standardization of Progressive Matrices, 1938. Br. J. Med. Psychol. 1941, 19, 137–150. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The Control of the False Discovery Rate in Multiple Testing under Dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 21 February 2023).
- Allaire, J. RStudio: Integrated Development Environment for R. Boston MA 2012, 770, 165–171. [Google Scholar]
- Zelazo, P.D.; Craik, F.I.M.; Booth, L. Executive Function across the Life Span. Acta Psychol. 2004, 115, 167–183. [Google Scholar] [CrossRef]
- Cepeda, N.J.; Kramer, A.F.; Gonzalez de Sather, J. Changes in Executive Control across the Life Span: Examination of Task-Switching Performance. Dev. Psychol. 2001, 37, 715–730. [Google Scholar] [CrossRef]
- Jurado, M.B.; Rosselli, M. The Elusive Nature of Executive Functions: A Review of Our Current Understanding. Neuropsychol. Rev. 2007, 17, 213–233. [Google Scholar] [CrossRef]
- Bopp, K.L.; Verhaeghen, P. Aging and Verbal Memory Span: A Meta-Analysis. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2005, 60, P223–P233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, D.C.; Polk, T.A.; Park, R.; Minear, M.; Savage, A.; Smith, M.R. Aging Reduces Neural Specialization in Ventral Visual Cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 13091–13095. [Google Scholar] [CrossRef] [Green Version]
- Salthouse, T.A. When Does Age-Related Cognitive Decline Begin? Neurobiol. Aging 2009, 30, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foltynie, T.; Brayne, C.E.G.; Robbins, T.W.; Barker, R.A. The Cognitive Ability of an Incident Cohort of Parkinson’s Patients in the UK. The CamPaIGN Study. Brain 2004, 127, 550–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmich, R.C.; Derikx, L.C.; Bakker, M.; Scheeringa, R.; Bloem, B.R.; Toni, I. Spatial Remapping of Cortico-Striatal Connectivity in Parkinson’s Disease. Cereb. Cortex 2010, 20, 1175–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Cognitive Impairment in Parkinson’s Disease: The Dual Syndrome Hypothesis. Neurodegener. Dis. 2013, 11, 79–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kray, J.; Eber, J.; Lindenberger, U. Age Differences in Executive Functioning across the Lifespan: The Role of Verbalization in Task Preparation. Acta Psychol. 2004, 115, 143–165. [Google Scholar] [CrossRef] [Green Version]
- Kray, J.; Lindenberger, U. Adult Age Differences in Task Switching. Psychol. Aging 2000, 15, 126–147. [Google Scholar] [CrossRef]
- Monsell, S. Task Switching. Trends Cogn. Sci. 2003, 7, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Arrington, C.M.; Logan, G.D. The Cost of a Voluntary Task Switch. Psychol. Sci. 2004, 15, 610–615. [Google Scholar] [CrossRef]
- Cools, R.; Barker, R.A.; Sahakian, B.J.; Robbins, T.W. Mechanisms of Cognitive Set Flexibility in Parkinson’s Disease. Brain 2001, 124, 2503–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwhof, F.; Bloem, B.R.; Reelick, M.F.; Aarts, E.; Maidan, I.; Mirelman, A.; Hausdorff, J.M.; Toni, I.; Helmich, R.C. Impaired Dual Tasking in Parkinson’s Disease Is Associated with Reduced Focusing of Cortico-Striatal Activity. Brain 2017, 140, 1384–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarnall, A.J.; Breen, D.P.; Duncan, G.W.; Khoo, T.K.; Coleman, S.Y.; Firbank, M.J.; Nombela, C.; Winder-Rhodes, S.; Evans, J.R.; Rowe, J.B.; et al. Characterizing Mild Cognitive Impairment in Incident Parkinson Disease: The ICICLE-PD Study. Neurology 2014, 82, 308–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.D.; Burroughs, M.; Apodaca, M.; Bunch, J. Greater Intraindividual Variability in Neuropsychological Performance Predicts Cognitive Impairment in de Novo Parkinson’s Disease. Neuropsychology 2020, 34, 24–30. [Google Scholar] [CrossRef] [PubMed]



| Clinical Data | PD01 | PD02 | PD03 | PD04 | PD05 | PD06 | PD07 | PD08 | PD09 | PD10 | PD11 | PD12 | PD13 | PD14 | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | f | m | m | m | m | m | m | f | m | f | m | m | f | f | ||
| Age | 65 | 61 | 55 | 57 | 60 | 79 | 77 | 79 | 74 | 68 | 77 | 63 | 68 | 66 | 67.8 | 7.9 |
| PD duration (years) | 2 | 6 | 2 | 2 | 1 | 6 | 7 | 5 | 10 | 8 | 4 | 10 | 3 | 6 | 5.4 | 2.8 |
| UPDRS-III | 16 | 24 | 40 | 33 | 26 | 28 | 28 | 30 | 42 | 30 | 49 | 31 | 43 | 49 | 33.5 | 9.4 |
| HY | 1 | 2 | 2.5 | 2 | 2 | 2.5 | 2.5 | 2 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.3 | 0.4 |
| LEDD | 400 | 300 | 1000 | / | / | 1200 | 300 | 300 | 200 | 400 | 250 | 900 | 200 | 800 | 520.8 | 337.6 |
| DA (mg) | / | / | / | 16 | 6 | / | / | / | / | / | / | / | / | / | 11.0 | 5.0 |
| SBP (mmHG) | 144 | 163 | 144 | 144 | 130 | 114 | 130 | 180 | 158 | 145 | 155 | 155 | 138 | 127 | 144.8 | 16.3 |
| DBP (mmHg) | 81 | 86 | 86 | 78 | 78 | 70 | 78 | 82 | 85 | 80 | 75 | 62 | 78 | 73 | 78.0 | 6.3 |
| HR (bpm) | 90 | 63 | 96 | 68 | 72 | 77 | 72 | 68 | 70 | 73 | 72 | 70 | 69 | 76 | 74.0 | 8.5 |
| Cognitive Assessment | Patient Group | Control Group | p-Score | p-Score (Adjusted) |
|---|---|---|---|---|
| TMa | 35.2 (12.8) | 36.7 (16.3) | 0.788 | 0.841 |
| TMb | 90.7 (34.5) | 82.9 (41.6) | 0.549 | 0.732 |
| TMc | 21.7 (8.6) | 20.2 (8.9) | 0.712 | 0.814 |
| WM | 14.1 (2.7) | 14.9 (2.3) | 0.393 | 0.629 |
| WMss | 2.8 (1.2) | 3.2 (0.9) | 0.173 | 0.624 |
| WMvs | 5.5 (1.6) | 5.8 (1.2) | 0.635 | 0.782 |
| VLTld | 7.8 (2.3) | 10.5 (3.9) | 0.046, * | 0.624 |
| RCFTld | 58.5 (15.0) | 62.1 (9.5) | 0.466 | 0.678 |
| TOL | 109.4 (18.4) | 103.3 (14.0) | 0.351 | 0.624 |
| TOLt | 97.5 (11.9) | 102.0 (9.7) | 0.303 | 0.624 |
| StrpInc | 23.5 (5.9) | 23.5 (7.0) | 1.000 | 1.000 |
| VFlex | 25.2 (10.1) | 31.5 (8.2) | 0.086 | 0.624 |
| Vfsem | 33.7 (6.4) | 37.9 (6.8) | 0.117 | 0.624 |
| VFsw | 12.2 (2.7) | 13.2 (2.8) | 0.347 | 0.624 |
| SPM | 3.8 (1.8) | 4.7 (2.0) | 0.251 | 0.624 |
| TVS | 105.5 (13.9) | 110.9 (12.8) | 0.306 | 0.624 |
| A/Patients | B/Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Stable | Flexible | Stable | Flexible | |||||
| RA | RT | RA | RT | RA | RT | RA | RT | |
| TMa | 0.31 | 0.34 | −0.06 | 0.26 | −0.59 | 0.19 | −0.52 | −0.06 |
| TMb | 0.03 | 0.31 | −0.30 | 0.19 | −0.84 | 0.38 | −0.77 | 0.08 |
| TMc | −0.09 | 0.40 | −0.35 | 0.28 | −0.65 | 0.08 | −0.57 | 0.01 |
| WM | 0.18 | −0.03 | 0.29 | −0.10 | 0.56 | −0.56 | 0.35 | −0.32 |
| WMss | 0.S0 | −0.23 | 0.53 | −0.30 | 0.33 | 0.11 | 0.27 | −0.09 |
| WMvs | 0.27 | −0.34 | 0.45 | −0.32 | −0.10 | 0.35 | −0.22 | −0.12 |
| VLTId | −0.39 | 0.04 | −0.12 | 0.13 | 0.63 | −0.30 | 0.50 | −0.13 |
| RCFTId | 0.24 | −0.29 | 0.42 | −0.34 | 0.29 | −0.05 | 0.06 | −0.33 |
| TOL | 0.31 | 0.21 | 0.07 | 0.21 | 0.35 | −0.17 | 0.3 | −0.50 |
| TOLt | 0.22 | −0.05 | 0.29 | 0.05 | 0.00 | 0.01 | −0.04 | 0.16 |
| Strplnc | −0.25 | 0.09 | −0.24 | 0.24 | 0.86 | −0.49 | 0.76 | −0.16 |
| VFlex | 0.04 | −0.16 | 0.25 | −0.08 | 0.49 | −0.13 | 0.33 | 0.29 |
| VFsem | −0.33 | −0.33 | −0.05 | −0.09 | 0.31 | −0.49 | 0.26 | 0.06 |
| VFsw | 0.12 | −0.40 | 0.25 | −0.26 | 0.23 | 0.09 | 0.22 | 0.43 |
| SPM | 0.06 | 0.18 | 0.00 | 0.08 | 0.69 | 0.06 | 0.63 | 0.20 |
| TVS | 0.42 | 0.02 | 0.19 | 0.01 | 0.68 | −0.53 | 0.47 | −0.38 |
| Legend | p < 0.001 | p < 0.01 | p < 0.05 | p < 0.05 | p < 0.01 | p < 0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resnik Robida, K.; Politakis, V.A.; Oblak, A.; Ozimič, A.S.; Burger, H.; Pirtošek, Z.; Bon, J. Detecting Subtle Cognitive Impairment in Patients with Parkinson’s Disease and Normal Cognition: A Novel Cognitive Control Challenge Task (C3T). Brain Sci. 2023, 13, 961. https://doi.org/10.3390/brainsci13060961
Resnik Robida K, Politakis VA, Oblak A, Ozimič AS, Burger H, Pirtošek Z, Bon J. Detecting Subtle Cognitive Impairment in Patients with Parkinson’s Disease and Normal Cognition: A Novel Cognitive Control Challenge Task (C3T). Brain Sciences. 2023; 13(6):961. https://doi.org/10.3390/brainsci13060961
Chicago/Turabian StyleResnik Robida, Karmen, Vida Ana Politakis, Aleš Oblak, Anka Slana Ozimič, Helena Burger, Zvezdan Pirtošek, and Jurij Bon. 2023. "Detecting Subtle Cognitive Impairment in Patients with Parkinson’s Disease and Normal Cognition: A Novel Cognitive Control Challenge Task (C3T)" Brain Sciences 13, no. 6: 961. https://doi.org/10.3390/brainsci13060961





