Histamine and Its Receptors in the Mammalian Inner Ear: A Scoping Review
Abstract
1. Introduction
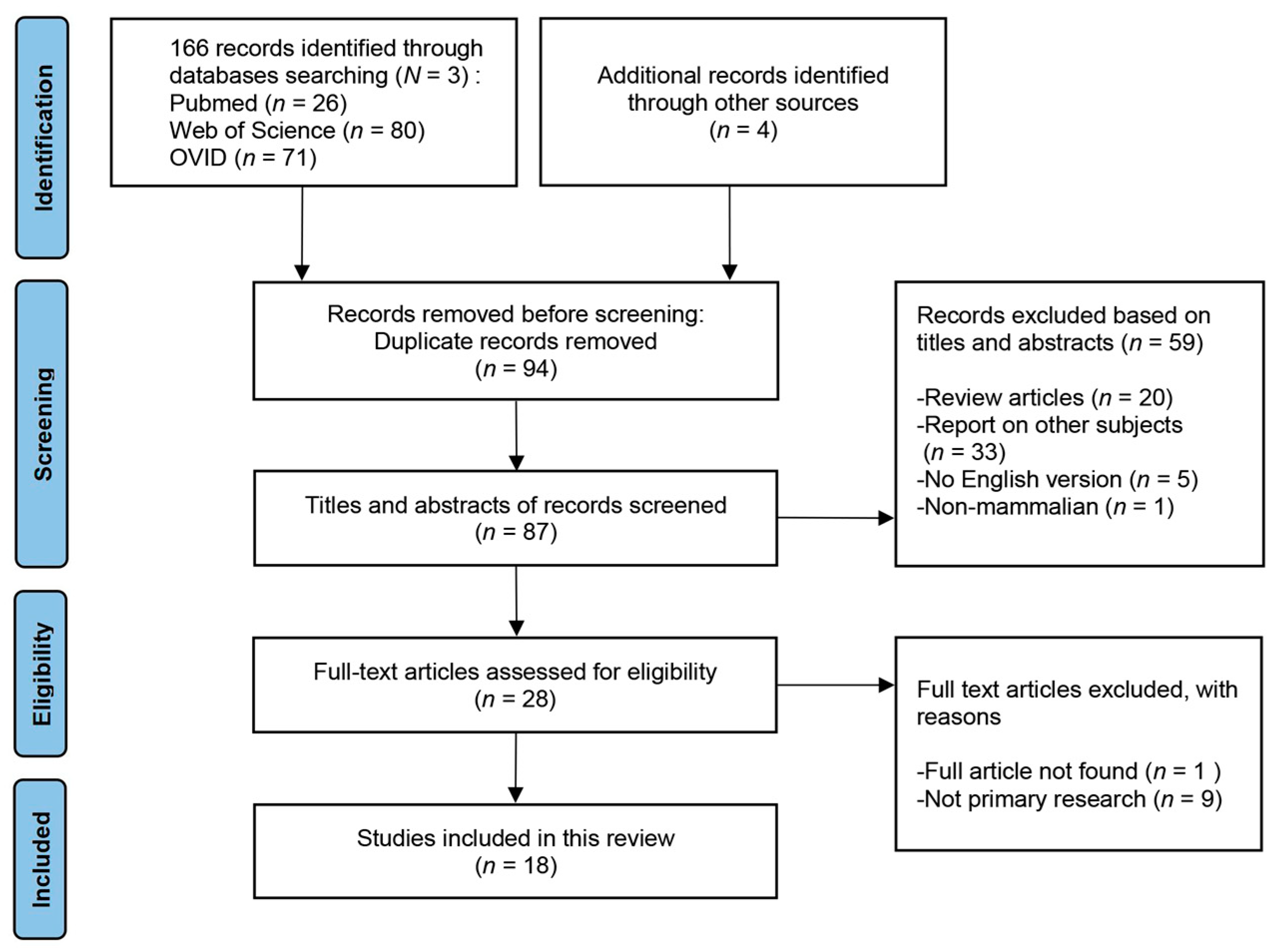
2. Materials and Methods
2.1. Search Strategy and Databases
2.2. Screening Process and Inclusion and Exclusion Criteria
2.3. Study Eligibility and Data Extraction
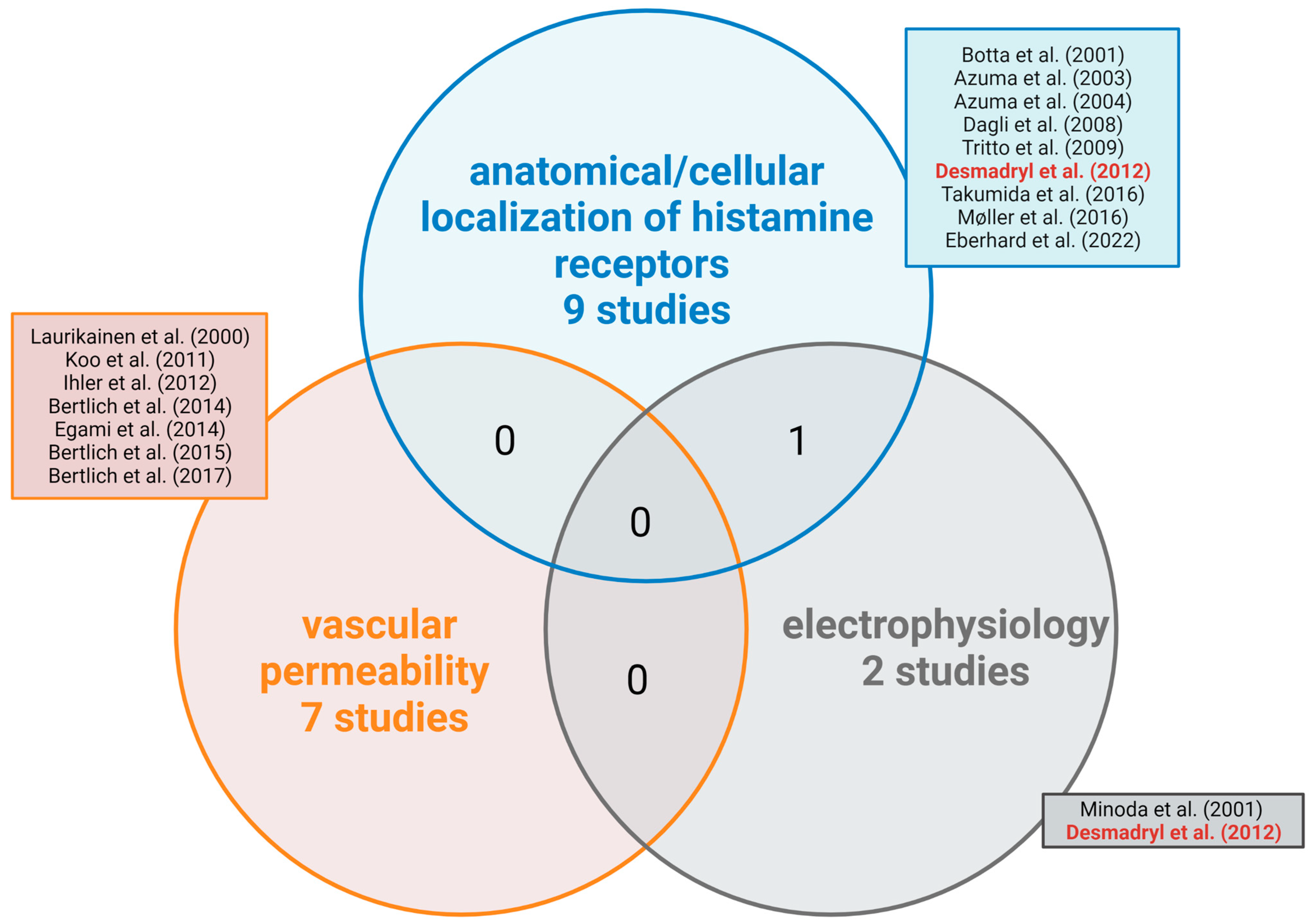
3. Results
3.1. Characteristics of Included Studies
| Author and Year | Location of Study | Species | Age or Weight | Purpose of the Study | Involved Histamine Receptors | Involved Agonists or Antagonists | Inner Ear Structures Focused on | Main Finding(s) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Laurikainen et al. (2000) [58] | Finland | Guinea pig | - | Function-related | 1 | 3 | Yes (betahistine) | Cochlea | The effect of intravenous betahistine on cochlear blood flow was found to be dose-dependent and greater in the cochlear vasculature than in the systemic vascular bed, as measured with laser Doppler. | ||
| Minoda et al. (2001) [59] | Japan | Guinea pig (Dunkin–Hartley) | 300–400 g | Function-related | 1 | 2 | Yes (pyrilamine and Cimetidine) | Cochlea | In low concentrations, histamine may act as an extracellular signal on inner hair cells or it may stimulate the afferent nerve by binding to their H1R and H2R. | ||
| Azuma et al. (2003) [38] | Japan | Rat (Wistar) | 200–280 g | Expression-related | 1 | 2 | 3 | No | Cochlea (modiolus) | H1, H2, and H3R mRNA were detected in the rat cochlear modiolus. | |
| Azuma et al. (2004) [39] | Japan | Rat (Wistar) | 200–280 g | Expression-related | 1 | 2 | 3 | No | Cochlea (spiral ganglion) | H1R, H2R, and H3R staining was observed in spiral ganglion cells in rat cochlea. Neurofilament-200 staining indicated that HR expression is specific to type I ganglion cells. | |
| Botta et al. (2008) [40] | Italy | Mouse (C57BL/6) | 3 weeks | Expression-related | 1 | 3 | No | Vestibule (semicircular canal) | Mouse vestibular epithelia express H1R. Conversely, no clear evidence for H3R expression was found. | ||
| Dagli et al. (2008) [41] | Turkey | Rabbit (New Zealand) | 2–3 kg | Expression-related | 1 | 2 | 3 | No | Endolymphatic sac | The endolymphatic sac of rabbits was positive for H1R, H2R and H3R. Cells positive for all receptors were found in the epithelial and subepithelial layers of the duct and the proximal endolymphatic sac. | |
| Tritto et al. (2009) [53] | Italy | Mouse (Swiss CD-1) | 3 weeks | Expression-related | 3 | No | Vestibule (vestibular ganglion) | Calyx and dimorphic neurons of mouse Scarpa’s ganglion express H3R. | |||
| Koo et al. (2011) [60] | USA | Mouse (C57BL/6) | 4–7 weeks | Function-related | No | Cochlea (the organ of Corti) | Systemic increases in serum levels of vasoactive peptides, like histamine, can modulate cochlear uptake of gentamicin, likely via permeability changes in the blood–labyrinth barriers. | ||||
| Desmadryl et al. (2012) [54] | France | Rat (Wistar or Long–Evans) | 2–8 days | Expression- and function-related | 3 | 4 | Yes (JNJ 10191584; JNJ 7777120; 4-methylhistamine; thioperamide; betahistine) | Vestibule (vestibular ganglion) | H4 and H3R transcripts are present in the rat vestibular ganglion, and H4R antagonists have a significant inhibitory effect on vestibular neuron activity. | ||
| Ihler et al. (2012) [61] | Germany | Guinea pig (Dunkin–Hartley) | 250–400 g | Function-related | 1 | 3 | Yes (betahistine) | Cochlea (stria vascularis) | The improved effects of higher doses of betahistine in the treatment of Meniere’s disease might be due to a corresponding increase in cochlear blood flow. | ||
| Bertlich et al. (2014) [62] | Germany | Guinea pig (Dunkin–Hartley) | 2–8 weeks | Function-related | 1 | 3 | Yes (betahistine) | Cochlea | Aminoethylpyridine and hydroxyethylpyridine are, like betahistine, able to increase cochlear blood flow significantly. The effect of aminoethylpyridine was greatest. Pyridylacetic acid had no effect on cochlear microcirculation. | ||
| Egami et al. (2014) [63] | Japan | Guinea pig (Dunkin–Hartley) | 250–300 g | Function-related | 1 | Yes (olopatadine hydrochloride) | Vestibule (endolymphatic sac) | The systemic sensitization with DNP-As produced allergy-induced experimental endolymphatic hydrops with type 1 hypersensitivity allergic reaction, and the development of these endolymphatic hydrops was prevented by H1R antagonists. | |||
| Bertlich et al. (2015) [64] | Germany | Guinea pig (Dunkin–Hartley) | 180–300 g | Function-related | 1 | 3 | Yes (betahistine) | Cochlea | Betahistine affects cochlear blood flow through histaminergic H3 heteroreceptors. | ||
| Takumida et al. (2016) [56] | Japan | Mouse (CBA/J) | 10 weeks | Expression-related | 1 | 2 | 3 | 4 | No | Cochlea and vestibule (the organ of Corti, spiral ganglion, vestibular ganglion, endolymphatic sac, macula) | All four types of histamine receptors are present in the inner ear. |
| Møller et al. (2016) [55] | Denmark | Human | - | Expression-related | 1 | 2 | 3 | 4 | No | Vestibule (endolymphatic sac) | The H1R was expressed in the epithelial lining of the endolymphatic sac, whereas H3R was expressed exclusively in the subepithelial capillary network. H2R and H4R were not expressed. |
| Bertlich et al. (2017) [65] | Germany | Guinea pig (Dunkin–Hartley) | 200–450 g | Function-related | 1 | 3 | Yes (betahistine) | Cochlea (stria vascularis) | Betahistine has an active effect on cochlear microcirculation, and its main mode of action is evidently active dilation of precapillary arterioles. | ||
| Eberhard et al. (2022) [57] | Denmark | Human | 48–69 years | Expression-related | 1 | 3 | No | Vestibule (saccule) | Intense expression of the H3R was found in the non-sensory epithelial lining cells of the human saccule, whereas there was no H1R expression. | ||
| Wang et al. (2022) [52] | China | Mouse (C57BL/6) | 4 and 48 weeks | Function-related | No | Cochlea | This study represents the first comprehensive comparison of cochlear metabolites between young and old mice, revealing significant upregulation of histamine as a notable metabolic change in the cochlea of the old C57BL/6 group. | ||||
3.2. Expression of Histamine Receptors in the Mammalian Cochlea
3.3. Expression of Histamine Receptors in the Mammalian Vestibular System
3.3.1. Semicircular Canals
3.3.2. Utricle and Saccule
3.3.3. Vestibular Ganglion
3.3.4. Endolymphatic Sac
4. Discussion
4.1. Histamine Alters Vascular Permeability
4.2. Electrophysiological Studies—The Role of Histamine in the Transmission of Electrical Signals of Sound
4.3. Histamine May Affect Hair Cell Synaptic Transmission by Binding to Histamine 3 Receptor
4.4. Clinical Application
4.5. Limitations
4.6. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parsons, M.E.; Ganellin, C.R. Histamine and its receptors. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S127–S135. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, P. The basics of histamine biology. Ann. Allergy Asthma Immunol. 2011, 106, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Y.; Liang, J.; Finkelman, F.D. Molecular Regulation of Histamine Synthesis. Front. Immunol. 2018, 9, 1392. [Google Scholar] [CrossRef] [PubMed]
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the nervous system. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.; Akdis, M.; Akdis, C.A. Regulation of the immune response and inflammation by histamine and histamine receptors. J. Allergy Clin. Immunol. 2011, 128, 1153–1162. [Google Scholar] [CrossRef]
- Tanaka, S.; Furuta, K. Roles of IgE and Histamine in Mast Cell Maturation. Cells 2021, 10, 2170. [Google Scholar] [CrossRef]
- Uvnäs, B. The molecular basis for the storage and release of histamine in rat mast cell granules. Life Sci. 1974, 14, 2355–2366. [Google Scholar] [CrossRef]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef]
- Ferstl, R.; Akdis, C.A.; O’Mahony, L. Histamine regulation of innate and adaptive immunity. FBL 2012, 17, 40–53. [Google Scholar] [CrossRef]
- Melvin, T.A.; Ramanathan, M., Jr. Role of innate immunity in the pathogenesis of allergic rhinitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 194–198. [Google Scholar] [CrossRef]
- Panula, P. Histamine receptors, agonists, and antagonists in health and disease. Handb. Clin. Neurol. 2021, 180, 377–387. [Google Scholar] [CrossRef]
- Cataldi, M.; Borriello, F.; Granata, F.; Annunziato, L.; Marone, G. Histamine receptors and antihistamines: From discovery to clinical applications. Chem. Immunol. Allergy 2014, 100, 214–226. [Google Scholar] [CrossRef]
- Sadek, B.; Stark, H. Cherry-picked ligands at histamine receptor subtypes. Neuropharmacology 2016, 106, 56–73. [Google Scholar] [CrossRef]
- Hargrove, L.; Graf-Eaton, A.; Kennedy, L.; Demieville, J.; Owens, J.; Hodges, K.; Ladd, B.; Francis, H. Isolation and characterization of hepatic mast cells from cholestatic rats. Lab. Investig. 2016, 96, 1198–1210. [Google Scholar] [CrossRef]
- Hattori, Y.; Hattori, K.; Matsuda, N. Regulation of the Cardiovascular System by Histamine. Handb. Exp. Pharmacol. 2017, 241, 239–258. [Google Scholar] [CrossRef]
- Wu, G.Y.; Zhuang, Q.X.; Zhang, X.Y.; Li, H.Z.; Wang, J.J.; Zhu, J.N. Facilitation of spinal α-motoneuron excitability by histamine and the underlying ionic mechanisms. Sheng Li Xue Bao 2019, 71, 809–823. [Google Scholar]
- Ramírez-Ponce, M.P.; Sola-García, A.; Balseiro-Gómez, S.; Maldonado, M.D.; Acosta, J.; Alés, E.; Flores, J.A. Mast Cell Changes the Phenotype of Microglia via Histamine and ATP. Cell. Physiol. Biochem. 2021, 55, 17–32. [Google Scholar] [CrossRef]
- MacGlashan, D., Jr. Histamine: A mediator of inflammation. J. Allergy Clin. Immunol. 2003, 112, S53–S59. [Google Scholar] [CrossRef]
- Orsini, J.A.; Spencer, P.A. Effects of a histamine type 2 receptor antagonist, BMY-26539-01, on equine gastric acid secretion. Can. J. Vet. Res. 2001, 65, 55–59. [Google Scholar]
- Wendell, S.G.; Fan, H.; Zhang, C. G Protein-Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action. Pharmacol. Rev. 2020, 72, 1–49. [Google Scholar] [CrossRef]
- Neumann, J.; Kirchhefer, U.; Dhein, S.; Hofmann, B.; Gergs, U. The Roles of Cardiovascular H(2)-Histamine Receptors under Normal and Pathophysiological Conditions. Front. Pharmacol. 2021, 12, 732842. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, E.; Kathmann, M. Role of the Histamine H(3) Receptor in the Central Nervous System. Handb. Exp. Pharmacol. 2017, 241, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Passani, M.B.; Giannoni, P.; Bucherelli, C.; Baldi, E.; Blandina, P. Histamine in the brain: Beyond sleep and memory. Biochem. Pharmacol. 2007, 73, 1113–1122. [Google Scholar] [CrossRef]
- Nakamura, T.; Itadani, H.; Hidaka, Y.; Ohta, M.; Tanaka, K. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem. Biophys. Res. Commun. 2000, 279, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, X.; Jiang, X.; Wilson, S.J.; Hofstra, C.L.; Blevitt, J.; Pyati, J.; Li, X.; Chai, W.; Carruthers, N.; et al. Cloning and pharmacological characterization of a fourth histamine receptor (H(4)) expressed in bone marrow. Mol. Pharmacol. 2001, 59, 420–426. [Google Scholar] [CrossRef]
- Nguyen, T.; Shapiro, D.A.; George, S.R.; Setola, V.; Lee, D.K.; Cheng, R.; Rauser, L.; Lee, S.P.; Lynch, K.R.; Roth, B.L.; et al. Discovery of a novel member of the histamine receptor family. Mol. Pharmacol. 2001, 59, 427–433. [Google Scholar] [CrossRef]
- Thurmond, R.L. The histamine H4 receptor: From orphan to the clinic. Front. Pharmacol. 2015, 6, 65. [Google Scholar] [CrossRef]
- Connelly, W.M.; Shenton, F.C.; Lethbridge, N.; Leurs, R.; Waldvogel, H.J.; Faull, R.L.; Lees, G.; Chazot, P.L. The histamine H4 receptor is functionally expressed on neurons in the mammalian CNS. Br. J. Pharmacol. 2009, 157, 55–63. [Google Scholar] [CrossRef]
- Ekdale, E.G. Form and function of the mammalian inner ear. J. Anat. 2016, 228, 324–337. [Google Scholar] [CrossRef]
- Nayagam, B.A.; Muniak, M.A.; Ryugo, D.K. The spiral ganglion: Connecting the peripheral and central auditory systems. Hear. Res. 2011, 278, 2–20. [Google Scholar] [CrossRef]
- Goutman, J.D.; Elgoyhen, A.B.; Gómez-Casati, M.E. Cochlear hair cells: The sound-sensing machines. FEBS Lett. 2015, 589, 3354–3361. [Google Scholar] [CrossRef]
- Day, B.L.; Fitzpatrick, R.C. The vestibular system. Curr. Biol. 2005, 15, R583–R586. [Google Scholar] [CrossRef]
- Khan, S.; Chang, R. Anatomy of the vestibular system: A review. NeuroRehabilitation 2013, 32, 437–443. [Google Scholar] [CrossRef]
- Keithley, E.M. Inner ear immunity. Hear. Res. 2022, 419, 108518. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Sung, C.Y.W.; Grati, M.; Chien, W. Immune responses in the mammalian inner ear and their implications for AAV-mediated inner ear gene therapy. Hear. Res. 2023, 432, 108735. [Google Scholar] [CrossRef]
- Miwa, T.; Okano, T. Role of Inner Ear Macrophages and Autoimmune/Autoinflammatory Mechanisms in the Pathophysiology of Inner Ear Disease. Front. Neurol. 2022, 13, 861992. [Google Scholar] [CrossRef]
- Shi, X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear. Res. 2016, 338, 52–63. [Google Scholar] [CrossRef]
- Azuma, H.; Sawada, S.; Takeuchi, S.; Higashiyama, K.; Kakigi, A.; Takeda, T. Expression of mRNA encoding the H1, H2, and H3 histamine receptors in the rat cochlea. Neuroreport 2003, 14, 423–425. [Google Scholar] [CrossRef]
- Azuma, H.; Sawada, S.; Takeuchi, S.; Higashiyama, K.; Kakigi, A.; Takeda, T. Immunohistochemical localization of histamine receptors in rat cochlea. Laryngoscope 2004, 114, 2249–2251. [Google Scholar] [CrossRef]
- Botta, L.; Tritto, S.; Perin, P.; Laforenza, U.; Gastaldi, G.; Zampini, V.; Zucca, G.; Valli, S.; Masetto, S.; Valli, P. Histamine H1 receptors are expressed in mouse and frog semicircular canal sensory epithelia. Neuroreport 2008, 19, 425–429. [Google Scholar] [CrossRef]
- Dagli, M.; Goksu, N.; Eryilmaz, A.; Mocan Kuzey, G.; Bayazit, Y.; Gun, B.D.; Gocer, C. Expression of histamine receptors (H(1), H(2), and H(3)) in the rabbit endolymphatic sac: An immunohistochemical study. Am. J. Otolaryngol. 2008, 29, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Szczepek, A.J.; Dudnik, T.; Karayay, B.; Sergeeva, V.; Olze, H.; Smorodchenko, A. Mast Cells in the Auditory Periphery of Rodents. Brain Sci. 2020, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Scholar, E. Histamine. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2009; pp. 1–6. [Google Scholar]
- Church, M.K.; Kolkhir, P.; Metz, M.; Maurer, M. The role and relevance of mast cells in urticaria. Immunol. Rev. 2018, 282, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Elieh-Ali-Komi, D.; Metz, M.; Siebenhaar, F.; Maurer, M. Understanding human mast cells: Lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 2022, 22, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lyu, Y.; Guo, J.; Liu, J.; Song, Y.; Fan, Z.; Li, X.; Li, N.; Zhang, D.; Wang, H. Bidirectional Transport of IgE by CD23 in the Inner Ear of Patients with Meniere’s Disease. J. Immunol. 2022, 208, 827–838. [Google Scholar] [CrossRef]
- Felix, H.; Oestreicher, E.; Felix, D.; Ehrenberger, K. Role of substance P in the peripheral vestibular and auditory system. Adv. Otorhinolaryngol. 2002, 59, 26–34. [Google Scholar]
- Ina, A.; Altintaş, D.U.; Yilmaz, M.; Uğuz, A.; Tuncer, U.; Kiroğlu, M.; Hergüner, O.; Bicakci, K. Congenital mastocytosis associated with neurosensory deafness. Pediatr. Dermatol. 2007, 24, 460–462. [Google Scholar] [CrossRef]
- Trevisan, G.; Pauluzzi, P.; Gatti, A.; Semeraro, A. Familial mastocytosis associated with neurosensory deafness. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 119–122. [Google Scholar] [CrossRef]
- Miyamura, K.; Kanzaki, Y.; Nagata, M.; Ishikawa, T. Provocation of nystagmus and deviation by type I allergy in the inner ear of the guinea pig. Ann. Allergy 1987, 58, 36–40. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, J.; Li, G.; Wang, J.; Liu, D.; Chen, L.; Song, X.; Cui, L.; Sun, Y. Application and prospect of quasi-targeted metabolomics in age-related hearing loss. Hear. Res. 2022, 424, 108604. [Google Scholar] [CrossRef]
- Tritto, S.; Botta, L.; Zampini, V.; Zucca, G.; Valli, P.; Masetto, S. Calyx and dimorphic neurons of mouse Scarpa’s ganglion express histamine H3 receptors. BMC Neurosci. 2009, 10, 70. [Google Scholar] [CrossRef]
- Desmadryl, G.; Gaboyard-Niay, S.; Brugeaud, A.; Travo, C.; Broussy, A.; Saleur, A.; Dyhrfjeld-Johnsen, J.; Wersinger, E.; Chabbert, C. Histamine H4 receptor antagonists as potent modulators of mammalian vestibular primary neuron excitability. Br. J. Pharmacol. 2012, 167, 905–916. [Google Scholar] [CrossRef]
- Møller, M.N.; Kirkeby, S.; Vikeså, J.; Nielsen, F.C.; Caye-Thomasen, P. Expression of histamine receptors in the human endolymphatic sac: The molecular rationale for betahistine use in Menieres disease. Eur. Arch. Otorhinolaryngol. 2016, 273, 1705–1710. [Google Scholar] [CrossRef]
- Takumida, M.; Takumida, H.; Anniko, M. Localization of histamine (H1, H2, H3 and H4) receptors in mouse inner ear. Acta Otolaryngol. 2016, 136, 537–544. [Google Scholar] [CrossRef]
- Eberhard, K.E.; Kirkeby, S.; Hansen, L.J.; Cayé-Thomasen, P. Neurotransmitter and neurotransmitter receptor expression in the saccule of the human vestibular system. Prog. Neurobiol. 2022, 212, 102238. [Google Scholar] [CrossRef]
- Laurikainen, E.; Miller, J.F.; Pyykkö, I. Betahistine effects on cochlear blood flow: From the laboratory to the clinic. Acta Otolaryngol. Suppl. 2000, 544, 5–7. [Google Scholar] [CrossRef]
- Minoda, R.; Toriya, T.; Masuyama, K.; Yumoto, E. The effects of histamine and its antagonists on the cochlear microphonic and the compound action potential of the guinea pig. Auris Nasus Larynx 2001, 28, 219–222. [Google Scholar] [CrossRef]
- Koo, J.W.; Wang, Q.; Steyger, P.S. Infection-mediated vasoactive peptides modulate cochlear uptake of fluorescent gentamicin. Audiol. Neurootol. 2011, 16, 347–358. [Google Scholar] [CrossRef]
- Ihler, F.; Bertlich, M.; Sharaf, K.; Strieth, S.; Strupp, M.; Canis, M. Betahistine exerts a dose-dependent effect on cochlear stria vascularis blood flow in guinea pigs in vivo. PLoS ONE 2012, 7, e39086. [Google Scholar] [CrossRef]
- Bertlich, M.; Ihler, F.; Sharaf, K.; Weiss, B.G.; Strupp, M.; Canis, M. Betahistine metabolites, aminoethylpyridine, and hydroxyethylpyridine increase cochlear blood flow in guinea pigs in vivo. Int. J. Audiol. 2014, 53, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Egami, N.; Kakigi, A.; Takeda, T.; Takeda, S.; Nishioka, R.; Hyodo, M.; Yamasoba, T. Type 1 allergy-induced endolymphatic hydrops and the suppressive effect of H1-receptor antagonist (olopatadine hydrochloride). Otol. Neurotol. 2014, 35, e104–e109. [Google Scholar] [CrossRef] [PubMed]
- Bertlich, M.; Ihler, F.; Freytag, S.; Weiss, B.G.; Strupp, M.; Canis, M. Histaminergic H3-Heteroreceptors as a Potential Mediator of Betahistine-Induced Increase in Cochlear Blood Flow. Audiol. Neurootol. 2015, 20, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Bertlich, M.; Ihler, F.; Weiss, B.G.; Freytag, S.; Strupp, M.; Jakob, M.; Canis, M. Role of capillary pericytes and precapillary arterioles in the vascular mechanism of betahistine in a guinea pig inner ear model. Life Sci. 2017, 187, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Ashina, K.; Tsubosaka, Y.; Nakamura, T.; Omori, K.; Kobayashi, K.; Hori, M.; Ozaki, H.; Murata, T. Histamine Induces Vascular Hyperpermeability by Increasing Blood Flow and Endothelial Barrier Disruption In Vivo. PLoS ONE 2015, 10, e0132367. [Google Scholar] [CrossRef]
- Park-Windhol, C.; D’Amore, P.A. Disorders of Vascular Permeability. Annu. Rev. Pathol. 2016, 11, 251–281. [Google Scholar] [CrossRef]
- Dvorak, H.F. Vascular permeability to plasma, plasma proteins, and cells: An update. Curr. Opin. Hematol. 2010, 17, 225–229. [Google Scholar] [CrossRef]
- Housley, G.D.; Norris, C.H.; Guth, P.S. Histamine and related substances influence neurotransmission in the semicircular canal. Hear. Res. 1988, 35, 87–97. [Google Scholar] [CrossRef]
- Bledsoe, S.C., Jr.; Sinard, R.J.; Allen, S.J. Analysis of histamine as a hair-cell transmitter in the lateral line of Xenopus laevis. Hear. Res. 1989, 38, 81–93. [Google Scholar] [CrossRef]
- Botta, L.; Mira, E.; Valli, S.; Perin, P.; Zucca, G.; Valli, P. Effects of betahistine on vestibular receptors of the frog. Acta Otolaryngol. 1998, 118, 519–523. [Google Scholar] [CrossRef]
- Chávez, H.; Vega, R.; Soto, E. Histamine (H3) receptors modulate the excitatory amino acid receptor response of the vestibular afferents. Brain Res. 2005, 1064, 1–9. [Google Scholar] [CrossRef]
- Botta, L.; Mira, E.; Valli, S.; Zucca, G.; Benvenuti, C.; Fossati, A.; Soto, E.; Guth, P.; Valli, P. Effects of betahistine and of its metabolites on vestibular sensory organs. Acta Otorhinolaryngol. Ital. 2001, 21, 24–30. [Google Scholar]
- Glowatzki, E.; Grant, L.; Fuchs, P. Hair cell afferent synapses. Curr. Opin. Neurobiol. 2008, 18, 389–395. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, T.Y.; Chang, C.Y.; Huang, S.K.; Wang, S.J. Ciproxifan, a histamine H(3) receptor antagonist and inverse agonist, presynaptically inhibits glutamate release in rat hippocampus. Toxicol. Appl. Pharmacol. 2017, 319, 12–21. [Google Scholar] [CrossRef]
- Kim, S.H.; Nam, G.S.; Choi, J.Y. Pathophysiologic Findings in the Human Endolymphatic Sac in Endolymphatic Hydrops: Functional and Molecular Evidence. Ann. Otol. Rhinol. Laryngol. 2019, 128, 76s–83s. [Google Scholar] [CrossRef]
- Lo, W.W.; Daniels, D.L.; Chakeres, D.W.; Linthicum, F.H., Jr.; Ulmer, J.L.; Mark, L.P.; Swartz, J.D. The endolymphatic duct and sac. AJNR Am. J. Neuroradiol. 1997, 18, 881–887. [Google Scholar]
- Nakashima, T.; Pyykkö, I.; Arroll, M.A.; Casselbrant, M.L.; Foster, C.A.; Manzoor, N.F.; Megerian, C.A.; Naganawa, S.; Young, Y.H. Meniere’s disease. Nat. Rev. Dis. Primers 2016, 2, 16028. [Google Scholar] [CrossRef]
- Tomoda, K.; Nagata, M.; Harada, N.; Iwai, H.; Yamashita, T. Effect of histamine on intracellular Ca2+ concentration in guinea pig isolated vestibular hair cells. Acta Otolaryngol. Suppl. 1997, 528, 37–40. [Google Scholar]
- Holmes, S.; Lalwani, A.K.; Mankekar, G. Is Betahistine Effective in the Treatment of Ménière’s Disease? Laryngoscope 2021, 131, 2639–2640. [Google Scholar] [CrossRef]
- Miller, D.J.; O’Dowd, A. Vascular smooth muscle actions of carnosine as its zinc complex are mediated by histamine H(1) and H(2) receptors. Biochemistry 2000, 65, 798–806. [Google Scholar]
- Lieberman, P. Histamine, antihistamines, and the central nervous system. Allergy Asthma Proc. 2009, 30, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Molina-Hernández, A.; Díaz, N.F.; Arias-Montaño, J.A. Histamine in brain development. J. Neurochem. 2012, 122, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Panula, P.; Sundvik, M.; Karlstedt, K. Developmental roles of brain histamine. Trends Neurosci. 2014, 37, 159–168. [Google Scholar] [CrossRef] [PubMed]

| Author and Year | Species | Age or Weight | Experimental Methods | H1R | Locations | H2R | Locations | H3R | Locations | H4R | Locations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Azuma et al. (2003) [38] | Rat (Wistar) | 200–280 g | PCR | ✓ | modiolus | ✓ | modiolus | ✓ | modiolus | n.d. | |
| Azuma et al. (2004) [39] | Rat (Wistar) | 200–280 g | IHC | ✓ | spiral ganglion | ✓ | spiral ganglion | ✓ | spiral ganglion | n.d. | |
| Botta et al. (2008) [40] | Mouse (C57BL/6) | 3 weeks | PCR; IF | ✓ | semicircular canal | n.d. | ✓ | semicircular canal | n.d. | ||
| Dagli et al. (2008) [41] | Rabbit (New Zealand) | 2000–3000 g | IHC | ✓ | endolymphatic sac | ✓ | endolymphatic sac | ✓ | endolymphatic sac | n.d. | |
| Tritto et al. (2009) [52] | Mouse (Swiss CD-1) | 3 weeks | PCR; IF | n.d. | n.d. | ✓ | vestibular ganglion | n.d. | |||
| Desmadryl et al. (2012) [53] | Rat (Wistar) | 2–8 days | PCR | n.d. | n.d. | ✓ | vestibular ganglion | ✓ | vestibular ganglion | ||
| Møller et al. (2016) [54] | Human | n.s. | IHC, microarrays | ✓ | endolymphatic sac (IHC, microarrays) | ✓ | endolymphatic sac (microarrays, expression greater than in dura) | ✓ | endolymphatic sac (IHC, microarrays) | ✓ | endolymphatic sac (microarrays, expression lesser than in dura) |
| Takumida et al. (2016) [55] | Mouse (CBA/J) | 10 weeks (25–30 g) | IHC; PCR | ✓ | stria vascularis; the organ of Corti; spiral ganglion; vestibular ganglion; vestibular epithelium; endolymphatic sac | ✓ | spiral ganglion; vestibular ganglion; vestibular epithelium; endolymphatic sac | ✓ | spiral ligament; the organ of Corti; spiral ganglion; vestibular ganglion; vestibular epithelium; endolymphatic sac | ✓ | spiral ligament; the organ of Corti; spiral ganglion; vestibular ganglion; vestibular epithelium; endolymphatic sac |
| Eberhard et al. (2022) [56] | Human | 48–69 years | IHC | neg. | saccule | n.d. | ✓ | saccule | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Domarecka, E.; Szczepek, A.J. Histamine and Its Receptors in the Mammalian Inner Ear: A Scoping Review. Brain Sci. 2023, 13, 1101. https://doi.org/10.3390/brainsci13071101
Kong L, Domarecka E, Szczepek AJ. Histamine and Its Receptors in the Mammalian Inner Ear: A Scoping Review. Brain Sciences. 2023; 13(7):1101. https://doi.org/10.3390/brainsci13071101
Chicago/Turabian StyleKong, Lingyi, Ewa Domarecka, and Agnieszka J. Szczepek. 2023. "Histamine and Its Receptors in the Mammalian Inner Ear: A Scoping Review" Brain Sciences 13, no. 7: 1101. https://doi.org/10.3390/brainsci13071101
APA StyleKong, L., Domarecka, E., & Szczepek, A. J. (2023). Histamine and Its Receptors in the Mammalian Inner Ear: A Scoping Review. Brain Sciences, 13(7), 1101. https://doi.org/10.3390/brainsci13071101







