Treadmill Training Plus Semi-Immersive Virtual Reality in Parkinson’s Disease: Results from a Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Participants
2.2. Procedures
2.2.1. Conventional Gait Training (Control Group)
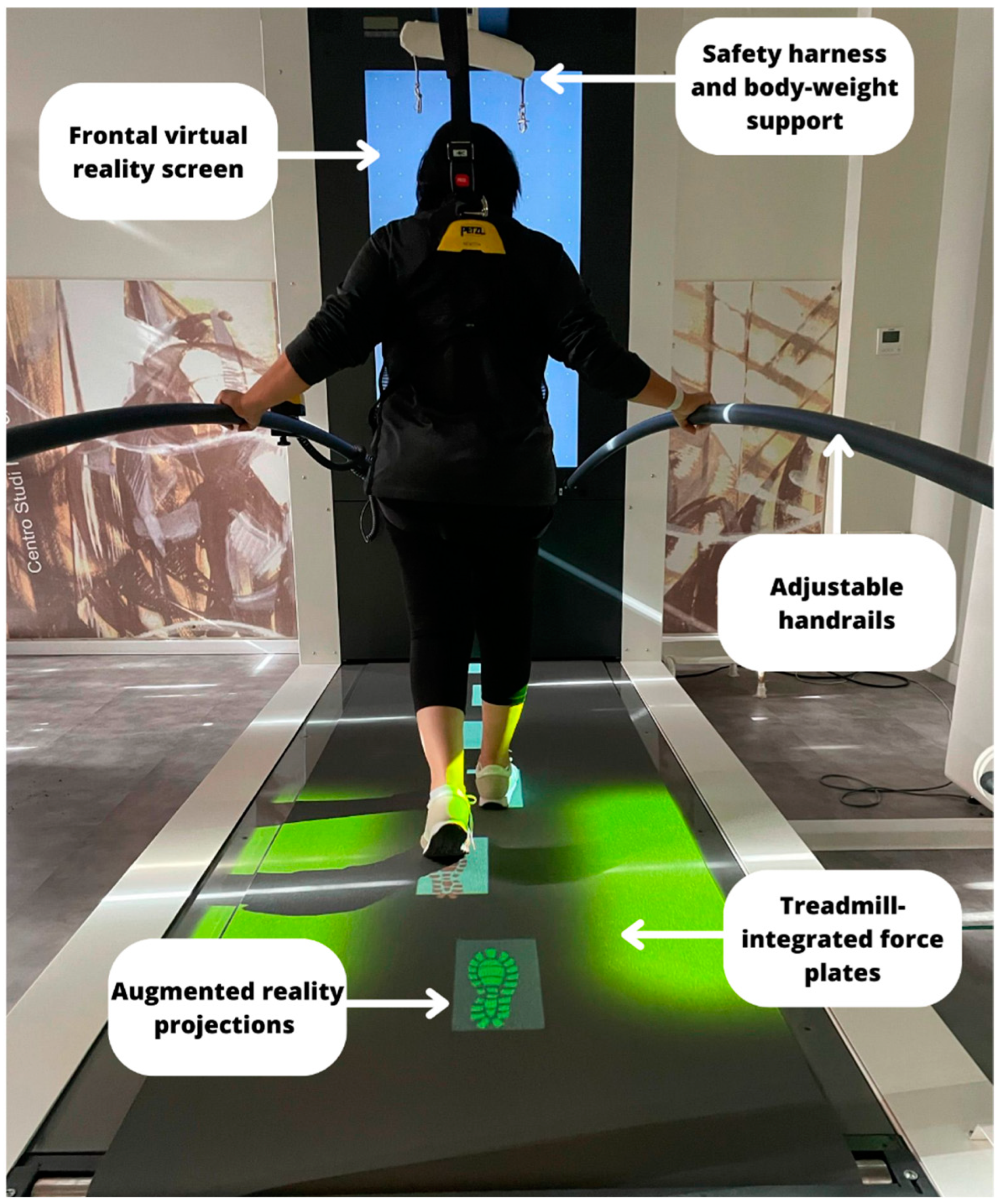
2.2.2. The C-Mill Gait Training (Experimental Group)
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s disease. Subcell. Biochem. 2012, 65, 389–455. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, L.; Di Santo, A.; Caminiti, M.L.; De Liso, A.; Shah, S.A.; Ricci, L.; Di Lazzaro, V. Gait Analysis in Parkinson’s Disease: An Overview of the Most Accurate Markers for Diagnosis and Symptoms Monitoring. Sensors 2020, 20, 3529. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, M.; De Nunzio, A.M.; Quartarone, A.; Militi, A.; Petralito, F.; Calabrò, R.S. Gait Analysis in Neurorehabilitation: From Research to Clinical Practice. Bioengineering 2023, 10, 785. [Google Scholar] [CrossRef]
- Gao, L.L.; Zhang, J.R.; Chan, P.; Wu, T. Levodopa Effect on Basal Ganglia Motor Circuit in Parkinson’s Disease. CNS Neurosci. Ther. 2017, 23, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.A.; Poletti, M. Neural and Behavioral Substrates of Subtypes of Parkinson’s Disease. Front. Syst. Neurosci. 2013, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, G.; Maravita, A.; Zollo, L.; Guglielmelli, E.; Di Lazzaro, V. Augmentation-Related Brain Plasticity. Front. Syst. Neurosci. 2014, 8, 109. [Google Scholar] [CrossRef]
- Radder, D.L.M.; Lígia Silva de Lima, A.; Domingos, J.; Keus, S.H.J.; van Nimwegen, M.; Bloem, B.R.; de Vries, N.M. Physiotherapy in Parkinson’s Disease: A Meta-Analysis of Present Treatment Modalities. Neurorehabil. Neural. Repair. 2020, 34, 871–880. [Google Scholar] [CrossRef]
- Ghai, S.; Ghai, I.; Schmitz, G.; Effenberg, A.O. Effect of Rhythmic Auditory Cueing on Parkinsonian Gait: A Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 506. [Google Scholar] [CrossRef]
- Muthukrishnan, N.; Abbas, J.J.; Shill, H.A.; Krishnamurthi, N. Cueing Paradigms to Improve Gait and Posture in Parkinson’s Disease: A Narrative Review. Sensors 2019, 19, 5468. [Google Scholar] [CrossRef]
- Naro, A.; Calabrò, R.S. What Do We Know about The Use of Virtual Reality in the Rehabilitation Field? A Brief Overview. Electronics 2021, 10, 1042. [Google Scholar] [CrossRef]
- Bonanno, M.; De Luca, R.; De Nunzio, A.M.; Quartarone, A.; Calabrò, R.S. Innovative Technologies in the Neurorehabilitation of Traumatic Brain Injury: A Systematic Review. Brain Sci. 2022, 12, 1678. [Google Scholar] [CrossRef]
- Feng, H.; Li, C.; Liu, J.; Wang, L.; Ma, J.; Li, G.; Gan, L.; Shang, X.; Wu, Z. Virtual Reality Rehabilitation Versus Conventional Physical Therapy for Improving Balance and Gait in Parkinson’s Disease Patients: A Randomized Controlled Trial. Med. Sci. Monit. 2019, 25, 4186–4192. [Google Scholar] [CrossRef] [PubMed]
- Canning, C.G.; Allen, N.E.; Nackaerts, E.; Paul, S.S.; Nieuwboer, A.; Gilat, M. Virtual Reality in Research and Rehabilitation of Gait and Balance in Parkinson Disease. Nat. Rev. Neurol. 2020, 16, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Sunzi, K.; Dai, F.; Liu, X.; Wang, Y.; Zhang, B.; He, L.; Ju, M. Effects of Virtual Reality Rehabilitation Training on Gait and Balance in Patients with Parkinson’s Disease: A Systematic Review. PLoS ONE 2019, 14, e0224819. [Google Scholar] [CrossRef] [PubMed]
- Gulcan, K.; Guclu-Gunduz, A.; Yasar, E.; Ar, U.; Sucullu Karadag, Y.; Saygili, F. The Effects of Augmented and Virtual Reality Gait Training on Balance and Gait in Patients with Parkinson’s Disease. Acta Neurol Belg. 2022, 28, 1–9. [Google Scholar] [CrossRef]
- Medical, B.V.M. C-Mill, The Cutting-Edge Balance and Gait Treadmill. 2022. Available online: https://www.motekmedical.com/solution/c-mill/ (accessed on 1 August 2022).
- Miranda-Cantellops, N.; Tiu, T.K. Berg Balance Testing. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Scura, D.; Munakomi, S. Tinetti Gait and Balance Test. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK578181/ (accessed on 20 November 2022).
- Caronni, A.; Picardi, M.; Redaelli, V.; Antoniotti, P.; Pintavalle, G.; Aristidou, E.; Gilardone, G.; Carpinella, I.; Lencioni, T.; Arcuri, P.; et al. The Falls Efficacy Scale International Is a Valid Measure to Assess the Concern about Falling and Its Changes Induced by Treatments. Clin. Rehabil. 2022, 36, 558–570. [Google Scholar] [CrossRef]
- Browne, W.; Nair, B.K.R. The Timed Up and Go test. Med. J. Aust. 2019, 210, 13–14.e1. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.M.; Fritz, S.L.; Krotish, D.E. Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults. J. Geriatr. Phys. Ther. 2013, 36, 24–30. [Google Scholar] [CrossRef]
- Agarwala, P.; Salzman, S.H. Six-Minute Walk Test: Clinical Role, Technique, Coding, and Reimbursement. Chest 2020, 157, 603–611. [Google Scholar] [CrossRef]
- Ravaud, J.F.; Delcey, M.; Yelnik, A. Construct Validity of The Functional Independence Measure (FIM): Questioning the Unidimensionality of The Scale and The “Value” of FIM Scores. Scand. J. Rehabil. Med. 1999, 31, 31–41. [Google Scholar]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society-Sponsored Revision of The Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale Presentation and Clinimetric Testing Results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 April 2022).
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Bekkers, E.M.J.; Mirelman, A.; Alcock, L.; Rochester, L.; Nieuwhof, F.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; Cereatti, A.; Croce, U.D.; et al. Do Patients with Parkinson’s Disease with Freezing of Gait Respond Differently Than Those without to Treadmill Training Augmented by Virtual Reality? Neurorehabil. Neural. Repair. 2020, 34, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Earhart, G.M. Dynamic Control of Posture Across Locomotor Tasks. Mov. Disord. 2013, 28, 1501–1508. [Google Scholar] [CrossRef]
- Pelosin, E.; Ponte, C.; Putzolu, M.; Lagravinese, G.; Hausdorff, J.M.; Nieuwboer, A.; Ginis, P.; Rochester, L.; Alcock, L.; Bloem, B.R.; et al. Motor-Cognitive Treadmill Training with Virtual Reality in Parkinson’s Disease: The Effect of Training Duration. Front. Aging Neurosci. 2022, 13, 753381. [Google Scholar] [CrossRef]
- Lu, Y.; Ge, Y.; Chen, W.; Xing, W.; Wei, L.; Zhang, C.; Yang, Y. The effectiveness of Virtual Reality for Rehabilitation of Parkinson Disease: An Overview of Systematic Reviews with Meta-Analyses. Syst. Rev. 2022, 11, 50. [Google Scholar] [CrossRef]
- Phu, S.; Vogrin, S.; Al Saedi, A.; Duque, G. Balance training using virtual reality improves balance and physical performance in older adults at high risk of falls. Clin. Interv. Aging 2019, 14, 1567–1577. [Google Scholar] [CrossRef]
- Kwon, S.H.; Park, J.K.; Koh, Y.H. A Systematic Review and Meta-Analysis on The Effect of Virtual Reality-Based Rehabilitation for People with Parkinson’s Disease. J. Neuroeng. Rehabil. 2023, 20, 94. [Google Scholar] [CrossRef]
- Wang, W.; Wong, S.S.-L.; Lai, F.H.-Y. The Effect of Virtual Reality Rehabilitation on Balance in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Electronics 2021, 10, 1003. [Google Scholar] [CrossRef]
- Duncan, L.R.; Hall, C.R.; Wilson, P.M.; Jenny, O. Exercise Motivation: A Cross-Sectional Analysis Examining Its Relationships with Frequency, Intensity, and Duration of Exercise. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 7. [Google Scholar] [CrossRef]
- Kefaliakos, A.; Pliakos, I.; Kiekkas, P.; Charalampidou, M.; Diomidous, M. Virtual Reality in the Rehabilitation of Patients with Neurological Disorders. Stud. Health Technol. Inform. 2016, 226, 45–47. [Google Scholar]
- Bonassi, G.; Lagravinese, G.; Bisio, A.; Ruggeri, P.; Pelosin, E.; Bove, M.; Avanzino, L. Consolidation and retention of motor skill after motor imagery training. Neuropsychologia 2020, 143, 107472. [Google Scholar] [CrossRef]
- Fan, H.; Luo, Z. Functional Integration of Mirror Neuron System and Sensorimotor Cortex Under Virtual Self-Actions Visual Perception. Behav. Brain Res. 2022, 423, 113784. [Google Scholar] [CrossRef]
- Lahuerta-Martín, S.; Llamas-Ramos, R.; Llamas-Ramos, I. Effectiveness of Therapies Based on Mirror Neuron System to Treat Gait in Patients with Parkinson’s Disease-A Systematic Review. J. Clin. Med. 2022, 11, 4236. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Leo, A.; De Luca, R.; Balletta, T.; Buda, A.; La Rosa, G.; Bramanti, A.; Bramanti, P. The Role of Virtual Reality in Improving Motor Performance as Revealed by EEG: A Randomized Clinical Trial. J. Neuroeng. Rehabil. 2017, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- McCrum, C.; Bhatt, T.S.; Gerards, M.H.G.; Karamanidis, K.; Rogers, M.W.; Lord, S.R.; Okubo, Y. Perturbation-Based Balance Training: Principles, Mechanisms and Implementation in Clinical Practice. Front. Sports Act. Living 2022, 4, 1015394. [Google Scholar] [CrossRef] [PubMed]
- Ivanenko, Y.; Gurfinkel, V.S. Human Postural Control. Front. Neurosci. 2018, 12, 171. [Google Scholar] [CrossRef]
- Mirelman, A.; Rochester, L.; Reelick, M.; Nieuwhof, F.; Pelosin, E.; Abbruzzese, G.; Dockx, K.; Nieuwboer, A.; Hausdorff, J.M. V-TIME: A Treadmill Training Program Augmented by Virtual Reality to Decrease Fall Risk in Older Adults: Study Design of a Randomized Controlled Trial. BMC Neurol. 2013, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.P.; de-Almeida, S.B.; Bonfadini, J.D.C.; Carneiro, A.H.S.; Luna, J.R.G.D.; Alencar, M.S.D.; Viana-Júnior, A.B.; Rodrigues, P.G.B.; de Sousa Pereira, I.; de Sá Roriz-Filho, J.; et al. Falls in Parkinson’s Disease: The Impact of Disease Progression, Treatment, and Motor Complications. Dement. Neuropsychol. 2022, 16, 153–161. [Google Scholar] [CrossRef] [PubMed]

| All Participants (N = 20) | Experimental Group (N = 10) | Control Group (N = 10) | p-Value | |
|---|---|---|---|---|
| Age | 65 ± 8.28 | 64.5 ± 10.84 | 65. 5 ± 10.36 | 0.75 |
| Gender | 0.06 | |||
| Female | 7 (35%) | 1 (10%) | 6 (60%) | |
| Male | 13 (65%) | 9 (90%) | 4 (40%) | |
| Hoehn & Yahr | 3 ± 8 | 2.5 ± 0.78 | 3.5 ± 0.36 | 0.07 |
| Years of illness | 10.8 ± 7.75 | 8.3 ± 4.09 | 13 ± 8.4 | 0.16 |
| 6MWT | 180.8 ± 144.4 | 265 ± 81.07 | 149.5 ± 105 | 0.48 |
| 10MWT | 6.7 ± 4.6 | 6.7 ± 4.8 | 6.7 ± 4.6 | 0.67 |
| TUG | ||||
| Right | 14.4 ± 10.6 | 12.1 ± 3.8 | 16.6 ± 14.5 | 0.72 |
| Left | 15.9 ± 12.5 | 14.3 ± 9.23 | 17.4 ± 15.4 | 0.67 |
| BBS | 35.7 ± 15.41 | 45.1 ± 15.60 | 36.3 ± 16.15 | 0.24 |
| TS | 15.9 ± 8.26 | 18.6 ± 7.9 | 13.2 ± 8.09 | 0.14 |
| FES-I | 38.2 ± 15.64 | 31.7 ± 12.64 | 44.8 ± 16.17 | 0.11 |
| UPDRS | 37.7 ± 14.77 | 37.6 ± 18.6 | 37.9 ± 10.56 | 0.67 |
| FIM | 102.9 ± 16.99 | 111.7 ± 18.12 | 94.1 ± 19.27 | 0.14 |
| CG Exercise Training | EG Exercise Training | |
|---|---|---|
| Setting | Conventional training with the manual guidance of the physiotherapist. | Innovative training with C-Mill treadmill equipped with semi-immersive VR |
| Gait training | Gait with obstacles (e.g., bricks, boxes) with different shapes and colours. Gait in tandem with paths created by physiotherapists, using chairs, boxes, traffic cones, sandbags. Walking at different speeds according to audio stimuli provided by therapist (e.g., music and voice of therapist). Walking with frequent direction changes according to audio (e.g., music and voice of therapist) and visual (e.g., coloured tape on the floor) stimuli. | Gait with virtual obstacles projected on the treadmill, with audio-visual stimuli according to target. Gait in tandem with virtual traffic cones, or virtual country paths projected on the treadmill, in addition to audio-visual stimuli. Walking on treadmill at different speeds based on virtual exercises (e.g., “Italian Alps”), projected on frontal screen. Walking on treadmill with frequent lateral direction changes according by virtual projections on the screen (e.g., Arkanoid). |
| Outcome Measures | Median (First–Third Quartile) at T0 | Median (First–Third Quartile) at T1 | p-Value * (T0–T1) | ES | |
|---|---|---|---|---|---|
| EG | 6MWT | 260 (183.7–325) | 360 (345–381) | 0.0005 | 0.93 |
| 10MWT | 5.78 (4.56–71.5) | 5.27 (4.06–5.85) | 0.11 | 0.33 | |
| TUG | |||||
| Right | 9.8 (9.24–15.93) | 8.6 (7.19–12.29) | 0.03 | 0.58 | |
| Left | 10.41 (9.45–18.17) | 9.36 (7.5–11.72) | 0.05 | 0.46 | |
| BBS | 43 (40–51.23) | 52.5 (50.25–55.25) | 0.006 | 1.16 | |
| TS | 20.5 (15–24.2) | 27 (23.5–28) | 0.002 | 0.89 | |
| FES-I | 29 (21.7–41.5) | 23 (21.5–33) | 0.03 | 0.46 | |
| UPDRS-III | 32 (20.75–58) | 25.5 (14.75–44.25) | 0.002 | 0.48 | |
| FIM | 110.5 (106.5–118.2) | 120.5 (117.5–123.7) | 0.004 | 1.13 | |
| CG | 6MWT | 45 (0–179.5) | 155 (0–294) | 0.01 | 0.41 |
| 10MWT | 6.43 (2.61–11.89) | 6.19 (2.6–8.8) | 0.09 | 0.20 | |
| TUG | |||||
| Right | 13.12 (5.67–25.8) | 11.35 (5.46–24.4) | 0.05 | 0.15 | |
| Left | 15.09 (6.05–25.8) | 12.75 (5.2–22.5) | 0.02 | 0.15 | |
| BBS | 25 (11.5–41.75) | 40 (13.25–47.25) | 0.004 | 0.50 | |
| TS | 11 (7–22.75) | 20.5 (8.75–25.25) | 0.01 | 0.59 | |
| FES-I | 49.5 (32.2–58.2) | 47 (28.5–53.25) | 0.09 | 0.10 | |
| UPDRS-III | 37 (28.25–46.25) | 29 (22.75–42) | 0.01 | 0.60 | |
| FIM | 95 (79.7–110.2) | 104.5 (82–113) | 0.005 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pullia, M.; Ciatto, L.; Andronaco, G.; Donato, C.; Aliotta, R.E.; Quartarone, A.; De Cola, M.C.; Bonanno, M.; Calabrò, R.S.; Cellini, R. Treadmill Training Plus Semi-Immersive Virtual Reality in Parkinson’s Disease: Results from a Pilot Study. Brain Sci. 2023, 13, 1312. https://doi.org/10.3390/brainsci13091312
Pullia M, Ciatto L, Andronaco G, Donato C, Aliotta RE, Quartarone A, De Cola MC, Bonanno M, Calabrò RS, Cellini R. Treadmill Training Plus Semi-Immersive Virtual Reality in Parkinson’s Disease: Results from a Pilot Study. Brain Sciences. 2023; 13(9):1312. https://doi.org/10.3390/brainsci13091312
Chicago/Turabian StylePullia, Massimo, Laura Ciatto, Giuseppe Andronaco, Concetta Donato, Rosario Ermes Aliotta, Angelo Quartarone, Maria Cristina De Cola, Mirjam Bonanno, Rocco Salvatore Calabrò, and Roberta Cellini. 2023. "Treadmill Training Plus Semi-Immersive Virtual Reality in Parkinson’s Disease: Results from a Pilot Study" Brain Sciences 13, no. 9: 1312. https://doi.org/10.3390/brainsci13091312






