Velocity-Selective Arterial Spin Labeling Perfusion in Monitoring High Grade Gliomas Following Therapy: Clinical Feasibility at 1.5T and Comparison with Dynamic Susceptibility Contrast Perfusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Imaging Protocol
2.3. Data Analysis
2.4. Statistical Analysis
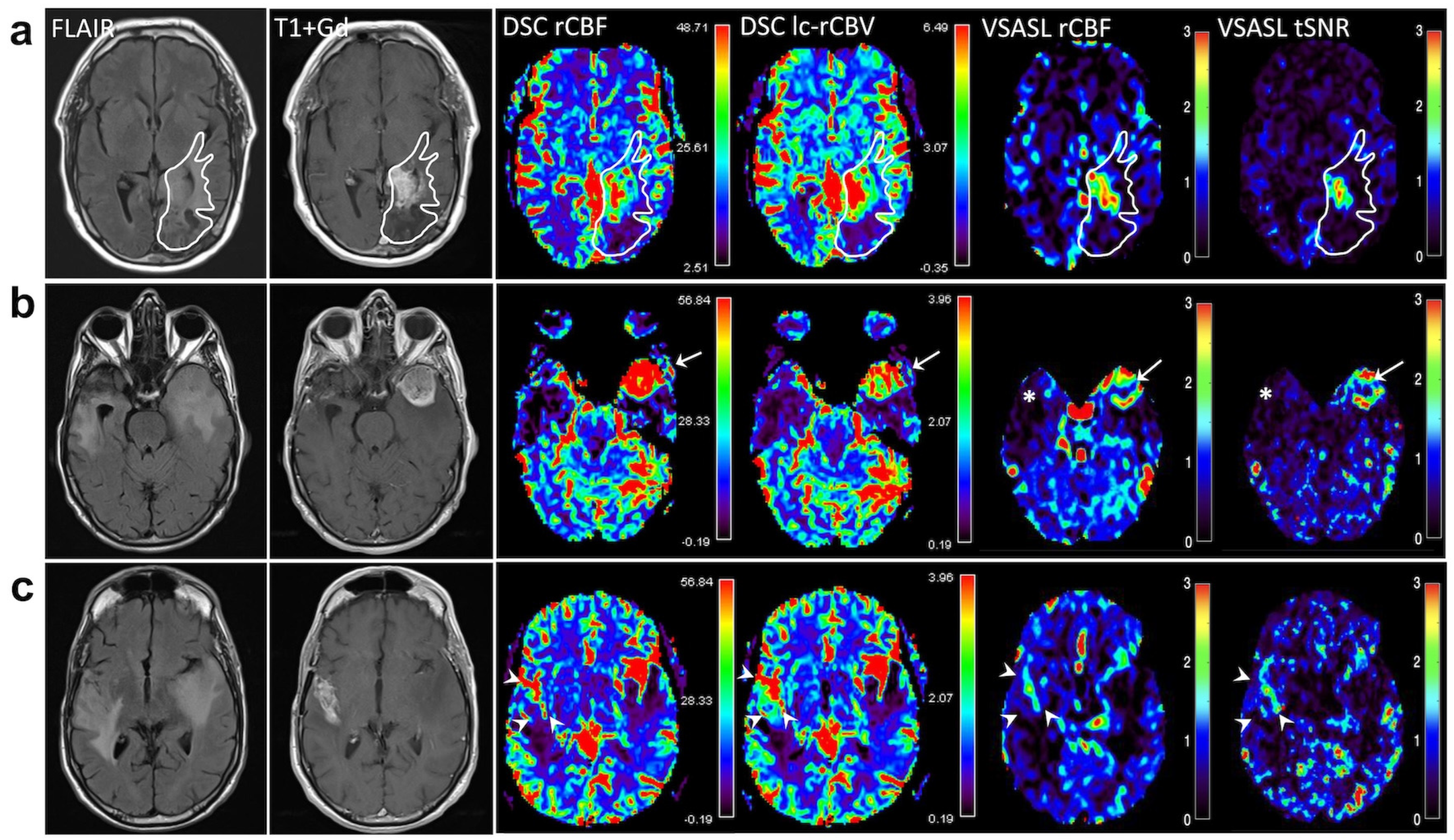
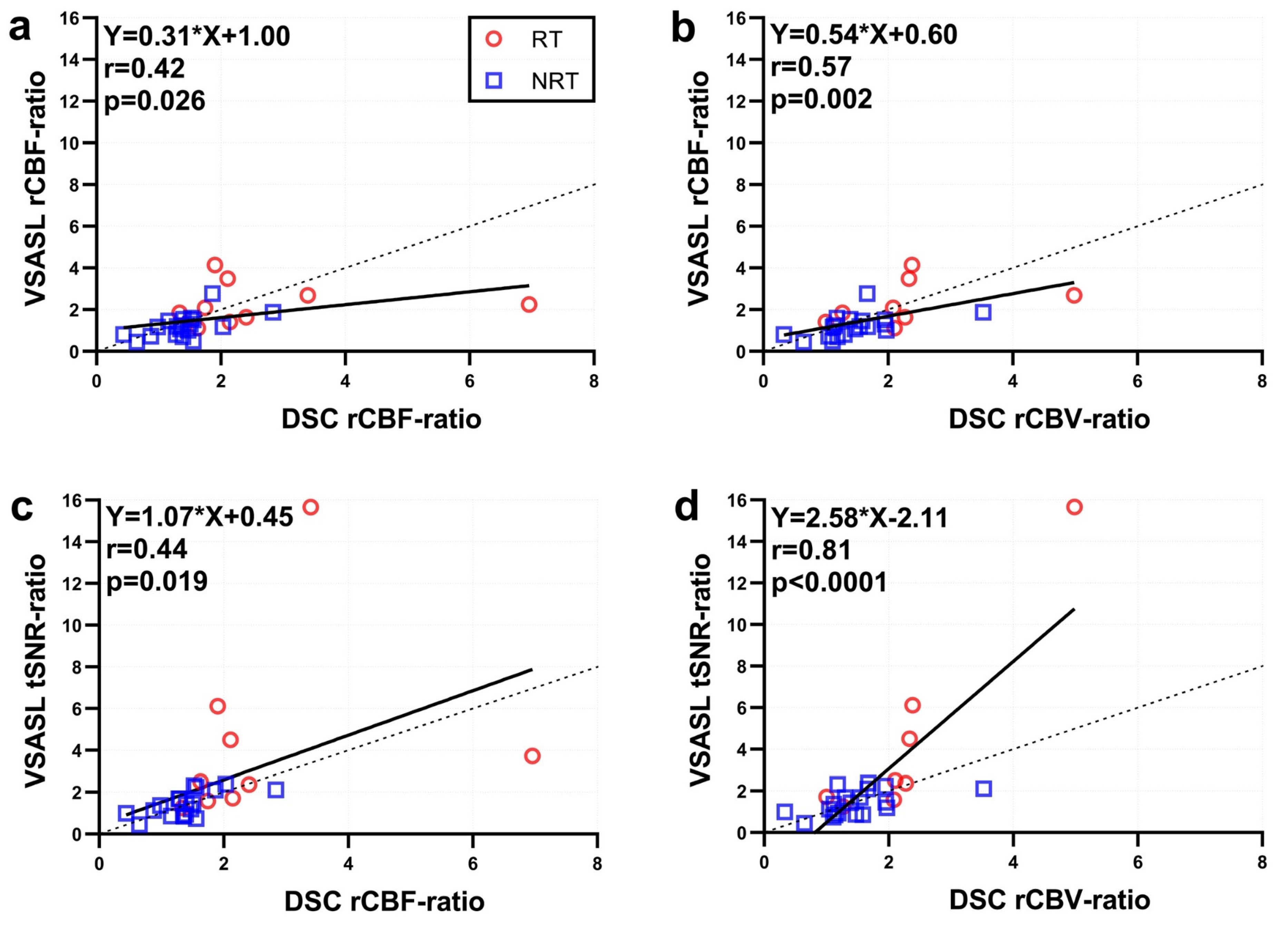
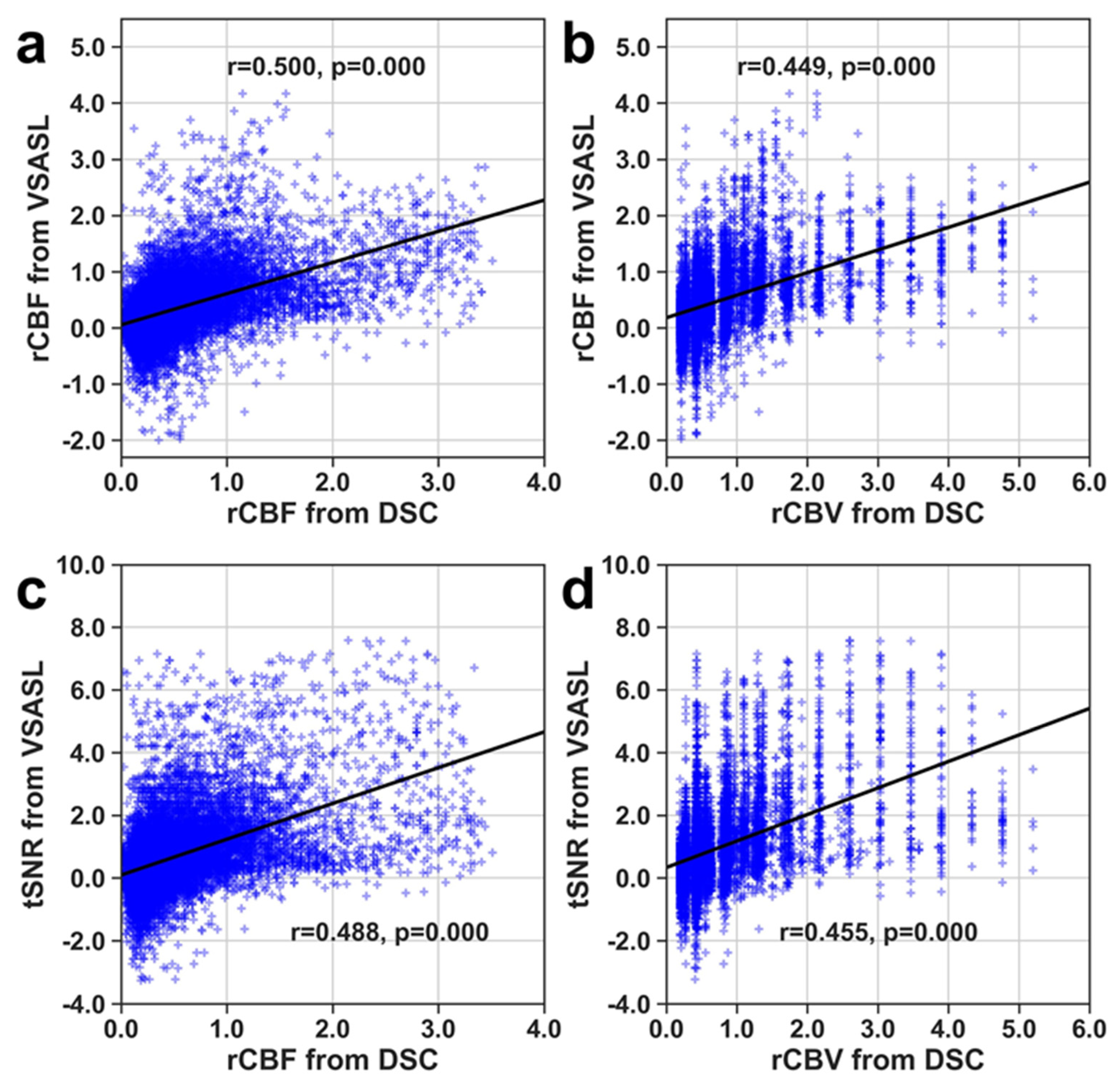
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boxerman, J.L.; Schmainda, K.M.; Weisskoff, R.M. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am. J. Neuroradiol. 2006, 27, 859–867. [Google Scholar]
- Mangla, R.; Ginat, D.T.; Kamalian, S.; Milano, M.T.; Korones, D.N.; Walter, K.A.; Ekholm, S. Correlation between progression free survival and dynamic susceptibility contrast MRI perfusion in WHO grade III glioma subtypes. J. Neurooncol. 2014, 116, 325–331. [Google Scholar] [CrossRef]
- Law, M.; Young, R.; Babb, J.; Rad, M.; Sasaki, T.; Zagzag, D.; Johnson, G. Comparing perfusion metrics obtained from a single compartment versus pharmacokinetic modeling methods using dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am. J. Neuroradiol. 2006, 27, 1975–1982. [Google Scholar]
- Schmainda, K.M.; Zhang, Z.; Prah, M.; Snyder, B.S.; Gilbert, M.R.; Sorensen, A.G.; Barboriak, D.P.; Boxerman, J.L. Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: Results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro-Oncology 2015, 17, 1148–1156. [Google Scholar] [CrossRef]
- van Dijken, B.R.J.; van Laar, P.J.; Holtman, G.A.; van der Hoorn, A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur. Radiol. 2017, 27, 4129–4144. [Google Scholar] [CrossRef] [PubMed]
- Marstrand, J.R.; Rostrup, E.; Rosenbaum, S.; Garde, E.; Larsson, H.B. Cerebral hemodynamic changes measured by gradient-echo or spin-echo bolus tracking and its correlation to changes in ICA blood flow measured by phase-mapping MRI. J. Magn. Reson. Imaging 2001, 14, 391–400. [Google Scholar] [CrossRef]
- Vonken, E.P.; van Osch, M.J.; Bakker, C.J.; Viergever, M.A. Simultaneous quantitative cerebral perfusion and Gd-DTPA extravasation measurement with dual-echo dynamic susceptibility contrast MRI. Magn. Reson. Med. 2000, 43, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Stokes, A.M.; Semmineh, N.; Quarles, C.C. Validation of a T1 and T2* leakage correction method based on multiecho dynamic susceptibility contrast MRI using MION as a reference standard. Magn. Reson. Med. 2016, 76, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Quarles, C.C.; Ward, B.D.; Schmainda, K.M. Improving the reliability of obtaining tumor hemodynamic parameters in the presence of contrast agent extravasation. Magn. Reson. Med. 2005, 53, 1307–1316. [Google Scholar] [CrossRef]
- Law, M.; Yang, S.; Babb, J.S.; Knopp, E.A.; Golfinos, J.G.; Zagzag, D.; Johnson, G. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am. J. Neuroradiol. 2004, 25, 746–755. [Google Scholar]
- Cha, S.; Knopp, E.A.; Johnson, G.; Wetzel, S.G.; Litt, A.W.; Zagzag, D. Intracranial mass lesions: Dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology 2002, 223, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.; Zaharchuk, G.; Thomas, D.L.; Lovblad, K.O.; Barkhof, F.; Golay, X. Arterial Spin Labeling Perfusion of the Brain: Emerging Clinical Applications. Radiology 2016, 281, 337–356. [Google Scholar] [CrossRef] [PubMed]
- White, C.M.; Pope, W.B.; Zaw, T.; Qiao, J.; Naeini, K.M.; Lai, A.; Nghiemphu, P.L.; Wang, J.J.; Cloughesy, T.F.; Ellingson, B.M. Regional and voxel-wise comparisons of blood flow measurements between dynamic susceptibility contrast magnetic resonance imaging (DSC-MRI) and arterial spin labeling (ASL) in brain tumors. J. Neuroimaging 2014, 24, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Jarnum, H.; Steffensen, E.G.; Knutsson, L.; Frund, E.T.; Simonsen, C.W.; Lundbye-Christensen, S.; Shankaranarayanan, A.; Alsop, D.C.; Jensen, F.T.; Larsson, E.M. Perfusion MRI of brain tumours: A comparative study of pseudo-continuous arterial spin labelling and dynamic susceptibility contrast imaging. Neuroradiology 2010, 52, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Vermeulen, M.J.; Bharatha, A.; Montanera, W.J.; Park, A.L. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA 2016, 316, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Ledneva, E.; Karie, S.; Launay-Vacher, V.; Janus, N.; Deray, G. Renal safety of gadolinium-based contrast media in patients with chronic renal insufficiency. Radiology 2009, 250, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Fraum, T.J.; Ludwig, D.R.; Bashir, M.R.; Fowler, K.J. Gadolinium-based contrast agents: A comprehensive risk assessment. J. Magn. Reson. Imaging 2017, 46, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.C.; Cronin, M.; Wu, W.C.; Inglis, B.; Frank, L.R.; Liu, T.T. Velocity-selective arterial spin labeling. Magn. Reson. Med. 2006, 55, 1334–1341. [Google Scholar] [CrossRef]
- Duhamel, G.; de Bazelaire, C.; Alsop, D.C. Evaluation of systematic quantification errors in velocity-selective arterial spin labeling of the brain. Magn. Reson. Med. 2003, 50, 145–153. [Google Scholar] [CrossRef]
- Qiu, D.; Straka, M.; Zun, Z.; Bammer, R.; Moseley, M.E.; Zaharchuk, G. CBF measurements using multidelay pseudocontinuous and velocity-selective arterial spin labeling in patients with long arterial transit delays: Comparison with xenon CT CBF. J. Magn. Reson. Imaging 2012, 36, 110–119. [Google Scholar] [CrossRef]
- Bolar, D.S.; Gagoski, B.; Orbach, D.B.; Smith, E.; Adalsteinsson, E.; Rosen, B.R.; Grant, P.E.; Robertson, R.L. Comparison of CBF Measured with Combined Velocity-Selective Arterial Spin-Labeling and Pulsed Arterial Spin-Labeling to Blood Flow Patterns Assessed by Conventional Angiography in Pediatric Moyamoya. AJNR Am. J. Neuroradiol. 2019, 40, 1842–1849. [Google Scholar] [CrossRef]
- Meakin, J.A.; Jezzard, P. An optimized velocity selective arterial spin labeling module with reduced eddy current sensitivity for improved perfusion quantification. Magn. Reson. Med. 2013, 69, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Meakin, J.A.; Jezzard, P.; Wong, E.C. An optimized design to reduce eddy current sensitivity in velocity-selective arterial spin labeling using symmetric BIR-8 pulses. Magn. Reson. Med. 2015, 73, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; van Zijl, P.C. Velocity-selective-inversion prepared arterial spin labeling. Magn. Reson. Med. 2016, 76, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, F.; Li, W.; van Zijl, P.C.; Lin, D.D.; Qin, Q. Improved velocity-selective-inversion arterial spin labeling for cerebral blood flow mapping with 3D acquisition. Magn. Reson. Med. 2020, 84, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, D.; Zhu, D.; Hillis, A.E.; Bakker, A.; Soldan, A.; Albert, M.S.; Lin, D.D.M.; Qin, Q. Test-retest reliability of 3D velocity-selective arterial spin labeling for detecting normal variations of cerebral blood flow. Neuroimage 2023, 271, 120039. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, W.; Xu, F.; Zhu, D.; Shin, T.; Qin, Q. Ensuring both velocity and spatial responses robust to B0/B1+ field inhomogeneities for velocity-selective arterial spin labeling through dynamic phase-cycling. Magn. Reson. Med. 2021, 85, 2723–2734. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, F.; Lin, D.D.; van Zijl, P.C.M.; Qin, Q. Quantitative measurement of cerebral blood volume using velocity-selective pulse trains. Magn. Reson. Med. 2017, 77, 92–101. [Google Scholar] [CrossRef]
- Qin, Q.; Qu, Y.; Li, W.; Liu, D.; Shin, T.; Zhao, Y.; Lin, D.D.; van Zijl, P.C.M.; Wen, Z. Cerebral blood volume mapping using Fourier-transform-based velocity-selective saturation pulse trains. Magn. Reson. Med. 2019, 81, 3544–3554. [Google Scholar] [CrossRef]
- Li, W.; Liu, D.; van Zijl, P.C.M.; Qin, Q. Three-dimensional whole-brain mapping of cerebral blood volume and venous cerebral blood volume using Fourier transform-based velocity-selective pulse trains. Magn. Reson. Med. 2021, 86, 1420–1433. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, D.; Xu, F.; Sedaghat, F.; Qin, Q. Prostate perfusion mapping using Fourier-transform based velocity-selective arterial spin labeling: Choice of cutoff velocity and comparison with brain. Magn. Reson. Med. 2023, 90, 1121–1129. [Google Scholar] [CrossRef]
- Li, W.; Xu, F.; Zhu, D.; van Zijl, P.C.M.; Qin, Q. T(2)-oximetry-based cerebral venous oxygenation mapping using Fourier-transform-based velocity-selective pulse trains. Magn. Reson. Med. 2022, 88, 1292–1302. [Google Scholar] [CrossRef]
- Qin, Q.; Shin, T.; Schar, M.; Guo, H.; Chen, H.; Qiao, Y. Velocity-selective magnetization-prepared non-contrast-enhanced cerebral MR angiography at 3 Tesla: Improved immunity to B0/B1 inhomogeneity. Magn. Reson. Med. 2016, 75, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.; Qin, Q.; Park, J.Y.; Crawford, R.S.; Rajagopalan, S. Identification and reduction of image artifacts in non-contrast-enhanced velocity-selective peripheral angiography at 3T. Magn. Reson. Med. 2016, 76, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, F.; Schar, M.; Liu, J.; Shin, T.; Zhao, Y.; van Zijl, P.C.M.; Wasserman, B.A.; Qiao, Y.; Qin, Q. Whole-brain arteriography and venography: Using improved velocity-selective saturation pulse trains. Magn. Reson. Med. 2018, 79, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.; Qin, Q. Characterization and suppression of stripe artifact in velocity-selective magnetization-prepared unenhanced MR angiography. Magn. Reson. Med. 2018, 80, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Li, W.; Liu, D.; Liu, G.; Pei, Y.; Shin, T.; Sedaghat, F.; Qin, Q. Non-contrast-enhanced abdominal MRA at 3 T using velocity-selective pulse trains. Magn. Reson. Med. 2020, 84, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhu, D.; Fan, H.; Lu, H.; Liu, D.; Li, W.; Qin, Q. Magnetic resonance angiography and perfusion mapping by arterial spin labeling using Fourier transform-based velocity-selective pulse trains: Examination on a commercial perfusion phantom. Magn. Reson. Med. 2021, 86, 1360–1368. [Google Scholar] [CrossRef]
- Hernandez-Garcia, L.; Nielsen, J.F.; Noll, D.C. Improved sensitivity and temporal resolution in perfusion FMRI using velocity selective inversion ASL. Magn. Reson. Med. 2019, 81, 1004–1015. [Google Scholar] [CrossRef]
- Franklin, S.L.; Bones, I.K.; Harteveld, A.A.; Hirschler, L.; van Stralen, M.; Qin, Q.; de Boer, A.; Hoogduin, J.M.; Bos, C.; van Osch, M.J.P.; et al. Multi-organ comparison of flow-based arterial spin labeling techniques: Spatially non-selective labeling for cerebral and renal perfusion imaging. Magn. Reson. Med. 2021, 85, 2580–2594. [Google Scholar] [CrossRef]
- Guo, J.; Das, S.; Hernandez-Garcia, L. Comparison of velocity-selective arterial spin labeling schemes. Magn. Reson. Med. 2021, 85, 2027–2039. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Alsop, D.C.; Bolar, D.S.; Hernandez-Garcia, L.; Meakin, J.; Liu, D.; Nayak, K.S.; Schmid, S.; van Osch, M.J.P.; Wong, E.C.; et al. Velocity-selective arterial spin labeling perfusion MRI: A review of the state of the art and recommendations for clinical implementation. Magn. Reson. Med. 2022, 88, 1528–1547. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Kong, D.; Wen, H.; Ou, X.; Rui, Q.; Wang, X.; Lin, D.D.; Qin, Q.; Wen, Z. Perfusion measurement in brain gliomas using velocity-selective arterial spin labeling: Comparison with pseudo-continuous arterial spin labeling and dynamic susceptibility contrast MRI. Eur. Radiol. 2022, 32, 2976–2987. [Google Scholar] [CrossRef]
- Thompson, G.; Mills, S.J.; Coope, D.J.; O’Connor, J.P.; Jackson, A. Imaging biomarkers of angiogenesis and the microvascular environment in cerebral tumours. Br. J. Radiol. 2011, 84, S127–S144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; King, A.D.; Zhou, H.; Leung, S.F.; Abrigo, J.; Chan, Y.L.; Hu, C.W.; Yeung, D.K.; Ahuja, A.T. Evolution of radiation-induced brain injury: MR imaging-based study. Radiology 2010, 254, 210–218. [Google Scholar] [CrossRef]
- Hu, L.S.; Baxter, L.C.; Smith, K.A.; Feuerstein, B.G.; Karis, J.P.; Eschbacher, J.M.; Coons, S.W.; Nakaji, P.; Yeh, R.F.; Debbins, J.; et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: Direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am. J. Neuroradiol. 2009, 30, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.S.; Kim, S.T.; Kim, E.H.; Lim, D.H.; Kim, W.S.; Suh, Y.L.; Lee, J.I.; Park, K.; Kim, J.H.; Nam, D.H. Diagnostic dilemma of pseudoprogression in the treatment of newly diagnosed glioblastomas: The role of assessing relative cerebral blood flow volume and oxygen-6-methylguanine-DNA methyltransferase promoter methylation status. AJNR Am. J. Neuroradiol. 2011, 32, 382–387. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Chen, Y.; Lv, X.; Lv, Y.; Zhou, J.; Xi, S.; Dou, W.; Qian, L.; Zheng, H.; et al. Diagnostic performance of multiparametric MRI in the evaluation of treatment response in glioma patients at 3T. J. Magn. Reson. Imaging 2020, 51, 1154–1161. [Google Scholar] [CrossRef]
- Razek, A.; El-Serougy, L.; Abdelsalam, M.; Gaballa, G.; Talaat, M. Differentiation of residual/recurrent gliomas from postradiation necrosis with arterial spin labeling and diffusion tensor magnetic resonance imaging-derived metrics. Neuroradiology 2018, 60, 169–177. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Withey, S.B.; Lateef, S.; MacPherson, L.; Pinkey, B.; Peet, A.C. A comparison of pseudo-continuous arterial spin labelling and dynamic susceptibility contrast MRI with and without contrast agent leakage correction in paediatric brain tumours. Br. J. Radiol. 2019, 92, 20170872. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Xu, F.; Liu, D.; Hillis, A.E.; Lin, D.; van Zijl, P.C.M.; Qin, Q. Evaluation of 3D stack-of-spiral turbo FLASH acquisitions for pseudo-continuous and velocity-selective ASL-derived brain perfusion mapping. Magn. Reson. Med. 2023, 90, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Boxerman, J.L.; Quarles, C.C.; Hu, L.S.; Erickson, B.J.; Gerstner, E.R.; Smits, M.; Kaufmann, T.J.; Barboriak, D.P.; Huang, R.H.; Wick, W.; et al. Consensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas. Neuro-Oncology 2020, 22, 1262–1275. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambrecht, S.; Liu, D.; Dzaye, O.; Kamson, D.O.; Reis, J.; Liebig, T.; Holdhoff, M.; Van Zijl, P.; Qin, Q.; Lin, D.D.M. Velocity-Selective Arterial Spin Labeling Perfusion in Monitoring High Grade Gliomas Following Therapy: Clinical Feasibility at 1.5T and Comparison with Dynamic Susceptibility Contrast Perfusion. Brain Sci. 2024, 14, 126. https://doi.org/10.3390/brainsci14020126
Lambrecht S, Liu D, Dzaye O, Kamson DO, Reis J, Liebig T, Holdhoff M, Van Zijl P, Qin Q, Lin DDM. Velocity-Selective Arterial Spin Labeling Perfusion in Monitoring High Grade Gliomas Following Therapy: Clinical Feasibility at 1.5T and Comparison with Dynamic Susceptibility Contrast Perfusion. Brain Sciences. 2024; 14(2):126. https://doi.org/10.3390/brainsci14020126
Chicago/Turabian StyleLambrecht, Sebastian, Dapeng Liu, Omar Dzaye, David O. Kamson, Jonas Reis, Thomas Liebig, Matthias Holdhoff, Peter Van Zijl, Qin Qin, and Doris D. M. Lin. 2024. "Velocity-Selective Arterial Spin Labeling Perfusion in Monitoring High Grade Gliomas Following Therapy: Clinical Feasibility at 1.5T and Comparison with Dynamic Susceptibility Contrast Perfusion" Brain Sciences 14, no. 2: 126. https://doi.org/10.3390/brainsci14020126





