Sex-Specific Association of Body Mass Index with Hippocampal Subfield Volume and Cognitive Function in Non-Demented Chinese Older Adults
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Cognitive Measurement
2.3. MRI Acquisition
2.4. Image Preprocessing
2.5. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics of Participants
3.2. Association between BMI and Hippocampal Subfield Volumes
3.3. Association between BMI and Cognitive Functions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, R.; Qi, J.; Lin, S.; Liu, X.; Yin, P.; Wang, Z.; Tang, R.; Wang, J.; Huang, Q.; Li, J.; et al. The China Alzheimer Report 2022. Gen. Psychiatry 2022, 35, e100751. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.; Rothenberg, E.; Blennow, K.; Steen, B.; Skoog, I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch. Int. Med. 2003, 163, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.F.; Elias, P.K.; Sullivan, L.M.; Wolf, P.A.; D’agostino, R.B. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol. Aging 2005, 26 (Suppl. S1), 11–16. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Ngandu, T.; Fratiglioni, L.; Viitanen, M.; Kåreholt, I.; Winblad, B.; Helkala, E.-L.; Tuomilehto, J.; Soininen, H.; Nissinen, A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch. Neurol. 2005, 62, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A.; Gunderson, E.P.; Barrett-Connor, E.; Quesenberry, C.P., Jr.; Yaffe, K. Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ 2005, 330, 1360. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.; Sharma, S.; Kelberman, M.A.; Ware, C.; Guo, N.; Qin, Z.; Weinshenker, D.; Parent, M.B. Obesity during preclinical Alzheimer’s disease development exacerbates brain metabolic decline. J. Neurochem. 2023. advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ajnakina, O.; Steptoe, A.; Cadar, D. Higher risk of dementia in English older individuals who are overweight or obese. Int. J. Epidemiol. 2020, 49, 1353–1365. [Google Scholar] [CrossRef]
- Nianogo, R.A.; Rosenwohl-Mack, A.; Yaffe, K.; Carrasco, A.; Hoffmann, C.M.; Barnes, D.E. Risk Factors Associated With Alzheimer Disease and Related Dementias by Sex and Race and Ethnicity in the US. JAMA Neurol. 2022, 79, 584–591. [Google Scholar] [CrossRef]
- Nyberg, C.K.; Fjell, A.M.; Walhovd, K.B. Level of body fat relates to memory decline and interacts with age in its association with hippocampal and subcortical atrophy. Neurobiol. Aging 2020, 91, 112–124. [Google Scholar] [CrossRef]
- Liang, F.; Fu, J.; Turner-McGrievy, G.; Wang, Y.; Qiu, N.; Ding, K.; Zeng, J.; Moore, J.B.; Li, R. Association of Body Mass Index and Plant-Based Diet with Cognitive Impairment among Older Chinese Adults: A Prospective, Nationwide Cohort Study. Nutrients 2022, 14, 3132. [Google Scholar] [CrossRef]
- Qu, Y.; Hu, H.-Y.; Ou, Y.-N.; Shen, X.-N.; Xu, W.; Wang, Z.-T.; Dong, Q.; Tan, L.; Yu, J.-T. Association of body mass index with risk of cognitive impairment and dementia: A systematic review and meta-analysis of prospective studies. Neurosci. Biobehav. Rev. 2020, 115, 189–198. [Google Scholar] [CrossRef]
- Li, G.; Hu, Y.; Zhang, W.; Wang, J.; Ji, W.; Manza, P.; Volkow, N.D.; Zhang, Y.; Wang, G.-J. Brain functional and structural magnetic resonance imaging of obesity and weight loss interventions. Mol. Psychiatry 2023, 28, 1466–1479. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.J.; Benedict, C.; Burgos, J.; Kempton, M.J.; Kullberg, J.; Nordenskjöld, R.; Kilander, L.; Nylander, R.; Larsson, E.-M.; Johansson, L.; et al. Late-life obesity is associated with smaller global and regional gray matter volumes: A voxel-based morphometric study. Int. J. Obes. 2013, 37, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Debette, S.; Beiser, A.; Hoffmann, U.; DeCarli, C.; O’Donnell, C.J.; Massaro, J.M.; Au, R.; Himali, J.J.; Wolf, P.A.; Fox, C.S.; et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann. Neurol. 2010, 68, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Gunstad, J.; Paul, R.H.; Cohen, R.A.; Tate, D.F.; Spitznagel, M.B.; Grieve, S.; Gordon, E. Relationship between body mass index and brain volume in healthy adults. Int. J. Neurosci. 2008, 118, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.K.; Tuulari, J.J.; Hirvonen, J.; Lepomäki, V.; Parkkola, R.; Hiltunen, J.; Hannukainen, J.C.; Soinio, M.; Pham, T.; Salminen, P.; et al. Obesity is associated with white matter atrophy: A combined diffusion tensor imaging and voxel-based morphometric study. Obesity 2013, 21, 2530–2537. [Google Scholar] [CrossRef] [PubMed]
- Kurth, F.; Levitt, J.G.; Phillips, O.R.; Luders, E.; Woods, R.P.; Mazziotta, J.C.; Toga, A.W.; Narr, K.L. Relationships between gray matter, body mass index, and waist circumference in healthy adults. Hum. Brain Mapp. 2013, 34, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Raji, C.A.; Ho, A.J.; Parikshak, N.N.; Becker, J.T.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Leow, A.D.; Toga, A.W.; Thompson, P.M. Brain structure and obesity. Hum. Brain Mapp. 2010, 31, 353–364. [Google Scholar] [CrossRef]
- Walther, K.; Birdsill, A.C.; Glisky, E.L.; Ryan, L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum. Brain Mapp. 2010, 31, 1052–1064. [Google Scholar] [CrossRef]
- Ward, M.A.; Carlsson, C.M.; Trivedi, M.A.; Sager, M.A.; Johnson, S.C. The effect of body mass index on global brain volume in middle-aged adults: A cross sectional study. BMC Neurol. 2005, 5, 23. [Google Scholar] [CrossRef]
- Chenji, S.; Cox, E.; Jaworska, N.; Swansburg, R.M.; MacMaster, F.P. Body mass index and variability in hippocampal volume in youth with major depressive disorder. J. Affect. Disord. 2021, 282, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Willette, A.A.; Kapogiannis, D. Does the brain shrink as the waist expands? Ageing Res. Rev. 2015, 20, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Gourley, D.D.; Dekhtyar, M.; Haley, A.P. Cognition, Brain Structure, and Brain Function in Individuals with Obesity and Related Disorders. Curr. Obes. Rep. 2020, 9, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Subramaniapillai, M.; McIntyre, R.S. A review of the neurobiology of obesity and the available pharmacotherapies. CNS Spectr. 2017, 22 (Suppl. S1), 29–38. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, I.A.; Jansen, P.R.; Lamb, H.J. Obesity, Brain Volume, and White Matter Microstructure at MRI: A Cross-sectional UK Biobank Study. Radiology 2019, 292, 270. [Google Scholar] [CrossRef] [PubMed]
- Janowitz, D.; Wittfeld, K.; Terock, J.; Freyberger, H.J.; Hegenscheid, K.; Völzke, H.; Habes, M.; Hosten, N.; Friedrich, N.; Nauck, M.; et al. Association between waist circumference and gray matter volume in 2344 individuals from two adult community-based samples. NeuroImage 2015, 122, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Whitmer, R.A.; Gustafson, D.R.; Barrett-Connor, E.; Haan, M.N.; Gunderson, E.P.; Yaffe, K. Central obesity and increased risk of dementia more than three decades later. Neurology 2008, 71, 1057–1064. [Google Scholar] [CrossRef]
- Yaffe, K.; Kanaya, A.; Lindquist, K.; Simonsick, E.M.; Harris, T.; Shorr, R.I.; Tylavsky, F.A.; Newman, A.B. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama 2004, 292, 2237–2242. [Google Scholar] [CrossRef]
- Fotuhi, M.; Do, D.; Jack, C. Modifiable factors that alter the size of the hippocampus with ageing. Nat. Rev. Neurol. 2012, 8, 189–202. [Google Scholar] [CrossRef]
- Erten-Lyons, D.; Woltjer, R.L.; Dodge, H.; Nixon, R.; Vorobik, R.; Calvert, J.F.; Leahy, M.; Montine, T.; Kaye, J. Factors associated with resistance to dementia despite high Alzheimer disease pathology. Neurology 2009, 72, 354–360. [Google Scholar] [CrossRef]
- Vasta, R.; Augimeri, A.; Cerasa, A.; Nigro, S.; Gramigna, V.; Nonnis, M.; Rocca, F.; Zito, G.; Quattrone, A.; Neuroimaging, F.T.A.D. Hippocampal Subfield Atrophies in Converted and Not-Converted Mild Cognitive Impairments Patients by a Markov Random Fields Algorithm. Curr. Alzheimer Res. 2016, 13, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, L.; Yang, H.; Luo, X.; Fan, G. Do multiple system atrophy and Parkinson’s disease show distinct patterns of volumetric alterations across hippocampal subfields? An exploratory study. Eur. Radiol. 2019, 29, 4948–4956. [Google Scholar] [CrossRef] [PubMed]
- Bastos-Leite, A.; van Waesberghe, J.; Oen, A.; van der Flier, W.; Scheltens, P.; Barkhof, F. Hippocampal sulcus width and cavities: Comparison between patients with Alzheimer disease and nondemented elderly subjects. AJNR Am. J. Neuroradiol. 2006, 27, 2141–2145. [Google Scholar] [PubMed]
- Shing, Y.L.; Rodrigue, K.M.; Kennedy, K.M.; Fandakova, Y.; Bodammer, N.; Werkle-Bergner, M.; Lindenberger, U.; Raz, N. Hippocampal subfield volumes: Age, vascular risk, and correlation with associative memory. Front. Aging Neurosci. 2011, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Zammit, A.R.; Ezzati, A.; Zimmerman, M.E.; Lipton, R.B.; Lipton, M.L.; Katz, M.J. Roles of hippocampal subfields in verbal and visual episodic memory. Behav. Brain Res. 2017, 317, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.S.; Gannon, O.J.; Salinero, A.E.; Zuloaga, K.L. Contributions of sex to cerebrovascular function and pathology. Brain Res. 2019, 1710, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Gannon, O.J.; Naik, J.S.; Riccio, D.; Mansour, F.M.; Abi-Ghanem, C.; Salinero, A.E.; Kelly, R.D.; Brooks, H.L.; Zuloaga, K.L. Menopause causes metabolic and cognitive impairments in a chronic cerebral hypoperfusion model of vascular contributions to cognitive impairment and dementia. Biol. Sex Differ. 2023, 14, 34. [Google Scholar] [CrossRef]
- Mazure, C.M.; Swendsen, J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016, 15, 451–452. [Google Scholar] [CrossRef]
- Ystad, M.A.; Lundervold, A.J.; Wehling, E.; Espeseth, T.; Rootwelt, H.; Westlye, L.T.; Andersson, M.; Adolfsdottir, S.; Geitung, J.T.; Fjell, A.M.; et al. Hippocampal volumes are important predictors for memory function in elderly women. BMC Med. Imaging 2009, 9, 17. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Collins, D.L.; Pruessner, M.; Evans, A.C. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. J. Neurosci. 2001, 21, 194–200. [Google Scholar] [CrossRef]
- Li, W.; van Tol, M.-J.; Li, M.; Miao, W.; Jiao, Y.; Heinze, H.-J.; Bogerts, B.; He, H.; Walter, M. Regional specificity of sex effects on subcortical volumes across the lifespan in healthy aging. Hum. Brain Mapp. 2014, 35, 238–247. [Google Scholar] [CrossRef]
- Li, C.; Wu, W.; Jin, H.; Zhang, X.; Xue, H.; He, Y.; Xiao, S.; Jeste, D.V.; Zhang, M. Successful aging in Shanghai, China: Definition, distribution and related factors. Int. Psychogeriatr. 2006, 18, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wu, W.; Wang, J.; Feng, W.; Wu, X.; Li, C. Cinical research Reliability and validity of the Repeatable Battery for the Assessment of Neuropsychological Status in community-dwelling elderly. Arch. Med. Sci. 2011, 5, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Katzman, R.; Zhang, M.; Wang, Z.; Liu, W.T.; Yu, E.; Wong, S.-C.; Salmon, D.P.; Grant, I. A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J. Clin. Epidemiol. 1988, 41, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Ségonne, F.; Dale, A.M.; Busa, E.; Glessner, M.; Salat, D.; Hahn, H.K.; Fischl, B. A hybrid approach to the skull stripping problem in MRI. Neuroimage 2004, 22, 1060–1075. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Cao, X.; Jiang, J.; Li, T.; Wang, J.; Yang, Z.; Li, C. Atrophy of hippocampal subfield CA2/3 in healthy elderly men is related to educational attainment. Neurobiol. Aging 2019, 80, 21–28. [Google Scholar] [CrossRef]
- Shi, Z.; Cao, X.; Hu, J.; Jiang, L.; Mei, X.; Zheng, H.; Chen, Y.; Wang, M.; Cao, J.; Li, W.; et al. Retinal nerve fiber layer thickness is associated with hippocampus and lingual gyrus volumes in nondemented older adults. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 99, 109824. [Google Scholar] [CrossRef]
- Murphy, D.G.M.; DeCarli, C.; Mclntosh, A.R.; Daly, E.; Mentis, M.J.; Pietrini, P.; Szczepanik, J.; Schapiro, M.B.; Grady, C.L.; Horwitz, B.; et al. Sex differences in human brain morphometry and metabolism: An in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch. Gen. Psychiatry 1996, 53, 585–594. [Google Scholar] [CrossRef]
- Neu, S.C.; Pa, J.; Kukull, W.; Beekly, D.; Kuzma, A.; Gangadharan, P.; Wang, L.S.; Romero, K.; Arneric, S.P.; Redolfi, A.; et al. Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol. 2017, 74, 1178–1189. [Google Scholar] [CrossRef]
- Elias, M.F.; Elias, P.K.; Sullivan, L.M.; Wolf, P.A.; D’Agostino, R.B. Lower cognitive function in the presence of obesity and hypertension: The Framingham heart study. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, A.M.; Lindquist, K.; Harris, T.B.; Launer, L.; Rosano, C.; Satterfield, S.; Yaffe, K. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Arch. Neurol. 2009, 66, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Narayan, K.M.V.; Boyle, J.P.; Thompson, T.J.; Sorensen, S.W.; Williamson, D.F. Lifetime risk for diabetes mellitus in the United States. JAMA 2003, 290, 1884–1890. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Sainsbury, A. The Stock Conference 2008 Working Group Sex differences in obesity and the regulation of energy homeostasis. Obes. Rev. 2009, 10, 154–167. [Google Scholar] [CrossRef]
- Harris, G.; Baer, D. Gender differences in body fat utilization during weight gain, loss, or maintenance. In Obesity: Epidemiology, Pathophysiology, and Prevention; CRC Press: Boca Raton, FL, USA, 2007; pp. 239–245. [Google Scholar]
- Yagi, S.; Galea, L.A.M. Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 2019, 44, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.C. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus 2012, 22, 656–669. [Google Scholar] [CrossRef]
- Hamson, D.K.; Roes, M.M.; Galea, L.A. Sex Hormones and Cognition: Neuroendocrine Influences on Memory and Learning. Compr. Physiol. 2016, 6, 1295–1337. [Google Scholar]
- Laukka, E.J.; Lövdén, M.; Herlitz, A.; Karlsson, S.; Ferencz, B.; Pantzar, A.; Keller, L.; Graff, C.; Fratiglioni, L.; Bäckman, L. Genetic effects on old-age cognitive functioning: A population-based study. Psychol. Aging 2013, 28, 262–274. [Google Scholar] [CrossRef]
- Altmann, A.; Tian, L.; Henderson, V.W.; Greicius, M.D. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 2014, 75, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhong, M.B.; Zhang, L.; Zhang, B.; Cai, D. Sex Differences in Alzheimer’s Disease: Insights From the Multiomics Landscape. Biol. Psychiatry 2022, 91, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Hohman, T.J.; Dumitrescu, L.; Barnes, L.L.; Thambisetty, M.; Beecham, G.; Kunkle, B.; Gifford, K.A.; Bush, W.S.; Chibnik, L.B.; Mukherjee, S.; et al. Sex-Specific Association of Apolipoprotein E With Cerebrospinal Fluid Levels of Tau. JAMA Neurol. 2018, 75, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Buch, S.; Chen, Y.; Jella, P.; Ge, Y.; Haacke, E.M. Vascular mapping of the human hippocampus using Ferumoxytol-enhanced MRI. NeuroImage 2022, 250, 118957. [Google Scholar] [CrossRef] [PubMed]
- Wisse, L.E.; Biessels, G.J.; Heringa, S.M.; Kuijf, H.J.; Luijten, P.R.; Geerlings, M.I. Hippocampal subfield volumes at 7T in early Alzheimer’s disease and normal aging. Neurobiol. Aging 2014, 35, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, X.; Yin, C.; He, M.; Li, S.; Han, Y. Trajectories of the Hippocampal Subfields Atrophy in the Alzheimer’s Disease: A Structural Imaging Study. Front. Neurosci. 2019, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, J.; Zhu, X.; Zhu, Y.; Qin, J.; Zhang, N.; Ju, S. Increased hippocampal fissure width is a sensitive indicator of rat hippocampal atrophy. Brain Res. Bull. 2018, 137, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Malykhin, N.V.; Huang, Y.; Hrybouski, S.; Olsen, F. Differential vulnerability of hippocampal subfields and anteroposterior hippocampal subregions in healthy cognitive aging. Neurobiol. Aging 2017, 59, 121–134. [Google Scholar] [CrossRef]
- Iglesias, J.E.; Augustinack, J.C.; Nguyen, K.; Player, C.M.; Player, A.; Wright, M.; Roy, N.; Frosch, M.P.; McKee, A.C.; Wald, L.L.; et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. NeuroImage 2015, 115, 117–137. [Google Scholar] [CrossRef]
- Izzo, J.; Andreassen, O.A.; Westlye, L.T.; van der Meer, D. The association between hippocampal subfield volumes in mild cognitive impairment and conversion to Alzheimer’s disease. Brain Res. 2020, 1728, 146591. [Google Scholar] [CrossRef]
- Murray, A.N.; Chandler, H.L.; Lancaster, T.M. Multimodal hippocampal and amygdala subfield volumetry in polygenic risk for Alzheimer’s disease. Neurobiol. Aging 2021, 98, 33–41. [Google Scholar] [CrossRef]
- Hibar, D.P.; Adams, H.H.H.; Jahanshad, N.; Chauhan, G.; Stein, J.L.; Hofer, E.; Renteria, M.E.; Bis, J.C.; Arias-Vasquez, A.; Ikram, M.K.; et al. Novel genetic loci associated with hippocampal volume. Nat. Commun. 2017, 8, 13624. [Google Scholar] [CrossRef]
- Mestre, Z.; Bischoff-Grethe, A.; Wierenga, C.E.; Jernigan, T.; Eichen, D.M.; Chang, L.; Ernst, T.; Boutelle, K.N. Associations Between Body Weight, Hippocampal Volume, and Tissue Signal Intensity in 12- to 18-Year-Olds. Obesity 2020, 28, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.P.; Moody, J.N.; Roca, J.G.; Hayes, S.M. Body mass index is associated with smaller medial temporal lobe volume in those at risk for Alzheimer’s disease. Neuroimage Clin. 2020, 25, 102156. [Google Scholar] [CrossRef] [PubMed]
- Gunstad, J.; Paul, R.H.; Cohen, R.A.; Tate, D.F.; Gordon, E. Obesity is associated with memory deficits in young and middle-aged adults. Eat. Weight Disord. 2006, 11, e15–e19. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.P.; Liu, D.; Samuels, L.R.; Shah, A.S.; Gifford, K.A.; Hohman, T.J.; Jefferson, A.L. Late-Life Body Mass Index, Rapid Weight Loss, Apolipoprotein E ε4 and the Risk of Cognitive Decline and Incident Dementia. J. Nutr. Health Aging 2017, 21, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Cheng, Y.; Li, Q.; Tang, Y.; Shen, Y.; Li, T.; Feng, W.; Cao, X.; Wu, W.; Wang, J.; et al. Cross-sectional study of the association of cognitive function and hippocampal volume among healthy elderly adults. Shanghai Arch. Psychiatry 2014, 26, 280–287. [Google Scholar] [CrossRef] [PubMed]
- England, H.B.; Gillis, M.M.; Hampstead, B.M. Hampstead, RBANS memory indices are related to medial temporal lobe volumetrics in healthy older adults and those with mild cognitive impairment. Arch. Clin. Neuropsychol. 2014, 29, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Duff, K.; Dixon, A.M.; Embree, L.; Hoffman, J.M. Change on the Repeatable Battery for the Assessment of Neuropsychological Status and its relationship to brain amyloid. J. Clin. Exp. Neuropsychol. 2023, 45, 105–117. [Google Scholar] [CrossRef] [PubMed]
- de Guevara, C.M.L.; del Paso, G.A.R.; Fernández Serrano, M.J.; Montoro, C.I. Fibromyalgia Syndrome and Cognitive Decline: The Role of Body Mass Index and Clinical Symptoms. J. Clin. Med. 2022, 11, 3404. [Google Scholar] [CrossRef]
- Kaup, A.R.; Mirzakhanian, H.; Jeste, D.V.; Eyler, L.T.; Sæther, S.G.; Rø, A.D.B.; Larsen, J.B.; Vaaler, A.; Kondziella, D.; Reitan, S.K.; et al. A review of the brain structure correlates of successful cognitive aging. J. Neuropsychiatry 2011, 23, 6–15. [Google Scholar] [CrossRef]
- Ezzati, A.; Katz, M.J.; Zammit, A.R.; Lipton, M.L.; Zimmerman, M.E.; Sliwinski, M.J.; Lipton, R.B. Differential association of left and right hippocampal volumes with verbal episodic and spatial memory in older adults. Neuropsychologia 2016, 93 Pt B, 380–385. [Google Scholar] [CrossRef]
- Bryant, A.N.; Ford, K.-L.; Kim, G. Racial/ethnic variations in the relation between body mass index and cognitive function among older adults. Am. J. Geriatr. Psychiatry 2014, 22, 653–660. [Google Scholar] [CrossRef]


| Characteristics | Entire Sample (n = 66) | Females (n = 35) | Males (n = 31) | p a |
|---|---|---|---|---|
| Age (year) | 68.90 ± 3.04 | 68.76 ± 2.96 | 69.06 ± 3.17 | 0.687 |
| Education (year) | 12.20 ± 2.80 | 12.09 ± 2.79 | 12.32 ± 2.84 | 0.864 |
| BMI (kg/m2) | 23.44 ± 3.291 | 23.16 ± 2.40 | 23.77 ± 4.09 | 0.467 |
| CMMSE | 28.26 ± 1.34 | 28.43 ± 1.24 | 28.06 ± 1.44 | 0.658 |
| RBANS total scale score | 95.636 ± 10.98 | 97.51 ± 11.71 | 93.52 ± 9.85 | 0.141 |
| Immediate memory | 86.35 ± 13.18 | 87.83 ± 12.67 | 84.68 ±13.75 | 0.336 |
| Visuospatial/ | ||||
| constructional ability | 102.80 ± 15.96 | 102.46 ± 16.42 | 103.19 ± 15.69 | 0.853 |
| Language | 97.38 ± 8.54 | 97.74 ± 9.66 | 96.97 ± 7.20 | 0.540 |
| Attention | 101.48 ± 13.09 | 104.06 ± 14.21 | 98.58 ± 11.23 | 0.090 |
| Delayed memory | 97.59 ± 12.22 | 99.97 ± 11.63 | 94.90 ± 12.50 | 0.197 |
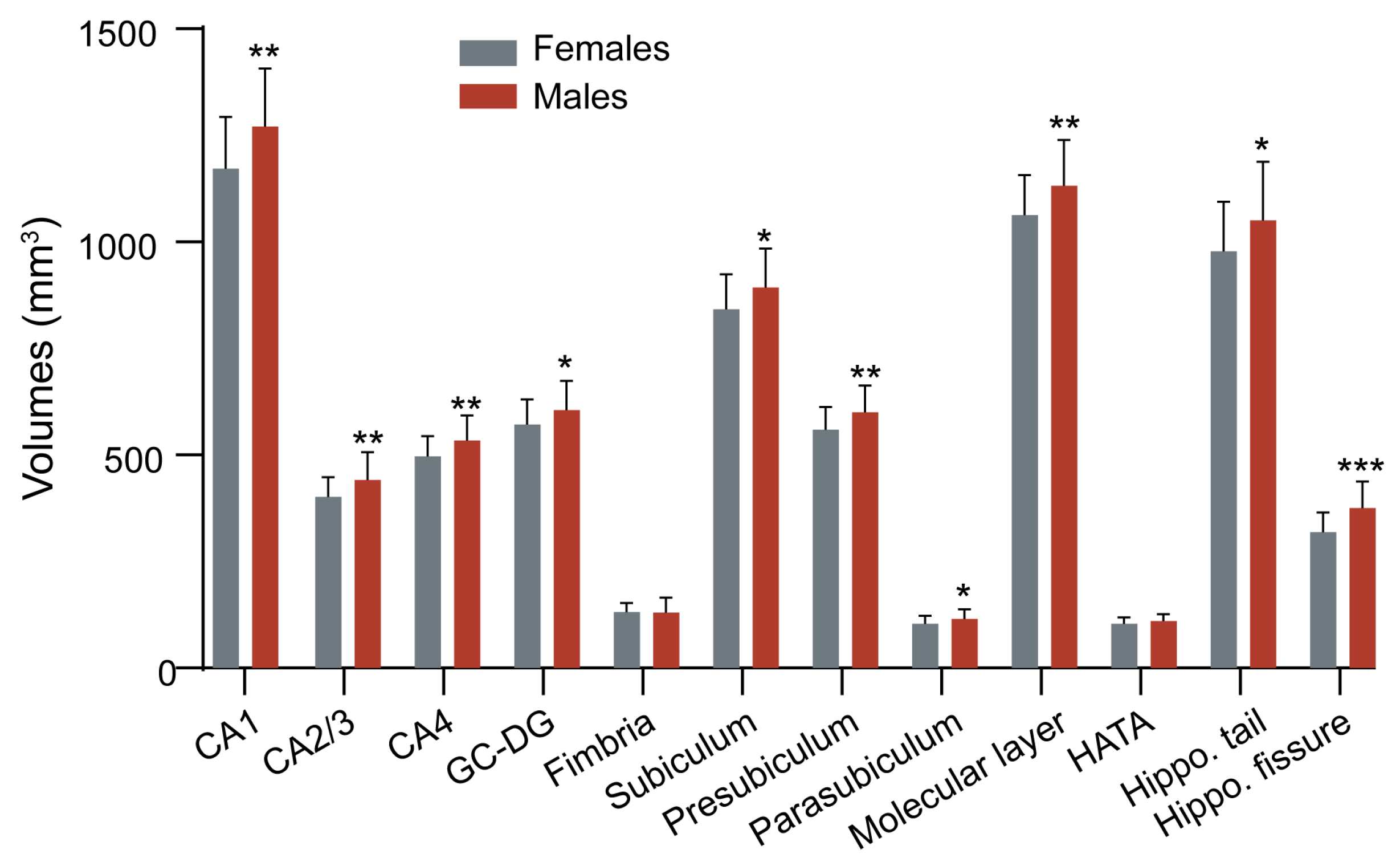
| Whole hippocampus volume (mm3) | 6642.11 ± 617.06 | 6425.65 ± 536.95 | 6886.51 ± 617.93 | 0.002 * |
| eTIV (cm3) | 1487.84 ± 163.39 | 1378.04 ± 96.94 | 1611.81 ± 131.32 | 0.000 * |
| Hippocampal Subfields | Overall (n = 66) a | Males (n = 31) b | Females (n = 35) b | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| CA1 | 0.051 | 0.694 | −0.096 | 0.629 | 0.286 | 0.112 |
| CA2/3 | 0.166 | 0.197 | 0.108 | 0.585 | 0.264 | 0.144 |
| CA4 | 0.016 | 0.899 | −0.132 | 0.505 | 0.249 | 0.170 |
| GC–DG | −0.051 | 0.692 | −0.229 | 0.240 | 0.183 | 0.315 |
| Fimbria | −0.167 | 0.195 | −0.081 | 0.680 | −0.314 | 0.080 |
| Subiculum | −0.063 | 0.626 | −0.216 | 0.269 | 0.200 | 0.272 |
| Presubiculum | −0.053 | 0.682 | −0.032 | 0.870 | 0.029 | 0.877 |
| Parasubiculm | −0.048 | 0.712 | −0.041 | 0.836 | −0.113 | 0.537 |
| Molecular layer | −0.004 | 0.976 | −0.158 | 0.423 | 0.263 | 0.146 |
| HATA | −0.086 | 0.506 | −0.156 | 0.428 | −0.041 | 0.823 |
| Hippocampal tail | 0.043 | 0.741 | −0.046 | 0.815 | 0.296 | 0.100 |
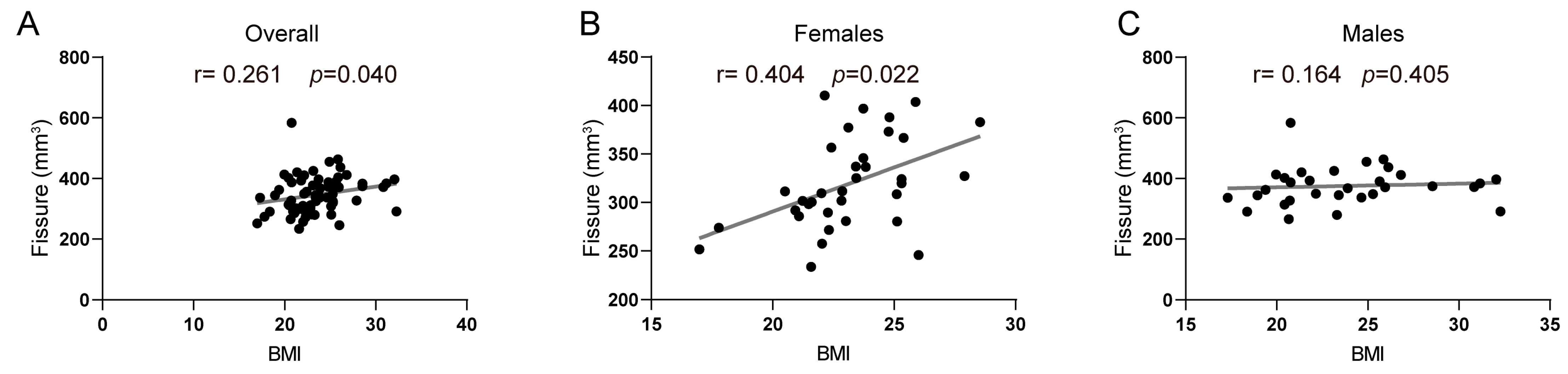
| Hippocampal fissure | 0.261 | 0.040 * | 0.164 | 0.405 | 0.404 | 0.022 * |
| Whole hippocampus | 0.004 | 0.974 | −0.136 | 0.489 | 0.256 | 0.157 |
| Cognitive Function | Overall (n = 66) a | Males (n = 31) b | Females (n = 35) b | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Immediate memory | 0.049 | 0.708 | 0.214 | 0.273 | −0.186 | 0.308 |
| List learning | 0.056 | 0.667 | 0.144 | 0.465 | −0.030 | 0.869 |
| Story memory | −0.025 | 0.849 | 0.147 | 0.454 | −0.277 | 0.124 |
| Visuospatial/ | ||||||
| constructional ability | −0.139 | 0.282 | −0.012 | 0.951 | −0.329 | 0.066 |
| Figure copy | −0.204 | 0.112 | −0.206 | 0.293 | −0.094 | 0.611 |
| Line orientation | 0.002 | 0.988 | 0.124 | 0.528 | −0.300 | 0.095 |
| Language | 0.117 | 0.364 | 0.227 | 0.245 | −0.071 | 0.700 |
| Picture naming | 0.069 | 0.597 | 0.211 | 0.282 | −0.007 | 0.972 |
| Semantic fluency | 0.169 | 0.188 | 0.221 | 0.258 | −0.053 | 0.775 |
| Attention | −0.123 | 0.340 | 0.140 | 0.477 | −0.252 | 0.163 |
| Digit span | −0.106 | 0.412 | −0.045 | 0.821 | 0.034 | 0.854 |
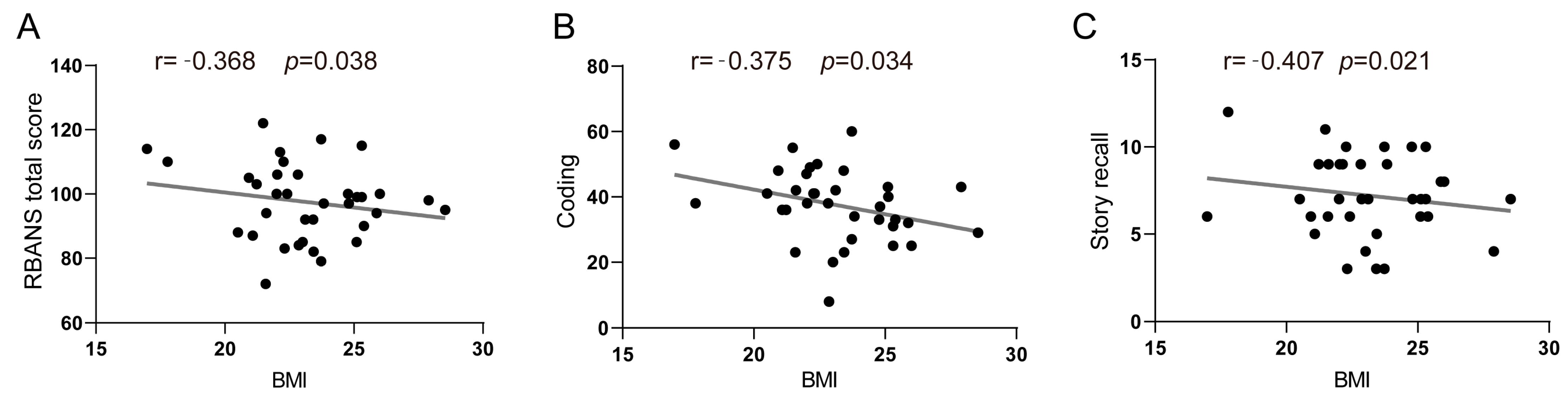
| Coding | −0.082 | 0.526 | 0.244 | 0.212 | −0.375 | 0.034 * |
| Delayed memory | −0.013 | 0.921 | 0.129 | 0.514 | −0.178 | 0.330 |
| List recall | −0.009 | 0.944 | 0.023 | 0.906 | −0.056 | 0.759 |
| List recognition | −0.050 | 0.699 | 0.139 | 0.481 | −0.320 | 0.074 |
| Story recall | 0.019 | 0.881 | 0.166 | 0.400 | −0.407 | 0.021 * |
| Figure recall | 0.126 | 0.330 | 0.176 | 0.369 | 0.003 | 0.989 |
| RBANS total scale score | −0.056 | 0.665 | 0.252 | 0.196 | −0.368 | 0.038 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Jiang, L.; Wei, K.; Yang, J.; Cao, X.; Li, C. Sex-Specific Association of Body Mass Index with Hippocampal Subfield Volume and Cognitive Function in Non-Demented Chinese Older Adults. Brain Sci. 2024, 14, 170. https://doi.org/10.3390/brainsci14020170
Lin S, Jiang L, Wei K, Yang J, Cao X, Li C. Sex-Specific Association of Body Mass Index with Hippocampal Subfield Volume and Cognitive Function in Non-Demented Chinese Older Adults. Brain Sciences. 2024; 14(2):170. https://doi.org/10.3390/brainsci14020170
Chicago/Turabian StyleLin, Shaohui, Lijuan Jiang, Kai Wei, Junjie Yang, Xinyi Cao, and Chunbo Li. 2024. "Sex-Specific Association of Body Mass Index with Hippocampal Subfield Volume and Cognitive Function in Non-Demented Chinese Older Adults" Brain Sciences 14, no. 2: 170. https://doi.org/10.3390/brainsci14020170






